Efficiency analysis of IVF programs using "Ural'skaya odnoshagovaya" Ural-m-1step culture media
Komarova E.M., Kazakova I.A., Lisovskaya T.V., Ivanov A.V., Mayasina E.N., Kozhevnikova A.I., Ivanov I.V., Fedoseeva O.B., Vartanyan E.V., Levin V.A., Gzgzyan A.M., Kogan I.Yu.
The procedures of the embryological stage of in vitro fertilization (IVF) program mainly determine the effectiveness of this technology for infertility treatment. Currently, in Russia it is possible to buy culture media for IVF programs from various manufacturers.
Objective: To assess the effectiveness of IVF programs with the use of "Ural'skaya odnoshagovaya" Ural-m-1step culture medium (TU 21.20.23–001–47571069–2022), LLC "Incammedic" (Russia).
Materials and methods: Two study groups were formed: the main group, which included 240 sibling IVF and IVF/ICSI protocols, with fertilization and embryo cultivation carried out on the Ural-m-1step medium (Russia), and comparison group, which consisted of 240 sibling IVF and IVF/ICSI protocols, with fertilization and embryo cultivation performed on the Vitrolife line of media (Sweden). To stimulate ovulation in patients of both groups we used protocols with GnRH antagonists; to support the luteal phase, a micronized progesterone preparation (Iprozhin, Italfarmaco, Italy/France) was introduced at a dose of 600 mg per day in 2 doses vaginally.
In the main group, 119 embryo transfers into the uterine cavity were performed on the 5th–6th day of cultivation, whereas the comparison group included 103 embryo transfers.
Results: According to the key quality indicators adopted by the Vienna and the Maribor consensuses, the effectiveness of IVF programs using the compared media did not statistically differ, p>0.05. The percentage of fertilization, the proportion of blastocysts, the number of blastocysts of excellent quality obtained on the 5th–6th day of cultivation in IVF and IVF/ICSI cycles in the main and comparison groups also did not differ. The pregnancy/implantation rate corresponded to the target ones, according to the Vienna and the Maribor consensuses, and did not have significant differences in the main and comparison groups, both in “fresh” cycles: 57/119 (54.8%) and 47/103 (48.8%), respectively, p=0.338, and in cryoprotocols: 62/119 (58.8%) and 56/103 (59.6%), respectively, p=0.660.
Conclusion: The results of the study indicate that the outcomes of IVF programs performed with in combination with the Ural-m-1step medium (TU 21.20.23–001–47571069–2022), LLC "Incammedik" (Russia), are comparable to those using the Vitrolife media (Sweden), which proves the interchangeability of these medical products in clinical practice.
Authors’ contributions: Komarova E.M. – material collection and analysis on the clinical basis of D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, St. Petersburg; Kazakova I.A., Mayasina E.N. – material collection and analysis on the clinical basis of Clinical Institute of Reproductive Medicine, Yekaterinburg; Lisovskaya T.V. – writing the text; Ivanov A.V. – material collection and analysis on the clinical basis of City Mariinsky Hospital, St. Petersburg; Kozhevnikova A.I. – statistical data processing; Ivanov I.V., Levin V.A. – research concept and design; Fedoseeva O.B. – consistency of all parts of the manuscript; Gzgzyan A.M., Vartanyan E.V. – manuscript editing; Kogan I.Yu. – approval of the final version.
Conflicts of interest: The authors declare no conflicts of interest in connection with the publication of this article.
Funding: The study was performed without external funding.
Ethical Approval: The study was approved by the local ethics committee of the Clinical Institute of Reproductive Medicine (Yekaterinburg) on February 13, 2024 (Protocol #2).
