The effect of myo-inositol on the follicular fluid metabolomic profile in women with predicted poor ovarian response undergoing in vitro fertilization: association with oocyte quality
Zakovryashin E.A., Korneeva I.E., Chagovets V.V., Mityurina E.V., Novoselova A.V., Sannikova E.S., Nazarenko T.A., Levin V.A.
Objective: To evaluate the outcomes of IVF and the follicular fluid metabolomic profile in patients with predicted poor ovarian response who received myo-inositol (MI) 1200 mg, alpha-lactalbumin (α-LA), and folic acid 400 mcg at the preconception stage.
Materials and methods: The study included 80 women with a predicted poor ovarian response, belonging to groups 3 and 4 of the POSEIDON classification, who were planning to undergo IVF as part of their infertility treatment. Patients in group 1 received MI 1200 mg, α-LA, and folic acid 400 mcg (Inofert Forte) daily for three months before ovarian stimulation. The women in group 2 received 400 mcg of folic acid. Amino acid and ceramide levels in the follicular fluid were analyzed.
Results: Group 1 showed a significant increase in the number of mature oocytes, zygotes, total blastocysts, and good-quality blastocysts compared with group 2. The use of MI + α-LA resulted in a statistically insignificant increase in the clinical pregnancy rate (27.0% in group 1 versus 10.8% in group 2, p<0.075, OR=3.05 [95% CI: 0.86–10.84]) and ongoing pregnancy rate (21.6% versus 8.1%, respectively, p<0.102, OR=3.13 [95% CI: 0.76–12.88]). The metabolomic profile of women in group 1 was characterized by increased levels of isoleucine, valine, leucine, alpha-aminobutyric acid, histidine, threonine, and proline, along with decreased levels of ceramides Cer (d18:0/24:1), Cer (d18:0/16:0), Cer (d18:0/22:0), Cer (d18:1/23:0), Cer (d18:1/25:0), Cer (d18:2/23:0), and Cer (d18:0/24:0).
Conclusion: Adjuvant therapy with MI before gonadotropic stimulation in patients with a poor ovarian response influences lipid and ceramide metabolism in the follicular microenvironment. This therapy also results in a greater number of mature oocytes and high-quality blastocysts, which positively affects the clinical outcomes of IVF infertility treatment.
Authors’ contributions: Korneeva I.E., Nazarenko T.A. – conception and design of the study; Zakovryashin E.A., Chagovets V.V., Mityurina E.V., Novoselova A.V. – material collection and processing; Zakovryashin E.A., Chagovets V.V., Novoselova A.V., Levin V.A. – statistical analysis; Zakovryashin E.A., Levin V.A. – drafting of the manuscript; Korneeva I.E., Chagovets V.V., Mityurina E.V., Sannikova E.S., Nazarenko T.A. – editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Patient consent for publication: All patients provided informed consent for the publication of their data.
Authors' data sharing statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Zakovryashin E.A., Korneeva I.E., Chagovets V.V., Mityurina E.V., Novoselova A.V., Sannikova E.S., Nazarenko T.A., Levin V.A. The effect of myo-inositol on the follicular fluid metabolomic profile in women with
predicted poor ovarian response undergoing in vitro fertilization: association with oocyte quality.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (4): 94-103 (in Russian)
https://dx.doi.org/10.18565/aig.2025.108
Keywords
Poor ovarian response refers to a clinical scenario in which reduced ovarian reserve leads to low oocyte retrieval in women undergoing in vitro fertilization (IVF) treatment for infertility. Approximately a quarter of patients seeking fertility treatment are diagnosed with follicular reserve depletion, resulting in lower cumulative live birth rates than in women with normal ovarian reserve [1]. To enhance the clinical outcomes of IVF programs for patients with poor ovarian response, the preliminary use of myo-inositol (MI) has been proposed in international literature [2–4]. MI serves as a substrate for universal secondary messengers involved in regulating various biochemical processes in the human body, including key metabolic pathways during the gonadotropin-dependent stage of folliculogenesis. Studies examining the effect of MI have focused solely on changes in embryological stage parameters and clinical outcomes when drawing conclusions about the effectiveness of therapy. Alpha-lactalbumin (α-LA), a water-soluble and thermally stable protein found in mammalian milk, enhances the bioavailability of MI by improving intestinal absorption [5]. A deeper understanding of the mechanisms and effects of MI on ovarian function can be achieved through metabolomic analysis of the follicular fluid. This fluid consists of blood plasma effusion and secretion from granulosa and theca cells, creating a relatively autonomous microenvironment for oocyte development. The metabolic characteristics of the oocyte and surrounding somatic cells are reflected in the composition of follicular fluid, making the study of its components relevant for predicting egg quality [6, 7]. Thus, investigating the metabolomic profile of follicular fluid in patients with poor ovarian response to MI treatment may help identify new markers of drug efficacy.
The study aimed to evaluate the outcomes of IVF and the follicular fluid metabolomic profile in patients with predicted poor ovarian response who received MI 1200 mg, alpha-lactalbumin (α-LA), and folic acid 400 mcg (Inofert Forte) at the preconception stage.
