In vitro maturation as a fertility preservation strategy in cancer patients
Syrkasheva A.G., Dobrokhotova Yu.E., Gokhberg Ya.A., Troshina M.N., Sorokin Yu.A., Lapina I.A.
Objective: To describe a case series on the use of in vitro maturation (IVM) technology in patients with cancer.
Materials and methods: This study included 34 patients aged 23–43 years who presented to the IVF Department of the Medsi on Solyanka Clinical and Diagnostic Center for fertility preservation programs. Transvaginal puncture of the antral follicles was performed, followed by aspiration of the follicular fluid. The resulting oocyte-cumulus complexes (OCCs) were matured for 24–48 h in an embryology laboratory.
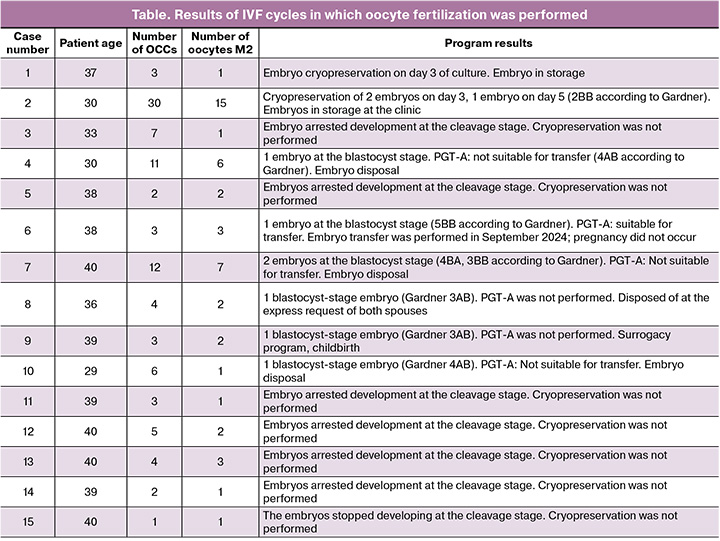
Results: IVM were performed in 34 patients. In one case, no oocytes were retrieved, and in five cases, mature oocytes were not obtained after the culture. A total of 185 OCCs were retrieved, averaging 5.4 OCCs per patient. Additionally, 95 mature oocytes at the MII stage were retrieved, averaging 2.9 per patient, resulting in an average maturation rate of 51.8%. Oocyte cryopreservation was conducted in 13 patients, resulting in a total of 47 oocytes, or an average of 3.6 oocytes per patient. Fertilization was performed using oocytes obtained after IVM in 15 patients. In 10 cases, embryos were not produced (due to lack of cleavage or embryos being unsuitable for transfer according to the PGT-A results). In five cases, embryos suitable for transfer to the uterine cavity were obtained (either euploid according to PGT-A or PGT-A was not performed). Embryo storage continued in two cases, while one patient discontinued embryo storage. Two thawed embryos were transferred: one into the patient's uterine cavity, which did not result in pregnancy, and the other into a surrogate mother's uterus, resulting in pregnancy and delivery.
Conclusion: IVM is a promising approach for fertility preservation in patients with cancer in whom ovarian stimulation is not feasible. The effectiveness of this technique is influenced by the patient's baseline parameters, primarily age and ovarian reserve. However, further research is required to standardize the approaches and enhance the efficacy of this technology.
Authors' contributions: Syrkasheva A.G., Lapina I.A. – conception and design of the study; Gokhberg Ya.A. – data collection and processing; Syrkasheva A.G., Sorokin Yu.A. – statistical analysis; Gokhberg Ya.A., Syrkasheva A.G., Troshina M.N. – drafting of the manuscript; Dobrokhotova Yu.E. – editing of the manuscript
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Syrkasheva A.G., Dobrokhotova Yu.E., Gokhberg Ya.A., Troshina M.N., Sorokin Yu.A.,
Lapina I.A. In vitro maturation as a fertility preservation strategy in cancer patients.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (10): 83-89 (in Russian)
https://dx.doi.org/10.18565/aig.2025.210
Keywords
Improving the quality of life of patients with cancer is a priority in modern medicine. In recent decades, the medical community has made significant strides in the early detection and treatment of malignant neoplasms, leading to improved prognoses and a notable increase in the life expectancy of these patients. According to updated data from the World Health Organization, the most common cancers among women of reproductive age are breast cancer (BC), endometrial cancer, cervical cancer (CC), ovarian cancer, and other oncological entities [1, 2]. Furthermore, recent epidemiological observations indicate that at least one in five cancer cases is diagnosed in women who have not yet achieved reproductive function [3].
