Feasibility of preimplantation genetic testing for aneuploidy in patients undergoing in vitro fertilization after conservative treatment of atypical hyperplasia and early endometrial cancer
Relevance: Atypical endometrial hyperplasia (AEH) and early endometrial cancer (EC) are frequently detected in female patients before the realization of their reproductive potential. Patients of older reproductive age with AEH and early EC have an increased risk of chromosomal pathology in offspring. Objective: To investigate the feasibility of preimplantation genetic testing for aneuploidy (PGT-A) of embryos in patients with a history of AEH and early EC undergoing IVF. Materials and methods: Fifty patients aged 25–43 years (median 35 years) who underwent IVF after conservative hormonal treatment for AEH and early EC constituted the study group. The control group included 40 patients with tubal-peritoneal and mild male factor infertility. We studied the pregnancy rate (PR) in patients of different age groups and the association between pregnancy rate and PGT-A during IVF. Results: Euploid blastocysts were obtained from 32% (32/100) of patients in the study group, of whom 74% (17/23) were aged <35 and 19% (15/77) ≥35 years. The rate of euploid embryos in the control group was comparable. The PR among all the patients in the study group was 32% (19/60); among them it was 38% (12/32) and 25% (7/28) in women <35 and ≥35 years, respectively. In the control group, PR was 41% (22/54) among all patients, including 46% (13/28) in patients of young reproductive age and 35% (9/26) in women ≥35 years. There was a correlation between PGT-A and PR. Among all patients in the study group who did and did not undergo PGT-A, PR was 47% (7/15) and 27% (12/45), respectively. In the control group, PR was 47% (9/19) among all patients who underwent PGT-A and 34% (12/35) among patients who refused PGT-A. Conclusion: The PGT-A of embryos during IVF can exclude chromosomal abnormalities in the retrieved embryos and increase PR by transferring an euploid embryo into the uterine cavity. Authors’ contributions: Krasnopolskaya K.V., Novikova O.V. – conception and design of the study; Krasnopolskaya K.V., Shishkina A.V., Shostenko L.V. – data collection and analysis; Shishkina A.V., Shostenko L.V., Isakova K.M., Rau D.I. – manuscript drafting; Krasnopolskaya K.V., Novikova O.V. – manuscript editing. Conflicts of interest: The authors have no conflicts of interest to declare. Funding: There was no funding for this study. Ethical Approval: The study was approved by the Research Ethics Committee of the Moscow Regional Research Institute of Obstetrics and Gynecology. Patient Consent for Publication: All patients provided informed consent for the publication of their data. Authors’ Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator. For citation: Krasnopolskaya K.V., Novikova O.V., Isakova K.M., Shostenko L.V., Rau D.I., Shishkina A.V. Feasibility of preimplantation genetic testing for aneuploidy in patients undergoing in vitro fertilization after conservative treatment of atypical hyperplasia and early endometrial cancer. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; 1: 76-82 (in Russian) https://dx.doi.org/10.18565/aig.2022.245Krasnopolskaya K.V., Novikova O.V., Isakova K.M., Shostenko L.V., Rau D.I., Shishkina A.V.
Keywords
Preimplantation genetic testing for aneuploidies (PGT-A) remains one of the most widely discussed topics in reproductive medicine. In some coun-tries, according to various authors, the proportion of in vitro fertilization (IVF) programs with PGT-A reaches 40% [1]. Both supporters and oppo-nents of this technique have shown considerable interest in its effectiveness and feasibility [1, 2]. One of the pressing issues in assisted reproduction is that couples with a high risk of chromosomal abnormalities in offspring must face the problem of not getting pregnant, not conceiving, or the need to terminate pregnancy after prenatal diagnosis at gestational stage [3].
Morphological evaluation of blastocysts is the main method to select embryos for transfer into the uterine cavity. However, to date, morphology is known to not necessarily correlate with euploidy of the selected embryos. Besides, the extent to which the presence of aneuploidy in embryos affects their morphology remains unclear. The exclusion of aneuploidy is essential in embryo selection because the presence of chromosomal abnormalities rep-resents one of the main causes of implantation and pregnancy failure [4–6]. Despite the high cost of PGT-A, at present, its use in IVF programs is high-ly warranted for patients with an increased risk to produce aneuploid em-bryos, especially women of late reproductive age, as it provides a wider range of opportunities to avoid the risk of having a child with chromosomal abnormalities [4].