Materials and methods
This prospective study included 80 patients with a predicted poor ovarian response who met the criteria of groups 3 and 4 of the POSEIDON classification (antral follicle count less than 5 and/or anti-Müllerian hormone (AMH) level less than 1.2 ng/ml). The inclusion criteria were as follows: age 21–38 years, body mass index (BMI) between 18.5 and 29.9 kg/m², and follicle-stimulating hormone (FSH) level on the 2nd or 3rd day of the menstrual cycle <15 IU/ml. Exclusion criteria included age <21 or >38 years, BMI <18.5, or ≥30 kg/m²; FSH level on days 2–3 of the menstrual cycle >15 IU/ml; severe pathozoospermia in the spouse (e.g., cryptozoospermia, surgical sperm retrieval), use of donor gametes, and contraindications for embryo transfer in a stimulated cycle (e.g., intrauterine pathology, hydrosalpinx). Participants were divided into two equal groups using the envelope randomization method. Patients in group 1 (n=40) received capsules containing myo-inositol (1200 mg), α-lipoic acid, and folic acid (400 mcg) (Inofert Forte) daily before starting the IVF program. Patients in group 2 (control, n=40) received folic acid at a daily dose of 400 mcg for three months prior to the IVF protocol.
The state of ovarian reserve was assessed based on the blood serum AMH concentration and antral follicle count on the 2nd or 3rd day of the menstrual cycle, as determined by ultrasound examination (US). AMH and FSH concentrations were measured using a Cobas 411 electrochemiluminescence analyzer (Roche, Germany).
Ovarian stimulation was performed according to a protocol involving gonadotropin-releasing hormone (GnRH) antagonists, starting on the 2nd or 3rd day of the menstrual cycle, with recombinant FSH (follitropin-alpha) at an initial dose of 225 IU/day administered subcutaneously. GnRH antagonist (cetrorelix, 0.25 mg/day, subcutaneously) was administered once the leading follicle reached a diameter of 14 mm. When at least one follicle reached a diameter of 17–18 mm, a trigger for final oocyte maturation (human chorionic gonadotropin (hCG), 10, 000 IU intramuscularly, once) was administered. Thirty-five to thirty-six hours after trigger administration, transvaginal puncture (TVP) of the ovaries was performed. Oocyte-cumulus complexes were retrieved from the aspirate and denuded in HEPES buffer. The composition of the follicular fluid metabolites was analyzed using high-performance liquid chromatography coupled with tandem mass spectrometry (HPLC-MS/MS). Follicular fluid aspirate was collected in tubes and delivered to the laboratory for preliminary preparation within 30 minutes. The samples were centrifuged at 14,000 G and +4°C for 10 min. The supernatant was collected and stored at -80°C. A modified Folch method was used for sample preparation for ceramide and amino acid extraction. The ceramide analysis was conducted using an Agilent 1260 Infinity I liquid chromatograph (Agilent Tech. Inc., USA) coupled with a QTRAP 5500 mass spectrometer (ABSciex, Canada). The levels of the 20 amino acids were measured using an Agilent 1260 Infinity II liquid chromatograph (Agilent Tech. Inc., USA), coupled with an Agilent 6460 triple quadrupole tandem mass spectrometer (Agilent Tech. Inc., USA).
Oocyte fertilization was performed using intracytoplasmic sperm injection (ICSI). Zygotes with two pronuclei (2pn) were counted 17–19 h after manipulation. On the 5th day of culture, embryo quality was assessed based on cavity size, the formation of trophectoderm cells, and the inner cell mass according to the classification proposed by Gardner D. (1999). Blastocysts were categorized into three groups: good quality, low quality, and early blastocysts of small size. Starting on the first day after the TVP of the ovaries, micronized progesterone was administered vaginally at a daily dose of 600 mg to support the luteal phase (Iprozhin, Italfarmaco, Italy/France). Embryo transfer was performed on the 5th day of culture in the presence of blastocysts. Serum β-hCG level was measured 14 days after embryo transfer and was considered positive at ≥50 IU/L. Following biochemical confirmation of pregnancy, a transvaginal ultrasound was conducted 4–6 weeks after embryo transfer to assess pregnancy viability.
The study endpoints included total gonadotropin dose, duration of stimulation, number of oocyte-cumulus complexes, number of oocytes at the metaphase II stage, number of zygotes, number and quality of blastocysts, clinical and progressive pregnancy rates per embryo transfer (%), and early reproductive loss rates per embryo transfer (%).