Patient Consent for Publication: All patients signed an informed consent to participate in the study in the form of consent to use a particular culture medium registered in the Russian Federation at the embryological stage of ART programs, informed consent for treatment using assisted reproductive technologies, informed consent for the processing with personal data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Komarova E.M., Kazakova I.A., Lisovskaya T.V., Ivanov A.V., Mayasina E.N., Kozhevnikova A.I., Ivanov I.V., Fedoseeva O.B., Vartanyan E.V., Levin V.A., Gzgzyan A.M., Kogan I.Yu. Efficiency analysis of IVF programs using "Ural'skaya odnoshagovaya" Ural-m-1step culture media.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (8): 172-178 (in Russian)
https://dx.doi.org/10.18565/aig.2025.194
Keywords
One of the important stages of in vitro fertilization (IVF) programs, which determines the effectiveness of this technology, is embryo cultivation [1, 2]. This is why attempts are constantly being made to improve culture media by selecting the optimal composition [3–5]. In particular, improvement of the quality of modern nutrient media has been achieved by the inclusion of albumin, growth factors, cytokines and antioxidants [6]. Currently, in Russia it is possible to buy culture media for IVF programs from various manufacturers [7, 8]. Over the past three years, due to unstable situation worldwide, there have unfortunately been frequent failures in the supply of culture media to Russia, the shelf-life of which, according to the manufacturers' instructions, usually does not exceed 6 months. As a result, medical organizations involved in the treatment of infertility using ART received these consumables with a remaining shelf-life of up to 2-3 months. At the same time, the cost of imported culture media has increased significantly over the past 3 years. In this regard, in order to provide accessible, timely and high-quality medical care to infertile couples using ART methods, the use of culture media from different manufacturers with the possibility of replacing them is relevant. The creation of a high-quality domestic culture medium for the embryological stage of the IVF program was a fundamental solution for preserving this medical technology in the treatment of infertile couples (in accordance with the State Guarantee Program for the Free Provision of Medical Care to the Russian Citizens1).
It should be noted that there is data in the literature on the advantage of using single-step culture media for culturing embryos in comparison with sequential media, which is due not only to the possibility of their use for various embryological procedures, a higher percentage of blastocyst production, but also to a higher risk of pregnancy [5]. However, no clear data was obtained on the increase in the implantation rate when using certain one-step media [6].
Currently, the domestic Ural-m-1step media, manufactured by OOO Inkammedik, for embryological stage of ART programs is quickly being introduced into clinical practice, and the accumulated experience of its use enables to conduct a comparative analysis of the effectiveness of IVF programs when using it in comparison with previously registered foreign analogues.
The aim of the study: to assess the effectiveness of IVF programs with the use of Ural-m-1step culture medium (TU 21.20.23–001–47571069–2022), Incammedic LLC (Russia).
Materials and methods
The study was conducted in accordance with the approved unified study protocol from March 1 to December 27, 2024 at the following clinical sites: D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, Department of Assisted Reproductive Technologies (Head – A.M. Gzgzyan), St. Petersburg; Mariinsky City Hospital, Department of Assisted Reproductive Technologies (Head – A.V. Ivanov), St. Petersburg; Clinical Institute of Reproductive Medicine (General Director – I.G. Portnov), Yekaterinburg.
A sibling multicenter study of embryo stage of IVF program was conducted with the use of Ural-m-1step culture medium (registration number 2024/21885 of 15.01.2024), Incammedic LLC (Russia), and with Vitrolife line of media: «Fertilization medium» (G-IVF)/ «Universal one-step medium» (G-TL), Vitrolife Sweden AB Gustaf Werners gata 2, SE-421 32 Västra Frölunda, Sweden (registration certificate # FSZ 2008/01194 of 04.10.2022)
Inclusion criteria for the first stage of study:
- patients aged 18–38 years old;
- diagnosed infertility according to ICD-10: N97.0 (female infertility, associated with anovulation); N97.1 (female infertility of tubal origin); N97.4 (female infertility, associated with male factor);
- infertility treatment using ART programs;
- use of protocols with gonadotropin-releasing hormone (GnRH) antagonists for ovulation stimulation;
- informed consent to participate in the study.