This study aimed to investigate the feasibility of preimplantation genetic testing for aneuploidy (PGT-A) of embryos in patients with a history of AEH and early EC undergoing IVF.
Materials and methods
The study enrolled 50 patients who underwent IVF at the Department of Reproduction, Moscow Regional Scientific Research Institute of Obstetrics and Gynecology, after conservative hormonal treatment of AEH and early EC. Forty patients with tubal-peritoneal and mild male factor infertility were included in the control group.
All patients in the study group underwent conservative hormonal thera-py before entering an assisted reproductive technology (ART) program. At the first stage, they underwent hysteroscopy with fractional diagnostic curet-tage and received an intrauterine levonorgestrel-containing system (52 mg levonorgestrel). The efficacy of therapy was monitored every 3 months by hysteroscopy and fractional diagnostic curettage. Patients were recommend-ed to undergo IVF programs and implement reproductive plans when cure was achieved, namely, when the absence of endometrial atypia was deter-mined by histological examination after 6 months from the start of treat-ment. During IVF programs, PGT-A of the embryos was recommended for patients of late reproductive age (35 years and older) due to the increased risk of age-related chromosomal abnormalities in the fetus. To prevent re-currence of AEH and EC, the levonorgestrel-containing system was removed after the PGT-A results were obtained just before the embryo transfer. All patients underwent frozen-thaw embryo transfer after exclusion of disease recurrence.
In the control group of patients, PGT-A was also recommended in IVF programs for women over 35 years old.
The study evaluated the age at the time of entry into the IVF program, their ovarian reserve, the frequency of PGT-A, the number of uterine em-bryos suitable for transfer according to PGT-A and the pregnancy rate (PR).
Results
The study included patients aged 25 to 43 years at the time of entry into the IVF program, with a median age of 35 years. Among patients with a his-tory of AEH, the proportion of women of advanced reproductive age was 53% (16/30) and among patients with early EC, 70% (14/20). In the control group, the proportion of women of advanced reproductive age was 55% (22/40) (Table 1).
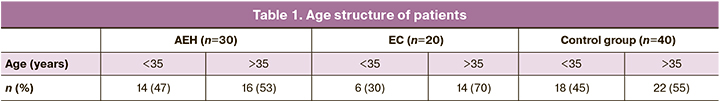
Most of the patients (66%) had preserved the ovarian reserve with anti-Müllerian hormone (AMH) of 1.2 ng/ml, while 34% of the patients had di-minished ovarian reserve (AMH1.2 ng/ml). Patients with early AEH and EC did not differ in terms of their ovarian reserve status. In the control group as well, the majority of the patients (63%) had AMH over 1.2 ng/ml, and 37% of the patients had a diminished ovarian reserve.
PGT-A in IVF programs was performed in 27% of the women in the study study group. The proportion of patients of young reproductive age who underwent PGT-A was 12.5%, and 43% of patients 35 years and older. In the control group, PGT-A was performed in 35% of patients. The pro-portion of women under 35 years of age was 14% and 48% of women 35 years of age and older.
The yield of healthy blastocysts according to PGT-A among all patients in the study group was 32% (32/100), including 74% (17/23) under 35 years of age. The proportion of euploid embryos decreased almost fourfold to 19% (15/77) in patients aged 35 over. In women of young reproductive age with a history of AEH, the yield of healthy blastocysts was 78% (14/18); in women of late reproductive age, it was 19% (13/69). In patients with a his-tory of EC in young reproductive age, the yield of healthy blastocysts was 60% (3/5), in late reproductive age patients 25% (2/8). In the yield of control group, the euploid embryos among all patients was 40% (23/58), including 65% (15/23) under 35 years of age and 23% (8/35) aged over 35 years of age (Table 2).