Statistical analysis
Statistical analysis was performed using SPSS version 27.0.1.0. Continuous variables are expressed as means (M) and standard deviations (SD), presented as M (SD), and comparisons were conducted using Student's t-test. The distribution of continuous variables was tested for normality using the Shapiro–Wilk test, graphical presentations in the form of histograms and quantile plots, and analysis of asymmetry and kurtosis. For asymmetric data, results were described using the median (Me), lower (Q25), and upper (Q75) quartiles in the format Me [Q25; Q75], with the Mann–Whitney test used for analysis. Nominal data were assessed using the chi-squared test. To compare binary outcomes, the odds ratio (OR) with a 95% confidence interval (95% CI) was calculated. Statistical significance was set at p<0.05. MetaboAnalyst 6.0 was utilized for semiquantitative analysis of ceramides and amino acids. The data were logarithmized for normalization and automatically scaled. Ward clustering with Euclidean metrics was used to construct a heatmap. Statistically significant differences in metabolite levels between the groups were determined using the nonparametric Wilcoxon rank-sum test (p<0.05) and fold-change values (FC>1.2). Statistical significance was set at p<0.05. MetaboAnalyst 6.0 was used for semiquantitative analysis of ceramides and amino acids. The data were logarithmized for normalization and automatically scaled. Ward clustering with the Euclidean metric was used to construct a heat map. Statistically significant differences in metabolite levels between groups were determined using the nonparametric Wilcoxon rank-sum test (p<0.05) and the fold change values FC>1.2. For amino acids with statistically significant differences between groups, analysis of their involvement in metabolic pathways was performed using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database.
Results
The clinical and medical history data of the patients included in this study are presented in Table 1. The participants in both groups were comparable in age, body mass index, frequency and type of gynecological surgeries, obstetric outcomes, and infertility factors. A history of IVF was reported by 34/80 (42.5%) of the study participants, and only 6/34 (17.6%) achieved clinical pregnancy during treatment. Ovarian resection for benign tumors was previously performed in 8/40 (20.0%) patients in group 1 and 10/40 (25.0%) in group 2.
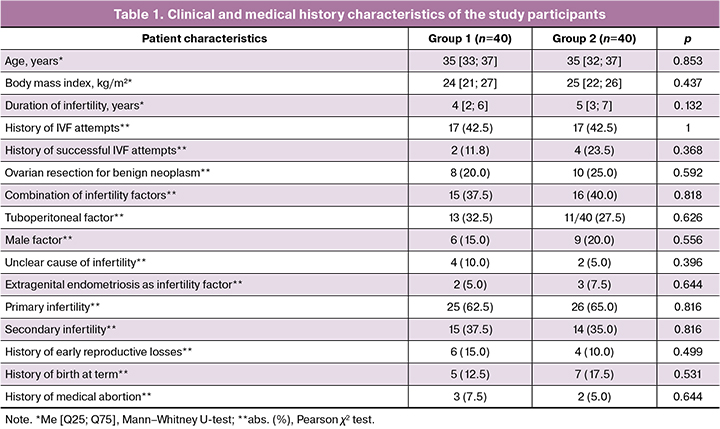
The ovarian reserve markers and hormonal profiles of patients are presented in Table 2. Thus, the mean antral follicle count and median AMH concentration, recorded on the 2nd–3rd day of the menstrual cycle, were equally reduced in the studied groups relative to normal ovarian reserve parameters. The basal FSH level in women from group 1 was comparable to that in patients from group 2 and amounted to 8.72 [7.82; 9.96] mIU/ml versus 8.53 [7.25; 11.12] mIU/ml (p=0.904), and the average estradiol level also did not differ and amounted to 120 [108; 134] pmol/l and 122 [110; 143] pmol/l, respectively (p=0.373).
Thus, at the beginning of the preparatory stage for ovarian stimulation, the clinical and medical history and laboratory data of the patients in both groups were comparable.
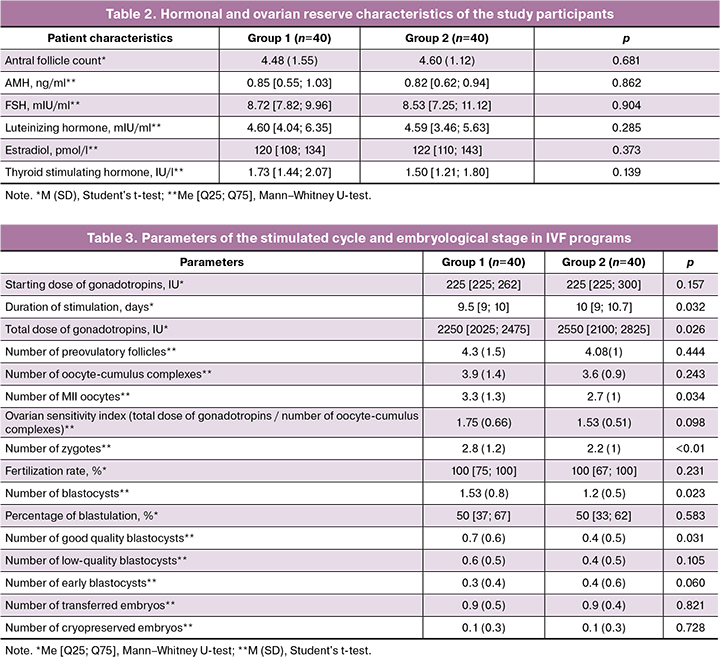
Characteristics of the stimulated cycle and embryological stages of the IVF program are shown in Table 3. The starting doses of gonadotropins were comparable in both groups, amounting to 225 [225; 262] IU and 225 [225; 300] IU, respectively (p=0.157), respectively. Dose adjustment of the drug was required in 7/40 (17.5%) women in group 2, which led not only to an increase in the total dose of gonadotropins (p=0.026), but also to an increase in the duration of ovarian stimulation to 10 [9; 10.7] days (p=0.032).