Gonadotoxic effects of antitumor therapy can result in iatrogenic premature ovarian failure. The American Society of Clinical Oncology (ASCO) guidelines recommend discussing fertility preservation with patients during the initial stage of cancer treatment, followed by referral to specialized reproductive medicine centers for the evaluation of possible strategies.
Significant advances in cryopreservation techniques have enabled the vitrification of oocytes and ovarian tissue, providing numerous options for preserving the fertility of patients with cancer. Consequently, a new field known as "oncofertility" has emerged in modern medicine, with reproductive medicine specialists encountering a new patient population with serious illnesses requiring urgent treatment. Oocyte vitrification remains the most well-studied and effective method for preserving fertility in patients with cancer. However, in certain situations, its use is not feasible; for example, some cancers are incompatible with the 12–14 day period required for ovarian stimulation or with the increased estradiol levels that accompany it. Ovarian tissue cryopreservation presents a possible alternative to ovarian stimulation; however, this method also has limitations, particularly concerning the potential risks associated with ovarian tissue transplantation.
In vitro maturation (IVM), which involves the maturation of immature oocytes in an embryology laboratory, has emerged as a promising method for preserving fertility in patients with cancer [4]. IVM offers new possibilities for fertility preservation, as it avoids ovarian stimulation and the associated increase in estrogen levels, which is especially crucial for hormone-dependent tumors [5]. In 2021, the American Society for Reproductive Medicine asserted that IVM could serve as an alternative treatment option for patients with estrogen-sensitive cancers or those with a limited window for fertility preservation programs [6]. Physiologically, IVM involves the maturation of oocyte-cumulus complexes (OCCs) isolated at the prophase I stage (germinal vesicle, GV), followed by meiosis I (MI) and reaching metaphase II (MII) [7]. This method includes two sequential stages: first, follicular fluid is collected, and OCCs are identified; second, oocytes are cultured in vitro until they reach full maturity. The culture time may vary based on the initial state of the oocyte: transitioning from GV to MII typically requires up to 48 h, whereas progression from MI to MII takes up to 24 h [5].
Although this technology is no longer considered experimental, the number of IVM cycles performed worldwide remains significantly lower than that of assisted reproductive technologies (ART) involving ovarian stimulation. The literature indicates that the effectiveness of IVM does not depend on the menstrual cycle phase (early follicular, late follicular, luteal) or on "priming" with gonadotropins before the procedure [8, 9]. The expected number of oocytes and embryos obtained is lower than that obtained with ovarian stimulation. Additionally, experts note that IVM technology is effective only under specific conditions related to antral follicle count (AFC) and anti-Müllerian hormone (AMH) levels, with threshold values of AMH ≥3.5 ng/ml and AFC ≥19 (necessary to obtain at least eight mature oocytes) [10]. Evaluating the clinical efficacy of IVM cycles in patients with cancer presents challenges due to the unique nature of the cancer process. Creux et al. analyzed 13 years of experience in fertility preservation for cancer patients and found that 23 (6.5%) of 353 women returned for oocyte/embryo thawing within the specified period. Among these, four patients had previously undergone an IVM program with oocyte vitrification, followed by thawing, fertilization, and embryo transfer into the uterine cavity; none achieved pregnancy after this procedure. Additionally, ten patients had previously undergone an IVM program with embryo vitrification, of which one became pregnant and gave birth, while another's pregnancy ended in spontaneous miscarriage at 7–8 weeks of gestation; in the remaining cases, pregnancy did not occur. It is important to note that the proportion of women seeking oocyte and embryo thawing in this patient group may be low, as corroborated by previous studies.
This study aimed to describe a case series of the use of IVM technology in patients with cancer.