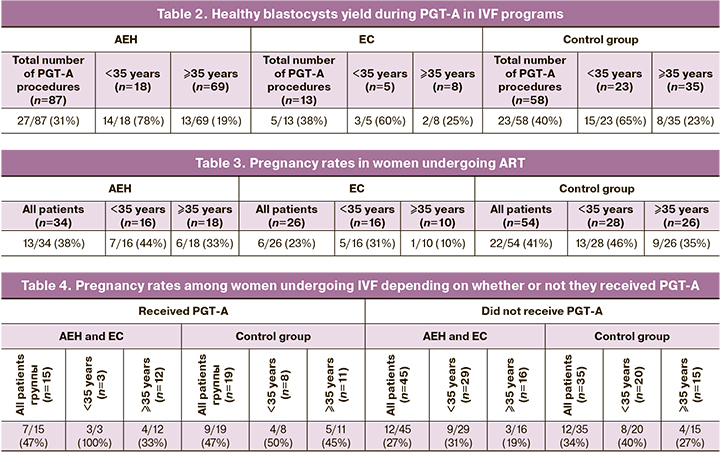
In the study group, a total of 60 embryos were transferred in 50 patients, resulting in 19 pregnancies (32%). PR was 38% (13/34) and 23% (6/26) in the AEH and early EC group, respectively. In the control group, 54 embryos were transferred in 40 patients, resulting in 22 pregnancies. The PR was 41% (22/54) (Table 3).
There was an association between PR and age at the time of admission to the IVF program. The PR was 38% (12/32) in patients under 35 years of age in the study group and 25% (7/28) in women 35 and older. In the AEH group, the PR in women of young reproductive age was 44% (7/16) and 33% (6/18) in the late reproductive age group. In the EC group, PR was 31% (5/16) in patients of young age and 10% (1/10) in late reproductive age. In the control group, PR was 46% (13/28) in patients of younger reproduc-tive age and 35% (9/26) in women aged 35 and older (Table 3).
Analysis of the relationship between PR and PGT-A in IVF showed an association between PGT-A and PR. Among all patients in the study group who did and did not undergo PGT-A, PR was 47% (7/15) and 27% (12/45), respectively.
All women under 35 years of age who underwent PGT-A became preg-nant, PR was 100% (3/3); among women 35 years of age and older, the rate was 33% (4/12). Notably, in IVF without PGT-A, PR was 31% (9/29) in women aged under 35 years of age and 19% (3/16) in those over 35. In the control group, PR was 47% (9/19) among all patients who used PGT-A and 34% (12/35) among patients who refused PGT-A. When PGT-A was per-formed in patients younger than 35 years of age, the PR was 50% (4/8); in women 35 years of age and older, the figure was 45% (5/11). In IVF pro-grams without PGT-A, PR was 40% (8/20) and 27% (4/15) in women under 35 years of age and 35 years and more, respectively (Table 4).
Discussion
Research towards preimplantation diagnosis of genetic disease has been conducted since the mid-1980s with the aim of helping couples who would prefer selection occur at this stage rather than during pregnancy [7]. Preim-plantation genetic diagnosis is a serious alternative to prenatal diagnosis for detecting genetic abnormalities in couples at risk of transmitting genetic dis-ease to offspring, as well as in women of late reproductive age with an in-creased risk of having embryos with chromosomal abnormalities [8, 9].
Preimplantation genetic screening increases the efficiency of ART, im-proves the quality of embryo selection, excludes the presence of aneuploidy, and reduces the risk of conceiving a child with a genetic abnormality [7, 10, 11]. Among the patients in the study group, 12.5% of patients under 35 years of age and 43% aged above 35in underwent PGT-A. According to our data, the yield of healthy blastocysts among all patients was 32% (32/100). The proportion of embryos suitable for transfer, according to PGT-A, was higher in patients of younger age [74% (17/23) vs 19% (15/77)]. In women with a history of AEH in the young reproductive age group, the yield of healthy blastocysts was 78% (14/18), whereas in late reproductive age women only 19% (13/69). Patients with a history of EC in young reproduc-tive age had a healthy blastocyst yield of 60% (3/5) and 25% (2/8) in late re-productive age patients. In the control group, PGT-A was performed in 35% of patients. As in the study group, PGT-A was most often performed in women of advanced reproductive age. The proportion of patients younger than 35 years of age who underwent embryo genetic tests was 14%, and women 35 years of age and older were 48%. In the control group, the eu-ploid embryo yield among all patients was 40% (23/58). This figure was al-so higher in women under 35 years of age [65% (15/23)], while in women of advanced reproductive age it was only 23% (8/35) (Table 2). The data sug-gest an undeniable influence of age on embryo ploidy. However, PGT-A is an expensive method; therefore, not all patients, including those with age-related risk, resort to embryo genetic testing. There were no significant dif-ferences in the proportion of healthy blastocysts between patients with AEH and a history of early EC and those in the control group. Therefore, PGT-A is recommended for all women of advanced reproductive age, regardless of the presence of endometrial pathology. However, it is worth noting that pa-tients with a history of AEH and early EC undergo lengthy hormonal treat-ment in preparation for pregnancy and undergo multiple intrauterine inter-ventions to monitor the effectiveness of therapy and establish cure rates. Women in this group have a high risk of cancer recurrence, so it is desirable to get pregnant as soon as possible after completion of treatment.