Exclusion criteria for the first stage of study:
- patients aged less 18 and over 38 years old;
- other forms of infertility;
- use of protocols with gonadotropin-releasing hormone (GnRH) agonists for ovulation stimulation;
- body mass index 30 and more kg/m2;
- I or II type diabetes mellitus;
- antiretroviral therapy or any other therapy intake that has been shown to have a negative gonadotoxic effect;
- use of donor gametes.
Of 1028 IVF and IVF/intracytoplasmic sperm injection (ICSI) protocols performed at three hospitals during the specified period, 695 were included in the study. 323 ART protocols were excluded from the study for the following reasons: patient over 35 years old (n=91); other forms of infertility (n=46); use of protocols with GnRH agonists for ovulation stimulation (n=52); programs with donor oocytes or spermatozoa (n=89); refusal to sign informed consent to participate in the study (n=45).
To stimulate ovulation, all patients were treated with GnRH antagonist protocols; to support the luteal phase, a micronized progesterone preparation (Iprozhin, Italfarmaco, Italy/France) was introduced 600 mg per day in 2 doses per vaginum, starting from the day following transvaginal ovarian puncture until the human chorionic gonadotropin test. After ovulation stimulation, 480 protocols were selected from 695 cases, in each of which at least 8 good-quality oocytes suitable for fertilization were obtained during ovarian puncture.
Subsequently, two study groups were formed from sibling (related) oocytes obtained in the same ovulation stimulation protocols: the main group, which included 240 sibling IVF and IVF/ICSI protocols, with fertilization and embryo cultivation carried out on the Ural-m-1step medium, Incammedik LLC (Russia), and the control group, with 240 sibling IVF and IVF/ICSI protocols, in which fertilization and embryo cultivation were performed on the Vitrolife line of media: Fertilization medium» (G-IVF)/ «Universal one-step medium» (G-TL), Vitrolife Sweden AB Gustaf Werners gata 2, SE-421 32 Västra Frölunda, Sweden. The formation of two study groups with the inclusion of sibling oocytes was made by the embryologist during the fertilization procedure.
The main group of 240 cycles included 119 embryo transfers into the uterine cavity performed on the 5th–6th day of cultivation, including 57 transfers in ovulation stimulation protocols and 62 transfers of cryopreserved embryos. 121 protocols were excluded from further analysis due to: embryo development arrest before the 5th–6th day of cultivation (n=47); absence of good-quality embryos suitable for transfer (n=68); transfer of more than one embryo (n=6).
In the control group of 240 cycles, transfer was performed in 103 cases (47 in ovulation stimulation protocols, 56 transfers of cryopreserved embryos). 137 protocols were excluded from further analysis because of: embryo development arrest before day 5–6 of cultivation (n=37); absence of good quality embryos suitable for transfer (n=62); transfer of more than one embryo (n=4).
The quality of embryos was assessed according to Gardner's morphological criteria in both groups [9]. One embryo of the best quality was selected for transfer.
While preparing for the transfer of cryopreserved embryos, luteal support was performed for a 5-day period prior to the day of transfer of the thawed blastocyst with micronized progesterone 600 mg per day in 2 doses per vaginum and continued until pregnancy was diagnosed.
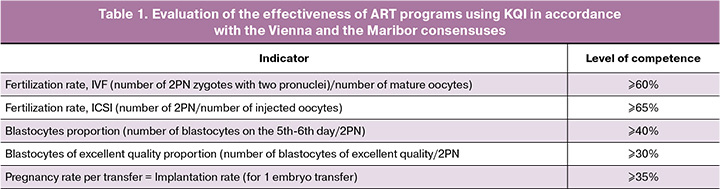
The effectiveness of ART programs was assessed using key quality indicators (KQI) in the embryology laboratory, adopted by the Vienna and the Maribor consensuses [10, 11], presented in Table 1.