When evaluating the results, no significant intergroup differences were found in the average number of preovulatory follicles and the number of obtained oocyte-cumulus complexes. However, patients in group 1, who received MI + α-LA at the preconception stage, had an advantage in the number of mature oocytes (3.3 (1.3) vs. 2.7 (1), p=0.034) and the number of zygotes (2.8 (1.2) vs. 2.2 (1), p<0.01). With comparable blastulation rates, women in group 1 showed a significant increase in the absolute number of embryos that reached day 5 of development (1.53 (0.8) versus 1.2 (0.5), p=0.023). It is important to note that this difference was also reflected in the number of blastocysts with high implantation potential – 0.7 (0.6) versus 0.4 (0.5) (p=0.031) in groups 1 and 2, respectively, while the number of low-quality blastocysts (0.6 (0.5) versus 0.4 (0.5) (p=0.105) and early blastocysts (0.3 (0.4) versus 0.4 (0.6) (p=0.060) did not differ between the groups.
The frequency of embryo transfer cancellation and mean number of transferred embryos in the studied groups were comparable. Two embryos were transferred in 5/40 (12.5%) women in group 1 and 3/40 (7.5%) patients in group 2 (p=0.756). The clinical results of the IVF programs are presented in Table 4.

The use of MI + α-LA at the stage of preparation for the IVF program allowed to increase the frequency of clinical pregnancy (27.0% versus 10.8% in groups 1 and 2, respectively, p<0.075, OR=3.05 (95% CI: 0.86–10.84) and progressive pregnancy (21.6% versus 8.1%, p<0.102, OR=3.13 (95% CI: 0.76–12.88), however, the improvement in indicators was not statistically significant. To study the effect of the adjuvant on the activity of various metabolic pathways involved in the growth and development of follicles, the levels of amino acids and lipids (ceramides) in the follicular fluid of patients in the examined groups were analyzed. The heat map made it possible to clearly demonstrated the features of intrafollicular metabolism that arose when using MI + α-LA at the preconception stage (Fig. 1). The color gradient in the range from -0.3 to +0.3 corresponds to the average level of metabolites in each group. When the data were presented visually, a pattern was clearly defined; in patients in group 1, there was an increase in amino acid levels and a decrease in ceramide levels in the follicular fluid.
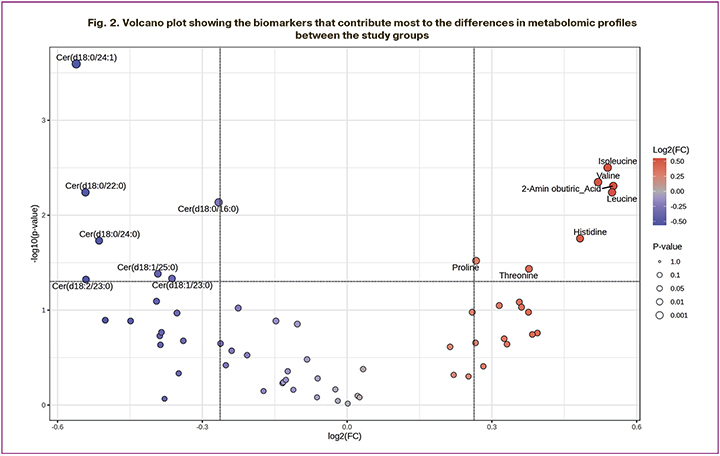
To identify differential biomarkers, we used a volcano plot, which allowed us to combine the range of change (Fold change, FC) and the statistical significance p-value (Fig. 2). For this test, the threshold p<0.05 and FC>1.2 were chosen. According to the graph, the metabolomic profile of follicular fluid of women receiving MI + α-LA is characterized by an increase in the levels of isoleucine, valine, leucine, alpha-aminobutyric acid, histidine, threonine, proline and a significant decrease in the levels of ceramides such as Cer (d18:0/24:1), Cer (d18:0/16:0), Cer (d18:0/22:0), Cer (d18:1/23:0), Cer (d18:1/25:0), Cer (d18:2/23:0), Cer (d18:0/24:0).
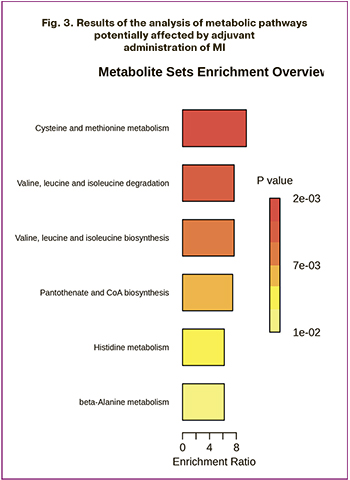
The final stage of the study involved analysis of metabolic pathways involving the identified biomarkers. Over-representation analysis (ORA) was used to determine the metabolic pathways that changed their activity when MI + α-LA was used (Fig. 3). Enrichment of the pathways of cysteine and methionine metabolism (p<0.01), valine, leucine, and isoleucine metabolism (p<0.01), pantoneate and coenzyme A biosynthesis (p<0.01), histidine metabolism (p=0.012), and beta-alanine metabolism (p=0.012) was revealed.