Materials and methods
The study design was a case series, with patient selection criteria based on participation in the IVF program for fertility preservation prior to cancer treatment between 2021 and 2024, conducted at the IVF Department of Medsi at Solyanka Clinical and Diagnostic Center. Thirty-four patients were included in this study.
All patients received a certificate from their oncologists confirming the absence of contraindications to treatment and underwent the necessary examinations in accordance with the regulations of the Russian Ministry of Health. All patients provided informed consent to participate in the study.
Transvaginal puncture of the antral follicles was performed under intravenous anesthesia, followed by aspiration of the follicular fluid. The resulting OCCs were matured for 24–48 h in an embryology laboratory.
Embryological stage
Oocyte cultivation and maturation: Immature oocytes at the GV stage were subjected to IVM for 24–48 h using specialized maturation media: Origio IVM (Origio, Denmark) supplemented with hormones (75 mIU follicle-stimulating hormone and 100 mIU human chorionic gonadotropin).
Oocytes obtained by puncture (Fig. 1) were placed in an Origio Planner incubator with a premixed gas mixture of 5% O₂, 6% CO₂, and nitrogen (N₂) at 37°C. Visual monitoring of maturity was conducted at 24- and 48-hour intervals. Oocytes that reached the MII stage were identified by the presence of the first polar body and were cryopreserved or fertilized.
Maturity was recorded during both the "first wave" (24 hours after OCC collection) and the "second wave" (48 h after antral follicular fluid aspiration). For the fertilization process, intracytoplasmic sperm injection (ICSI) was used to fertilize mature oocytes, followed by standard embryo culture to the blastocyst stage in Vitrolife single-stage G-TL medium. In some cases, cleavage-stage embryos were vitrified, whereas in others, embryos were cultured to the blastocyst stage. Trophectoderm biopsy and preimplantation genetic testing for aneuploidies (PGT-A) were conducted at the patient's request, using the NGS method.
Results
The study included 34 reproductive-age patients (aged 23–43 years) seeking fertility preservation while undergoing oncology treatment, with an average age of 36 years. The oncological profiles of the examined patients included the following diagnoses: breast cancer (67.6%, n=23), cervical cancer (23.5%, n=8), and other oncological diseases (8.9%, n=3). Consistent with numerous other domestic and international studies, breast cancer was the most common pathology, necessitating systemic chemotherapy, which has a potentially significant gonadotoxic effect.
All patients underwent antral follicle puncture without prior hormonal stimulation. A total of 185 oocyte-cumulus complexes (OCCs) were obtained, averaging 5.4 OCCs per patient. Of these, 95 mature oocytes at the MII stage were retrieved, resulting in an average of 2.9 mature oocytes per patient. The average maturation rate was 51.8%.
Notably, in five patients (12.8%), no mature oocytes were obtained through culture, making cryopreservation impossible. In 13 women (33.3%), mature oocytes at the MII stage were vitrified, with a total of 47 cryopreserved oocytes in this group, averaging 3.6 oocytes per patient. In the remaining cases, oocyte fertilization was performed (Fig. 2).
In 15 clinical cases (44.1%), a decision was made to fertilize the retrieved mature oocytes using ICSI, followed by cryopreservation of the embryos. The case series in which oocyte fertilization was performed is described below (table).
In seven cases (46.7%, cases 3, 5, 11, 12, 13, 14, and 15), a complete absence of embryo cleavage was observed despite the presence of mature oocytes. The average age of the patients in this subgroup was 38.5 years, suggesting a likely age-related decline in oocyte quality. The average number of mature oocytes in these patients was 1.6, indicating a decreased ovarian reserve. These data support the hypothesis that the effectiveness of IVM programs in patients of advanced reproductive age and/or with preexisting decreased ovarian reserve is extremely low, and this technique should not be recommended for this patient group.
In three cases (20.0%, cases 4, 7, and 10), blastocysts were obtained and PGT-A was performed. According to the PGT-A results, the embryos were deemed unsuitable for transfer and discarded. In two cases (13.3%, cases 1 and 2), embryos were cryopreserved on days 3 and 5 of culture and are currently stored at the clinic. In case 8, an embryo was cryopreserved without PGT-A; however, at the persistent request of both spouses, the embryo was discarded. In one case (case 6), PGT-A indicated that the embryo was suitable for transfer into the uterine cavity. A thawed embryo transfer cycle was performed; however, pregnancy did not occur.