Statistical analysis
All quantitative indicators were tested for distribution normality using Shapiro–Wilk test. Given that the distribution of quantitative indicators differed significantly from the norm, statistical analysis was conducted with the use of nonparametric criteria and description in the form of median and interquartile interval: Me (25%; 75%). Statistical significance of the differences between the features was assessed with Mann–Whitney test (U-criterium). Qualitative indicators were described with simple identification of the number of cases and proportion (percent) for each category. Statistical significance of the differences between these features was assessed with Pearson’s χ2 test. Differences in p<0,05 were considered statistically significant.
Results
The analysis of clinical and laboratory data of 480 patients, whose oocytes were used for the assessment of the effectiveness of embryo stage of ART programs with the formation of comparison groups, did not demonstrate statistically significant group differences (Table 2).
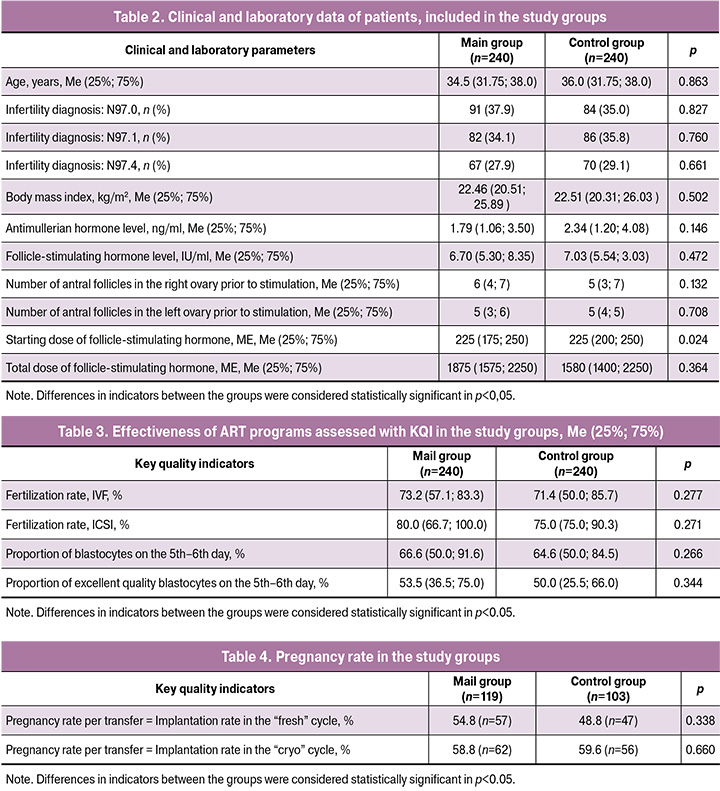
Fertilization rates in two groups after classical IVF and IVF/ICSI did not differ, however they corresponded to the level of competence according to the Vienna and the Maribor consensuses (Table 3). Proportion of blastocytes, obtained on the 5th–6th day of cultivation in the group with the use of Ural-m-1step medium, is similar to that in the group with Vitrolife line of media, and counts 66,6 (50,0; 91,6) and 64,6 (50,0; 84,5), accordingly, which did not have significant differences (p=0,266). Also close parameters were obtained in both groups for the number of excellent quality blastocytes, р=0,344 (Table 2).
Pregnancy/implantation rate corresponded to target ones according to the Vienna and the Maribor consensuses, but did not have statistically significant differences in the main as well as in the control groups, both in “fresh” cycles: 57/119 (54,8%) and 47/103 (48,8%), correspondingly, р=0,338, and in cryoprotocols: 62/119 (58,8%) and 56/103 (59,6%), correspondingly, р=0,660 (Table 4).
After transfer of the embryos, obtained with the use of Ural-m-1step medium (TU 21.20.23–001–47571069–2022), Incammedik LLC, Russia), no clinical manifestations associated with patient’s embryo contamination (temperature rise, local and/or general inflammation) or patient’s organism sensibilization (allergy reaction), were detected.