Discussion
Carboxyl polyalcohol MI serves as a precursor to secondary signaling molecules that are crucial for transmitting stimuli from membrane receptors of FSH and insulin to the structures of granulosa cells. Adequate levels of MI in the follicular microenvironment are essential for ensuring proper energy metabolism in granulosa cells, which directly affects oocyte competence [9].
Previous research indicates that using MI during the preparatory phase of ovarian stimulation in patients with a poor ovarian response can positively influence stimulated cycle parameters and embryological outcomes in IVF programs. Caprio F. et al. (2015) reported that combining MI and folic acid for three months prior to entering the IVF program resulted in a significant reduction in the total dose of gonadotropins for this cohort of women and an increase in the number of mature oocytes, compared to the use of folic acid alone [2]. Similarly, Mohammadi S. et al. (2021) confirmed that MI enhances ovarian receptivity to gonadotropic drugs, although they concluded that selected adjuvant therapy primarily affects the fertilization rate rather than the number of MII oocytes [3]. Nazari L. et al. (2020) examined a shorter MI regimen and found that after one month of therapy, there was a significant increase in the number of excellent quality blastocysts and a decrease in the number of blastocysts with low implantation potential in patients with reduced ovarian reserve undergoing IVF [4, 10]. Notably, Caprio F. et al. (2015) and Mohammadi S. et al. (2021) reported statistically insignificant improvements in clinical pregnancy rates among the MI group, whereas Nazari L. et al. (2020) observed positive trends in the rate of progressive pregnancies [2–4]. The findings of the present study partially corroborate data from these studies. We determined that MI + α-LA therapy, combined with folic acid (Inofert Forte, Italfarmaco, Italy), in patients with poor ovarian response significantly reduced the required dose of recombinant FSH and increased both the number of MII oocytes (p=0.034) and zygotes (p<0.01), which subsequently led to a greater number of good quality embryos (p=0.031). Additionally, in the adjuvant MI + α-LA group, we noted a trend toward improvement in both clinical and progressive pregnancy rates.
To compare the metabolic features of developing oocytes and granulosa cells with oral MI against the backdrop of enhanced embryological parameters and clinical outcomes in IVF, we analyzed the metabolomic composition of follicular fluid using HPLC-MS/MS. The strategy of employing MI + α-LA resulted in significant changes in the levels of 14 components within the follicular fluid: the levels of 7 amino acids (isoleucine, valine, leucine, alpha-aminobutyric acid, histidine, threonine, and proline) increased, while the content of 7 ceramides decreased. The observed alterations in amino acid metabolism suggested enhanced activity across five metabolic pathways: cysteine and methionine metabolism; valine, leucine, and isoleucine metabolism; pantothenate and coenzyme A biosynthesis; histidine metabolism; and beta-alanine metabolism. This analysis of metabolite levels has advanced our understanding of the role of individual substances in the formation of competent oocytes.
Hemmings K.E. et al. (2013) demonstrated the differences in valine and isoleucine consumption between mature and degenerating oocytes. Excessive depletion of these amino acids in the culture medium was characteristic of oocytes exhibiting abnormal metabolic processes, leading to developmental arrest at the GV and MI stages. In contrast, high levels of valine and isoleucine correlated with a greater likelihood of oocytes reaching the MII stage [11]. Subsequently, Bahrami M. et al. (2023) identified isoleucine and proline as key amino acids essential for meiotic division of oocytes [12]. In a larger study, Hood R.B. et al. (2023) analyzed follicular fluid samples from 125 women who underwent ovarian stimulation and found a positive correlation between active proline metabolism and the number of mature oocytes [13]. Lazzarino G. et al. (2021) reported that the follicular fluid of patients with infertility directly linked to female reproductive system dysfunction exhibited significantly lower levels of leucine, isoleucine, valine, and threonine compared to women seeking treatment for infertility solely due to male factors. The authors proposed these amino acids as biomarkers for predicting oocyte quality [14]. The increased levels of amino acids in the follicular fluid, identified as differential metabolites between the studied groups, may positively influence the number of embryos reaching the blastocyst stage and the size of their intracellular masses. Thus, proline accumulated by oocytes during successful fertilization of developing embryos regulates mitochondrial activity and the production of oxidative stress agents, functioning as a growth factor [15]. Threonine is crucial for normal stem cell proliferation and pluripotency maintenance [16]. Leucine is necessary for stable absorption of pyruvate by embryos; depletion of this amino acid halts stem cell division, disrupting their transition from the presynthetic to the synthetic stage of the interphase [17]. Notably, the enrichment of certain metabolic pathways following the use of inositol-containing supplements may contribute to the enhancement of oogenesis and embryogenesis. Elgebaly et al. (2021) demonstrated in an animal model that the addition of cysteine to the culture medium for oocyte maturation significantly increased the percentage of eggs reaching the MII stage [18]. Lee Y. et al. (2019) explored the effect of including alanine in the culture medium for immature oocytes [19]. While they did not observe a significant increase in the proportion of MII oocytes with the addition of amino acids, they did note a higher rate of embryo cleavage and blastulation frequency. Metabolomic profiling aims to identify biomarkers that, in addition to morphological characteristics, allow noninvasive assessments of blastocyst potential for successful implantation, eliminating the need for the traumatic procedure of trophectoderm cell biopsy for preimplantation genetic testing [20]. Wallace M. et al. (2012) found that decreased glucose levels and increased levels of proline, lactate, leucine, and isoleucine in the follicular fluid of infertile patients undergoing IVF were predictors of clinical pregnancy onset [21]. Therefore, the changes in the amino acid composition of follicular fluid observed after using MI + α-LA align with the findings of our study regarding the trend of improved clinical indicators in the adjuvant group.