In one case (number 9), a thawed embryo was transferred to the surrogate mother's uterus, resulting in a clinical pregnancy. The pregnancy proceeded uneventfully, and the surrogate mother delivered spontaneously at 38 weeks. A boy was born weighing 3,450 g and measuring 51 cm in height, with no visible birth defects.
Discussion
The issue of fertility preservation in patients with cancer is becoming increasingly important due to the rising life expectancy resulting from advances in early diagnosis and treatment. A key area of focus is improving methods to preserve the reproductive potential of women undergoing gonadotoxic treatment. IVM is one of the most promising technologies, particularly in situations where treatment delay is impossible or ovarian stimulation is contraindicated.
According to the updated ASCO guidelines, discussions about fertility preservation should be an integral part of the care pathway for patients diagnosed with malignancy, especially for women of reproductive age. These guidelines emphasize the importance of referring patients to ART clinics before the initiation of chemotherapy or radiation therapy. Notably, IVM allows oocyte retrieval without hormonal stimulation, producing mature eggs for subsequent cryopreservation. In some cases, the procedure can be performed within 2–3 days of the patient's initial presentation, which is crucial when urgent treatment is required [11].
Gotschel F. et al. [8] demonstrated that the IVM program, unlike standard ovarian stimulation protocols, does not require a specific phase of the menstrual cycle. This flexibility expands the feasibility of the procedure and alleviates the time constraints.
In a review article by Anbari F. et al. [12], strategic approaches to preserving fertility in women with cancer were discussed, including the prospects of combining IVM with other methods, such as ovarian tissue cryopreservation. This multimodal strategy may enhance the likelihood of pregnancy after treatment completion, particularly in patients with reduced ovarian reserve or those at high risk of gonadotoxic therapy.
Several original studies by Russian researchers have explored this technology in patients with cancer. In the study by Lapina I.A. et al. [13], data from five patients were analyzed. Forty-six oocytes were obtained via transvaginal ovarian puncture. After 28 h of culture in IVM medium, 30 (46%) oocytes reached the MII stage, 14 (22%) reached the MI stage, and 12 (18%) were at the prophase I stage. An additional oocyte reached the MII stage after a further 24 h of culture.
Assessing the effectiveness of IVM cycles in cancer patients is challenging because of the low proportion of women who seek oocyte thawing/fertilization or embryo thawing. This is influenced by both the characteristics of the cancer (therapy may extend over several years, ART is only possible after achieving stable remission, and there is the potential for a negative disease course, such as the patient’s death) and changes in women's perspectives on their reproductive plans. For instance, in our study, one patient chose to dispose of the embryo obtained before treatment, stating during the interview that she was not ready to carry a pregnancy or pursue a surrogacy program.
In our study, none of the 13 patients who vitrified their oocytes sought fertilization. Among the 15 cycles in which oocyte fertilization was performed, five resulted in embryos suitable for transfer (either euploid according to PGT-A or not performed). Embryo storage continued in two cases, whereas one patient discontinued storage. In two instances, a thawed embryo was transferred: in one case, into the patient's uterus, where pregnancy did not occur, and in the other, into a surrogate mother's uterus, resulting in a successful pregnancy and delivery.
The accumulated data confirm the value of IVM as a fertility preservation tool in patients with cancer. This technique is safe, flexible in timing, and eliminates the need for preoperative hormonal stimulation. This is particularly useful for patients with contraindications to traditional ART regimens. However, it is important to note that this technique currently has reduced efficacy compared with ovarian stimulation. The literature indicates that a prerequisite for IVM is the presence of preserved (or near-excessive) ovarian reserve: AMH ≥ 3.5 ng/ml and AFC ≥ 19 are necessary to obtain eight mature oocytes, while higher AMH and AFC levels are needed to secure a larger number of oocytes. However, eight mature oocytes are not always sufficient to achieve at least one healthy birth, especially in patients of advanced reproductive ages. Although the age threshold for using this technology has not been defined, it should be considered primarily for younger patients. Safety studies of the IVM technique have not demonstrated an increased risk of imprinting abnormalities or chromosomal aberrations in oocytes associated with this method [6]. The development of children born using IVM has been studied in a limited number of cases, and no significant differences have been found compared to children born through standard ART cycles; however, the small number of reported cases limits the reliability of these findings.