Discussion
The effectiveness of IVF and IVF/ICSI procedures using Ural-m-1step medium (TU 21.20.23–001–47571069–2022), Incammedik LLC, Russia, according to key performance indicators (KPI), corresponds to the effectiveness of IVF and IVF/ICSI procedures using Vitrolife line of media: «Fertilization medium» (G-IVF)/ «Universal one-step medium» (G-TL), Vitrolife Sweden AB Gustaf Werners gata 2, SE-421 32 Västra Frölunda, Sweden (р>0,05). Special attention was paid to such parameters as pregnancy rate per transfer and implantation rate (number of pregnancies, confirmed by ultrasound scan) to assess clinical effectiveness of the IVF and IVF/ICSI programs. As one of the inclusion criteria at this stage of the study was no more than one embryo transfer the indicated parameters were the same (Table 4.)
Absence of any clinical manifestations associated with contamination of transferred embryos, as well as with patient’s organism sensibilization to the components of Ural-m-1step medium (TU 21.20.23–001–47571069–2022), Incammedik LLC, Russia, demonstrates its safety when used as prescribed.
Conclusion
The results of the study show that when used with Ural-m-1step medium (TU 21.20.23–001–47571069–2022), Incammedik LLC, Russia, IVF outcomes correspond to those when used with Vitrolife line of media, Sweden.
The results of the comparative analysis of IVF programs effectiveness in combination with Ural-m-1step medium (TU 21.20.23–001–47571069–2022), Incammedik LLC, Russia, and Vitrolife line of media, Sweden, proved the interchangeability of these medical products in clinical practice.
____________________
1 "On the Program of State Guarantee for the Free Provision of Medical Care to Citizens for 2024 and for the Planning Period of 2025 and 2026". Decree # 2353 of the Russian Federation Government of December 28, 2023. https://www.garant.ru/products/ipo/prime/doc/408223431/
References
- Pivko J., Makarevich A., Olexiková L., Kubovičová E., Makovický P., Dujičková L. et al. The morphological and functional ultrastructure of cells in pre-implantation embryos. Gen. Physiol. Biophys. 2023; 42(4): 307-21. https://dx.doi.org/10.4149/gpb_2023011
- Coticchio G., Lagalla C., Sturmey R., Pennetta F., Borini A. The enigmatic morula: mechanisms of development, cell fate determination, self-correction and implications for ART. Hum. Reprod. Update. 2019; 25(4): 422-38. https://dx.doi.org/10.1093/humupd/dmz008
- Morbeck D.E., Krisher R.L., Herrick J.R., Baumann N.A., Matern D., Moyer T. Composition of commercial media used for human embryo culture. Fertil. Steril. 2014; 102(3): 759-766.e9. https://dx.doi.org/10.1016/j.fertnstert.2014.05.043
- Morbeck D.E., Baumann N.A., Oglesbee D. Composition of single-step media used for human embryo culture. Fertil. Steril. 2017; 107(4): 1055-1060.e1. https://dx.doi.org/10.1016/j.fertnstert.2017.01.007
- Zagers M.S., Laverde M., Goddijn M., de Groot J.J., Schrauwen F.A.P., Vaz F.M. et al. The composition of commercially available human embryo culture media. Hum. Reprod. 2025; 40(1): 30-40. https://dx.doi.org/10.1093/humrep/deae248
- Zander-Fox D.L., Pacella-Ince L., Morgan D.K., Green M.P. Mammalian embryo culture media: now and into the future. Reprod. Fertil. Dev. 2023; 36(2): 66-80. https://dx.doi.org/10.1071/RD23168