In the study group, PR was 38% (12/32) and 25% (7/28) in patients of younger and advanced reproductive age, respectively. In the AEH group, the pregnancy rate was 44% (7/16) in women under 35 years, whereas in wom-en 35 years and older the rate was only 33% (6/18). In the EC group, in pa-tients under 35 years of age, the PR was 31% (5/16), while in late reproduc-tive age the rate was also lower, 10% (1/10). When comparing PR with the control group of patients without a history of AEH and early EC, we ob-tained the following data. The PR in the control group was slightly higher than in the study group and was 46% (13/28) in patients of young reproduc-tive age and 35% (9/26) in women 35 years and older. This pattern may be related to multiple intrauterine interventions, prolonged hormonal treatment, and higher incidence of endometrial hypoplasia in patients with history of AEH and early EC.
According to our findings, there was a tendency for higher PR for em-bryo transfer after genetic diagnosis in both the study and control groups. Among all patients with a history of AEH and early EC who underwent ge-netic diagnosis of embryos, PR was 47% (7/15); among patients who re-fused PGT-A, the rate was significantly lower [27% (12/45)]. After PGT-A, all women aged under 35 became pregnant; among women 35 years of age and older, the rate was 33% (4/12). In IVF programs without genetic diag-nosis, the PR rate was 31% (9/29) in women under 35 years of age and 19% (3/16) in those 35 years and older. Patients in the control group who under-went embryo genetic diagnosis also had a higher PR than those without PGT-A [47% (9/19) vs 34% (12/35), respectively]. When PGT-A was per-formed in younger and older reproductive age patients, PR was 50% (4/8) and 45% (5/11), respectively. In IVF programs without PGT-A, PR was lower [40% (8/20) in women under 35 years of age and 27% (4/15) in those 35 years or older].
Further studies are required to obtain more data. However, our results suggest that the genetic diagnosis of embryos is warranted in women with a history of AEH and CE, especially in the older reproductive age, because af-ter PGT-A, euploid embryos are transferred into the uterine cavity, poten-tially provides a greater chance of implantation, allowing a slightly reduced risk of recurrence of the underlying pathology by reducing the time from the discontinuation of hormonal therapy to the onset of pregnancy.
According to our earlier data, patients in the study groups have a higher incidence of endometrial hypoplasia in IVF programs. In IVF studies with fresh embryo transfer, the incidence of endometrial thickness <7 mm on day of HCG administration varies between 1% and 9.1% [12]. In women with a history of AEH and early EC, the incidence of endometrial hypoplasia in HRT cycles is significantly higher (17%). A significant increase in the inci-dence of endometrial hypoplasia may be due to multiple surgical interven-tions on the endometrium, as well as long-term hormonal treatment of AEH and early EC. The frequency of intrauterine interventions, according to our data, ranged from 4 to 7 in each patient, from the diagnosis of endometrial disease to the planning of embryo transfer. The duration of the use of the levonorgestrel-containing intrauterine system prior to ART with embryo transfer was an average of 9.4 months.
Taking into account the high rate of endometrial hypoplasia in IVF and the risk of recurrence of AEH and early EC associated with estrogen therapy required for endometrial preparation for embryo transfer, the question of PGT-A performance on embryos becomes even more relevant for this cate-gory of patients.