This procedure should be continued in situations where other fertility preservation methods are not feasible, and ongoing monitoring of patients, the course of pregnancy and childbirth, and the health of the resulting children are essential.
Conclusion
IVM represents a promising approach in fertility preservation programs for patients with cancer when ovarian stimulation is not feasible. The effectiveness of this technique is primarily dependent on the patient's baseline parameters, particularly age and ovarian reserve. However, further research is required to standardize the approaches and enhance the effectiveness of this technology.
References
- Filho A.M., Laversanne M., Ferlay J., Colombet M., Piñeros M., Znaor A. et al. The GLOBOCAN 2022 cancer estimates: data sources, methods, and a snapshot of the cancer burden worldwide. Int. J. Cancer. 2025; 156(7): 1336-46. https://dx.doi.org/10.1002/ijc.35278
- Ferlay J., Colombet M., Soerjomataram I., Parkin D.M., Piñeros M., Znaor A. et al. Cancer statistics for the year 2020: an overview. Int. J. Cancer. 2021. https://dx.doi.org/10.1002/ijc.33588
- Rives N., Courbière B., Almont T., Kassab D., Berger C., Grynberg M. et al. What should be done in terms of fertility preservation for patients with cancer? The French 2021 guidelines. Eur. J. Cancer. 2022; 173: 146-66. https://dx.doi.org/10.1016/j.ejca.2022.05.013
- Михайлова Н.Д., Мишиева Н.Г., Кириллова А.О., Мартазанова Б.А., Джинчарадзе Л.Г. Дозревание ооцитов in vitro. Акушерство и гинекология. 2021; 11: 64-70. [Mikhailova N.D., Mishieva N.G., Kirillova A.O., Martazanova B.A., Jincharadze L.G. Maturation of oocytes in vitro. Obstetrics and Gynecology. 2021; (11): 64-70 (in Russian)]. https://dx.doi.org/10.18565/aig.2021.11.64-70
- Anderson R.A., Amant F., Braat D., D'Angelo A., Chuva de Sousa Lopes S.M., Demeestere I. et al.; ESHRE Guideline Group on Female Fertility Preservation. ESHRE guideline: female fertility preservation. Hum Reprod Open. 2020; 2020(4): hoaa052. https://dx.doi.org/10.1093/hropen/hoaa052
- Practice Committees of the American Society for Reproductive Medicine, the Society of Reproductive Biologists and Technologists, and the Society for Assisted Reproductive Technology. Electronic address: jgoldstein@asrm.org. In vitro maturation: a committee opinion. Fertil. Steril. 2021; 115(2): 298-304. https://dx.doi.org/10.1016/j.fertnstert.2020.11.018
- Jiang Y., He Y., Pan X., Wang P., Yuan X., Ma B. Advances in oocyte maturation in vivo and in vitro in mammals. Int. J. Mol. Sci. 2023; 24(10): 9059. https://dx.doi.org/10.3390/ijms24109059
- Gotschel F., Sonigo C., Becquart C., Sellami I., Mayeur A., Grynberg M. New insights on in vitro maturation of oocytes for fertility preservation. Int. J. Mol. Sci. 2024; 25(19): 10605. https://dx.doi.org/10.3390/ijms251910605
- Kedem A., Yerushalmi G.M., Brengauz M., Raanani H., Orvieto R., Hourvitz A. et al. Outcome of immature oocytes collection of 119 cancer patients during ovarian tissue harvesting for fertility preservation. J. Assist. Reprod. Genet. 2018; 35(5): 851-6. https://dx.doi.org/10.1007/s10815-018-1153-1