Given that follicular fluid contains a variety of components other than amino acids, including proteins, lipids, steroid hormones, antioxidants, and cytokines, a comprehensive analysis of its composition offers insights into a broader range of processes during the intrafollicular stage of the oocyte and granulosa cell life cycle [6]. In this study, we also assessed ceramide levels, which are a class of lipids. Lipids are complex organic molecules that are essential for follicular cells to construct membranes, maintain energy reserves, and function as signaling molecules that regulate cell growth and apoptosis [22]. Specifically, ceramides act as mediators of the mitochondrial apoptotic pathway. Their accumulation within the cell suppresses the activity of the anti-apoptotic protein Bcl-2 and increases the expression of the pro-apoptotic protein BAX gene, which disrupts the outer mitochondrial membrane and facilitates the formation of ceramide channels that allow cytochrome C to be released into the cytosol, ultimately triggering programmed cell death [23]. Lee S. et al. (2021) demonstrated that high temperatures induced an increase in ceramide levels in oocytes, resulting in mitochondrial dysfunction followed by apoptosis. However, inhibiting ceramide formation allows oocytes exposed to temperature stress to continue development, avoiding the initiation of apoptosis [24]. If ceramides function as secondary messengers in stress-associated intracellular reaction cascades, one could infer that the decrease in ceramide levels observed in this study in the MI + α-LA group indicates the establishment of more favorable conditions in the follicular microenvironment for the completion of meiotic maturation of oocytes. This, in turn, led to a significant increase in the number of MII oocytes compared with the standard preparation strategy for IVF.
Conclusion
Based on the results of this study, we conclude that the adjuvant use of the MI + α-LA (Inofert Forte) combination in patients with a poor ovarian response prior to ovarian stimulation in IVF programs results in a higher number of mature oocytes and high-quality blastocysts, positively influencing the clinical outcomes of infertility treatment. However, owing to the small sample size, these findings should be interpreted with caution, and larger studies are warranted. Women in the MI + α-LA group exhibited increased levels of isoleucine, valine, leucine, alpha-aminobutyric acid, histidine, threonine, and proline in the follicular fluid, alongside a decrease in ceramide levels. The observed improvements in the embryological parameters may be associated with these metabolic changes. The results of this study highlight the potential for using metabolomic analysis of follicular fluid, not only to identify biomarkers of gamete and embryo quality in patients undergoing IVF but also to evaluate the effectiveness of adjuvant agents and understand the diverse mechanisms underlying their actions.
References
- Roberts L.M., Herlihy N., Reig A., Titus S., Garcia-Milian R., Knight J. et al. Transcriptomic landscape of cumulus cells from patients <38 years old with a history of poor ovarian response (POR) treated with platelet-rich plasma (PRP). Aging (Albany NY). 2025; 17(2): 431-47. https://dx.doi.org/10.18632/aging.206202.
- Caprio F., D'Eufemia M.D., Trotta C., Campitiello M.R., Ianniello R., Mele D. et al. Myo-inositol therapy for poor-responders during IVF: a prospective controlled observational trial. J. Ovarian Res. 2015; 8: 37. https://dx.doi.org/10.1186/s13048-015-0167-x.
- Mohammadi S., Eini F., Bazarganipour F., Taghavi S.A., Kutenaee M.A. The effect of Myo-inositol on fertility rates in poor ovarian responder in women undergoing assisted reproductive technique: a randomized clinical trial. Reprod. Biol. Endocrinol. 2021; 19(1): 61. https://dx.doi.org/10.1186/s12958-021-00741-0.
- Nazari L., Salehpour S., Hosseini S., Saharkhiz N., Azizi E., Hashemi T. et al. Effect of myo-inositol supplementation on ICSI outcomes among poor ovarian responder patients: A randomized controlled trial. J. Gynecol. Obstet. Hum. Reprod. 2020; 49(5): 101698. https://dx.doi.org/10.1016/j.jogoh.2020.101698.