Conclusion
Women with AEH and early EC undergoing IVF represent a challenging group of patients due to the high incidence of endometrial hypoplasia, the need for hormone replacement therapy, and the risk of recurrent AEH and EC. The results of our study suggest that PGT-A should be used in this group of patients. The PGT-A of embryos during IVF can exclude chromo-somal abnormalities in the retrieved embryos and increase PR by transfer-ring an euploid embryo into the uterine cavity.
References
- Treff N.R., Marin D. The "mosaic" embryo: misconceptions and misinterpretations in preimplantation genetic testing for aneuploidy. Fertil. Steril. 2021; 116(5): 1205-11. https://dx.doi.org/10.1016/j.fertnstert.2021.06.027.
- Kuliev A., Rechitsky S. Preimplantation genetic testing: current challenges and future prospects. Expert Rev. Mol. Diagn. 2017; 17(12): 1071-88. https://dx.doi.org/10.1080/14737159.2017.1394186.
- Verlinsky Y., Kuliev A. Human preimplantation diagnosis: needs, efficiency and efficacy of genetic and chromosomal analysis. Baillieres Clin. Obstet. Gynaecol. 1994; 8(1): 177-96. https://dx.doi.org/10.1016/s0950-3552(05)80031-8.
- Fragouli E., Alfarawati S., Spath K., Wells D. Morphological and cytogenetic assessment of cleavage and blastocyst stage embryos. Mol. Hum. Reprod. 2014; 20(2): 117-26. https://dx.doi.org/10.1093/molehr/gat073.
- Greco E., Litwicka K., Minasi M.G., Cursio E., Greco P.F., Baril-lari P. Preimplantation genetic testing: where we are today. Int. J. Mol. Sci. 2020; 21(12): 4381. https://dx.doi.org/10.3390/ijms21124381.
- Gonzalez X.V., Odia R., Naja R., Serhal P., Saab W., Seshadri S., Ben-Nagi J. Euploid blastocysts implant irrespective of their morphology after NGS-(PGT-A) testing in advanced maternal age patients. J. Assist. Reprod. Genet. 2019; 36(8): 1623-9. https://dx.doi.org/10.1007/s10815-019-01496-9.
- Delhanty J.D. Preimplantation diagnosis. Prenat. Diagn. 1994; 14(13): 1217-27. https://dx.doi.org/10.1002/pd.1970141307.
- Dahdou E.M., Balayla J., Audibert F.; Genetics Committee. Technical update: preimplantation genetic diagnosis and screening. J. Obstet. Gynaecol. Can. 2015; 37(5): 451-63. https://dx.doi.org/10.1016/s1701-2163(15)30261-9.
- Малышева О.В., Бичевая Н.К., Гзгзян А.М., Глотов О.С., Кинунен А.А., Лобенская А.Ю., Мекина И.Д., Полякова И.В., Пуппо И.Л., Сайфитдинова А.Ф., Щербак С.Г., Коган И.Ю. Технологические платформы преимплантационного генетического тестирования на анеуплоидии: сравнительная эффективность диагностики хромосомной патологии. Акушерство и гинекология. 2020; 4: 65-71. https://dx.doi.org/10.18565/aig.2020.4.65-71. [Маlysheva О.V., Bichevaya N.K., Gzgzyan А.М., Glotov О.S., Kinunen А.А., Lobenskaya А.Yu., Меkina I.D., Polyakova I.V., Puppo I.L., Saifitdinova А.F., Shcherbak S.G., Kоgan I.Yu. Technological platforms for preimplantation genetic testing for aneuplody: comparative effectiveness of diagnosing chromosomal abnormalities. Obstetrics and Gynecology. 2020; 4: 65-71. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.4.65-71.
- Савостина Г.В., Перминова С.Г., Екимов А.Н., Веюкова М.А. Преимплантационное генетическое тестирование эмбрионов на анеуплоидии: возможности, проблемы и перспективы. Акушерство и гинекология. 2021; 11: 42-9. https://dx.doi.org/10.18565/aig.2021.11.42-49. [Savostina G.V., Perminova S.G., Ekimov A.N., Veyukova M.A. Preimplantation genetic testing for embryo aneuploidy: opportunities, problems, and prospects. Obstetrics and Gynecology. 2021; 11: 42-9. (in Russian)]. https://dx.doi.org/10.18565/aig.2021.11.42-49.