- Sonigo C., Simon C., Boubaya M., Benoit A., Sifer C., Sermondade N. et al. What threshold values of antral follicle count and serum AMH levels should be considered for oocyte cryopreservation after in vitro maturation? Hum. Reprod. 2016; 31(7): 1493-500. https://dx.doi.org/10.1093/humrep/dew102
- Su H.I., Lacchetti C., Letourneau J., Partridge A.H., Qamar R., Quinn G.P. et al. Fertility preservation in people with cancer: ASCO guideline update. J. Clin. Oncol. 2025; 43(12): 1488-515. https://dx.doi.org/10.1200/JCO-24-02782
- Anbari F., Khalili M.A., Mahaldashtian M., Ahmadi A., Palmerini M.G. Fertility preservation strategies for cancerous women: an updated review. Turk. J. Obstet. Gynecol. 2022; 19(2): 152-61. https://dx.doi.org/10.4274/tjod.galenos.2022.42272
- Лапина И.А., Доброхотова Ю.Э., Сорокин Ю.А., Малахова А.А., Чирвон Т.Г., Таранов В.В., Германович Н.Ю., Ковальская Е.В., Кайкова О.В., Гомзикова В.М., Твердикова М.А. Сохранение репродуктивного материала при помощи метода in vitro maturation у пациенток с онкологическими заболеваниями. Гинекология. 2022; 24(1): 41-6. [Lapina I.A., Dobrokhotova Yu.E., Sorokin Iu.A., Malakhova A.A., Chirvon T.G., Taranov V.V., Germanovich N.Iu., Koval'skaia E.V., Kaikova O.V., Gomzikova V.M., Tverdikova M.A. Preservation of reproductive material using the in vitro maturation method in patients with oncological diseases. Gynecology. 2022; 24(1): 41-6 (in Russian)]. https://dx.doi.org/10.26442/20795696.2022.1.201350
Received 06.08.2025
Accepted 07.10.2025
About the Authors
Anastasiya G. Syrkasheva, Dr. Med. Sci., Associate Professor, Department of Obstetrics and Gynecology, Institute of Surgery, Pirogov Russian National Research Medical University, Ministry of Health of Russia; Head of the Department of Assisted Reproductive Technologies, CDC «Medsi on Solyanka», 109028, Russia, Moscow, Solyanka str., 12, bld. 1, +7(926)363-17-20, anast.syrkasheva@gmail.com, https://orcid.org/0000-0002-7150-2230Yulia E. Dobrokhotova, Dr. Med. Sci., Professor, Head of the Department of Obstetrics and Gynecology, Institute of Surgery, Pirogov Russian National Research
Medical University, Ministry of Health of Russia, 117513, Russia, Moscow, Ostrovityanova str., 1, bld. 9, pr.dobrohotova@mail.ru, https://orcid.org/0000-0002-9091-4097
Yael A. Gokhberg, PhD, obstetrician-gynecologist, Department of Assisted Reproductive Technologies, CDC «Medsi on Solyanka», 109028, Russia, Moscow, Solyanka str.,
12, bld. 1, gohberg.ya@medsigroup.ru, https://orcid.org/0000-0003-3637-6096
Maria N. Troshina, Head of the Embryological Laboratory of the Department of Assisted Reproductive Technologies, CDC «Medsi on Solyanka», 109028, Russia, Moscow, Solyanka str., 12, bldg. 1, troshina.mn@medsigroup.ru
Yuri A. Sorokin, Head of the Center for Reproductive Health, CDC «Medsi on Solyanka», 109028, Russia, Moscow, Solyanka str., 12, bld. 1, Sorokin.Yua@medsigroup.ru, https://orcid.org/0000-0001-9305-323X
Irina A. Lapina, Dr. Med. Sci., Professor, Department of Obstetrics and Gynecology, Institute of Surgery, Pirogov Russian National Research Medical University,
Ministry of Health of Russia; obstetrician-gynecologist, CDC «Medsi on Solyanka», 109028, Russia, Moscow, Solyanka str., 12, bld. 1, lapina.ia@medsigroup.ru,
https://orcid.org/0000-0002-2875-6307
Corresponding author: Anastasiya G. Syrkasheva, anast.syrkasheva@gmail.com