- Kamenov Z., Gateva A., Dinicola S., Unfer V. Comparing the efficacy of Myo-inositol plus α-Lactalbumin vs. Myo-inositol alone on reproductive and metabolic disturbances of polycystic ovary syndrome. Metabolites. 2023; 13(6): 717. https://dx.doi.org/10.3390/ metabo13060717.
- Zhang Y., He C., He Y., Zhu Z. Follicular fluid metabolomics: tool for predicting IVF outcomes of different infertility causes. Reprod. Sci. 2025; 32(4): 921-34. https://dx.doi.org/10.1007/s43032-024-01664-y.
- Ибрагимова Л.К., Смольникова В.Ю., Эльдаров Ч.М., Бобров М.Ю., Агаджанян Д.С., Романов Е.А., Калинина Е.А. Особенности метаболомного профиля фолликулярной жидкости и сред культивирования эмбрионов пациенток с наружным генитальным эндометриозом. Акушерство и гинекология. 2021; 11: 114-24. [ Ibragimova L.K., Smol'nikova V.Yu., El'darov Ch.M., Bobrov M.Yu., Agadzhanyan D.S., Romanov E.A., Kalinina E.A. Metabolomic profile of follicular fluid and embryo culture media in patients with extragenital endometriosis. Obstetrics and Gynecology. 2021; (11): 114-24 (in Russian)]. https://dx.doi.org/10.18565/aig.2021.11.114-124.
- Gardner D.K., Schoolcraft W.B. Culture and transfer of human blastocysts. Curr. Opin. Obstet. Gynecol. 1999; 11(3): 307-11. https://dx.doi.org/10.1097/00001703-199906000-00013.
- Placidi M., Casoli G., Tatone C., Di Emidio G., Bevilacqua A. Myo-inositol and its derivatives: their roles in the challenges of infertility. Biology (Basel). 2024; 13(11): 936. https://dx.doi.org/10.3390/biology13110936.
- Вартанян Э.В., Цатурова К.А., Девятов Е.А., Михайлюкова А.С., Левин В.А., Сагамонова К.Ю., Громенко Д.С., Овсянникова Т.В., Эрлихман Н.М., Колосова Е.А., Сафронова Е.В., Фотина О.В., Красновская Е.В., Пожарищенская Т.Г., Аутлева С.Р., Гзгзян А.М., Нуриев И.Р., Воропаева Е.Е., Пестова Т.И., Здановский В.М., Ким Н.А., Котельников А.Н., Сафронов О.В., Назаренко Т.А., Ионова Р.М. Подготовка к лечению бесплодия методом экстракорпорального оплодотворения при сниженном овариальном резерве. Акушерство и гинекология. 2019; 8: 134-42. [Vartanyan E.V., Tsaturova K.A., Devyatova E.A., Mikhailyukova A.S., Levin V.A., Sagamonova K.Yu., Gromenko D.S., Ovsyannikova T.V., Erlikhman N.M., Kolosova E.A., Safronova E.V., Fotina O.V., Krasnovskaya E.V., Pozharischenskaya T.G., Autleva S.R., Gzgzyan A.M., Nuriev I.R., Voropaeva E.E., Pestova T.I., Zdanovsky V.M., Kim N.A., Kotelnikov A.N., Safronov O.V., Nazarenko T.A., Ionova R.M. Preparation for the in vitro fertilization treatment of infertility in diminished ovarian reserve. Obstetrics and Gynecology. 2019; (8): 134-42 (in Russian)]. https://dx.doi.org/10.18565/aig.2019.8.134-142.
- Hemmings K.E., Maruthini D., Vyjayanthi S., Hogg J.E., Balen A.H., Campbell B.K. et al. Amino acid turnover by human oocytes is influenced by gamete developmental competence, patient characteristics and gonadotrophin treatment. Hum. Reprod. 2013; 28(4): 1031-44. https://dx.doi.org/10.1093/humrep/des458.
- Bahrami M., Morris M.B., Day M.L. Glutamine, proline, and isoleucine support maturation and fertilisation of bovine oocytes. Theriogenology. 2023; 201: 59-67. https://dx.doi.org/10.1016/j.theriogenology.2023.02.019.
- Hood R.B., Liang D., Tan Y., Ford J.B., Souter I., Chavarro J.E. et al. Serum and follicular fluid metabolome and markers of ovarian stimulation. Hum. Reprod. 2023; 38(11): 2196-207. https://dx.doi.org/10.1093/humrep/dead189.
- Lazzarino G., Pallisco R., Bilotta G., Listorti I., Mangione R., Saab M.W. et al. Altered follicular fluid metabolic pattern correlates with female infertility and outcome measures of in vitro fertilization. Int. J. Mol. Sci. 2021; 22(16): 8735. https://dx.doi.org/10.3390/ijms22168735.
- Treleaven T., Hardy M.L.M., Guttman-Jones M., Morris M.B., Day M.L. In vitro fertilisation of mouse oocytes in L-Proline and L-Pipecolic acid improves subsequent development. Cells. 2021; 10(6): 1352. https://dx.doi.org/10.3390/cells10061352.