- Валиахметова Э.З., Кулакова Е.В., Скибина Ю.С., Грязнов А.Ю., Сысоева А.П., Макарова Н.П., Калинина Е.А. Неинвазивное тестирование преимплантационных эмбрионов человека in vitro как способ прогнозирования исходов программ экстракорпорального оплодотворения. Акушерство и гинекология. 2021; 5: 5-16. https://dx.doi.org/10.18565/aig.2021.5.5-16. [Valiakhmetova E.Z., Kulakova E.V., Skibina Yu.S., Gryaznov A.Yu., Sysoeva A.P., Makarova N.P., Kalinina E.A. Non-invasive testing of human preimplantation embryos in vitro as a way to predict the outcomes of in vitro fertilization programs. Obstetrics and Gynecology. 2021; 5: 5-16. (in Russian)]. https://dx.doi.org/10.18565/aig.2021.5.5-16.
- Liu K.E., Hartman M., Hartman A. Management of thin endometrium in assisted reproduction: a clinical practice guideline from the Canadian Fertility and Andrology Society. Reprod. Biomed. Online. 2019; 39(1): 49-62. https://dx.doi.org/10.1016/j.rbmo.2019.02.013.
Received 13.10.2022
Accepted 11.01.2022
About the Authors
Ksenia V. Krasnopolskaya, Dr. Med. Sci., Professor, Corr. Member of the RAS, Moscow Regional Scientific Research Institute of Obstetrics and Gynecology,101000, Russia, Moscow, Pokrovka str., 22a, Head of the Department of High Reproductive Technologies, Obstetrician-Gynecologist, PRIOR-CLINIC,
101000, Russia, Moscow, Potapovskiy pereulok, 4/1, +7(985)769-54-94, ksu0207@mail.ru, https://orcid.org/0000-0002-1275-9220
Olga V. Novikova, Dr. Med. Sci., Professor, Obstetrician-Gynecologist, Oncological Gynecologist, PRIOR-CLINIC; Department of Obstetrics and Gynecology,
M.F. Vladimirsky Moscow Regional Clinical Research Institute, +7(903)104-02-23, onov@bk.ru, https://orcid.org/0000-0003-2999-2018,
101000, Russia, Moscow, Potapovskiy pereulok, 4/1.
Kamila M. Isakova, PhD, Obstetrician-Gynecologist, Researcher, Moscow Regional Scientific Research Institute of Obstetrics and Gynecology; Chief Physician,
Clinic BioOptima, +7(903)677-39-89, isa-kama@yandex.ru, https://orcid.org/0000-0001-6194-1654, 101000, Russia, Moscow, Pokrovka str., 22a.
Lidiya V. Shostenko, Obstetrician-Gynecologist, Postgraduate Student at the Department of Reproduction, Moscow Regional Scientific Research Institute of Obstetrics and Gynecology, +7(916)216-94-83, lidsonnn@gmail.com, https://orcid.org/0000-0001-5002-9344, 101000, Russia, Moscow, Pokrovka str., 22a.
Anna V. Shishkina, Obstetrician-Gynecologist, Postgraduate Student at the Department of Reproduction, Moscow Regional Scientific Research Institute of Obstetrics and Gynecology, +7(915)304-94-58, anya.shostenko@yahoo.com, https://orcid.org/0000-0002-4733-5284, 101000, Russia, Moscow, Pokrovka str., 22a.
Daria I. Rau, Obstetrician-Gynecologist, Postgraduate Student at the Department of Reproduction, Moscow Regional Scientific Research Institute of Obstetrics and Gynecology, +7(915)304-94-58, dariarau23@gmail.com, 101000, Russia, Moscow, Pokrovka str., 22a.
Corresponding authors: Lidiya V. Shostenko, lidsonnn@gmail.com; Anna V. Shishkina, anya.shostenko@yahoo.com; Daria I. Rau, dariarau23@gmail.com