- Van Winkle L.J. Amino acid transport and metabolism regulate early embryo development: species differences, clinical significance, and evolutionary implications. Cells. 2021; 10(11): 3154. https://dx.doi.org/10.3390/cells10113154.
- Correia B., Sousa M.I., Branco A.F., Rodrigues A.S., Ramalho-Santos J. Leucine and arginine availability modulate mouse embryonic stem cell proliferation and metabolism. Int. J. Mol. Sci. 2022; 23(22): 14286. https://dx.doi.org/10.3390/ijms232214286.
- Elgebaly M.M., Hazaa A.B.M., Amer H.A., Mesalam A. L-Cysteine improves bovine oocyte developmental competence in vitro via activation of oocyte-derived growth factors BMP-15 and GDF-9. Reprod. Domest. Anim. 2022; 57(7): 734-42. https://dx.doi.org/10.1111/rda.14113.
- Lee Y., Shim J., Ko N., Kim H.J., Park J.K., Kwak K., Kim H., Choi K. Effect of alanine supplementation during in vitro maturation on oocyte maturation and embryonic development after parthenogenesis and somatic cell nuclear transfer in pigs. Theriogenology. 2019; 127: 80-7. https://dx.doi.org/10.1016/j.theriogenology.2019.01.001
- Гапоненко А.А., Митюрина Е.В., Франкевич В.Е. Метаболомный профиль фолликулярной жидкости как маркер качества ооцитов в программах вспомогательных репродуктивных технологий. Акушерство и гинекология. 2021; 11: 26-31. [Gaponenko A.A., Mityurina E.V., Frankevich V.E. The follicular fluid metabolomic profile as a marker for oocyte quality in assisted reproductive technology programs. Obstetrics and Gynecology. 2021; (11): 26-31 (in Russian)]. https://dx.doi.org/10.18565/aig.2021.11.26-31.
- Wallace M., Cottell E., Gibney M.J., McAuliffe F.M., Wingfield M., Brennan L. An investigation into the relationship between the metabolic profile of follicular fluid, oocyte developmental potential, and implantation outcome. Fertil. Steril. 2012; 97(5): 1078-84.e1-8. https://dx.doi.org/10.1016/j.fertnstert.2012.01.122.
- Luti S., Fiaschi T., Magherini F., Modesti P.A., Piomboni P., Governini L. et al. Relationship between the metabolic and lipid profile in follicular fluid of women undergoing in vitro fertilization. Mol. Reprod. Dev. 2020; 87(9): 986-97. https://dx.doi.org/10.1002/mrd.23415.
- Alizadeh J., da Silva Rosa S.C., Weng X., Jacobs J., Lorzadeh S., Ravandi A. et al. Ceramides and ceramide synthases in cancer: Focus on apoptosis and autophagy. Eur. J. Cell Biol. 2023; 102(3): 151337. https://dx.doi.org/10.1016/j.ejcb.2023.151337.
- Lee S., Kang H.G., Jeong P.S., Kim M.J., Park S.H., Song B.S. et al. Heat stress impairs oocyte maturation through ceramide-mediated apoptosis in pigs. Sci. Total Environ. 2021; 755(Pt 1): 144144. https://dx.doi.org/10.1016/j.scitotenv.2020.144144.
Received 21.04.2025
Accepted 25.04.2025
About the Authors
Evgeniy A. Zakovryashin, PhD student, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, +7(916)051-75-36, 324155hf@mail.ru, https://orcid.org/0009-0002-3908-7230Irina Е. Korneeva, Dr. Med. Sci., Professor, Leading Researcher at the Scientific and Educational Center of Auxiliary Reproductive Technologies with a Clinical Department named after Frederick Paulsen, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, https://orcid.org/0000-0002-0904-585X
Vitaliy V. Chagovets, PhD, Head of the Laboratory for Metabolomics and Bioinformatics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, https://orcid.org/0000-0002-5120-376X
Elena V. Mityurina, PhD, Senior Researcher at the Scientific and Educational Center of Auxiliary Reproductive Technologies with a Clinical Department
named after Frederick Paulsen, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, https://orcid.org/0000-0001-8830-2158
Anastasia V. Novoselova, Researcher at the Laboratory of Metabolomics and Bioinformatics, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4.
Elena S. Sannikova, Embryologist at the Scientific and Educational Center of Auxiliary Reproductive Technologies with a Clinical Department named after Frederick Paulsen, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology, Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, https://orcid.org/0009-0009-2351-3400
Tatiana A. Nazarenko, Dr. Med. Sci., Professor, Head of the Institute of Reproductive Medicine, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology, Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, https://orcid.org/0000-0002-5823-1667
Vitaly A. Levin, Endocrinologist, Ornament Health AG, Lucerne, Switzerland, https://orcid.org/0009-0000-8758-0842
Corresponding author: Evgeniy A. Zakovryashin, 324155hf@mail.ru