- Кириенко К.В., Апрышко В.П., Харитонова М.А., Трошина М.Н., Ермилова И.Ю., Калинина Е.С., Хряпенкова Т.Г., Страшнова А.Л., Воронич Н.С., Бирюков А.А., Болт А.И., Клепуков А.А., Савина Е.М., Миронова А.Г., Симоненко Е.Ю., Яковенко С.А. Оценка эффективности программ ВРТ: есть ли необходимость применения отдельных сред для эмбрионов разных стадий развития? Проблемы репродукции. 2016; 22(5): 56-60. [Kirienko K.V., Apryshko V.P., Kharitonova M.A., Troshina M.N., Ermilova I.Yu., Kalinina E.S., Khryapenkova T.G. , Strashnova A.L., Voronich N.S., Biryukov A.A., Bolt A.I., Klepukov A.A., Savina E.M. , Mironova A.G., Simonenko E.Yu., Yakovenko S.A. Evaluation of ART program using sequential and single-step media for embryo culture? Russian Journal of Human Reproduction. 2016; 22(5): 56-60. (in Russian)]. https://doi: 10.17116/repro201622556-60
- Петросян Я.А., Сыркашева А.Г., Макарова Н.П. Влияние эмбриологического этапа на эффективность программ вспомогательных репродуктивных технологий с переносом размороженного эмбриона. Акушерство и гинекология. 2020; 4: 127-32. [Petrosyan Ya.A., Syrkasheva A.G., Makarova N.P. Impact of the embryological stage on the effectiveness of assisted reproductive technology programs with frozen-thawed embryo transfer. Obstetrics and Gynecology. 2020; (4): 127-32 (in Russian)]. https://dx.doi.org/10.18565/aig.2020.4.127-132
- Gardner D.K., Scoolcraft W.B. Inner cell mass morphology. In: Jansen R., Mortimer D., eds. In vitro culture of human blastocysts. UK: Parthenon Publishing London; 1999: 378-88.
- ESHRE Special Interest Group of Embryology and Alpha Scientists in Reproductive Medicine. Electronic address: coticchio.biogenesi@grupposandonato.it. The Vienna consensus: report of an expert meeting on the development of ART laboratory performance indicators. Reprod. Biomed. Online. 2017; 35(5): 494-510. https://dx.doi.org/10.1016/j.rbmo.2017.06.015
- ESHRE Clinic PI Working Group; Vlaisavljevic V., Apter S., Capalbo A., D'Angelo A., Gianaroli L., Griesinger G. et al. The Maribor consensus: report of an expert meeting on the development of performance indicators for clinical practice in ART. Hum. Reprod. Open. 2021; 2021(3): hoab022. https://dx.doi.org/10.1093/hropen/hoab022
Received 17.07.2025
Accepted 11.08.2025
About the Authors
Evgenia M. Komarova, PhD (Bio), Junior Researcher, embryologist, ART Department, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology,199034, Russia, St. Petersburg, Mendeleevskaya Line, 3, evgmkomarova@gmail.com, https://orcid.org/0000-0001-5161-369X
Irina A. Kazakova, PhD (Bio), Head of the Clinical Embryology Laboratory, Clinical Institute of Reproductive Medicine, 620000, Russia, Yekaterinburg, B. Yeltsin str., 3, brykina_irina@mail.ru
Tatyana V. Lisovskaya, Dr. Med. Sci., Associate Professor, Deputy Director General for Scientific and Methodological Work, Clinical Institute of Reproductive Medicine, 620000, Russia, Yekaterinburg, B. Yeltsin str., 3, tv.lis@mail.ru, https://orcid.org/0000-0002-9747-9323
Andrey V. Ivanov, Head of Assisted Reproductive Technologies Department, City Mariinsky Hospital, 191014, Russia, St. Petersburg, Liteiny Ave., 56, dr.ivanovav@gmail.com
Elena N. Mayasina, PhD, Deputy Director General for Medical Unit, Clinical Institute of Reproductive Medicine, 620000, Russia, Yekaterinburg, B. Yeltsin str., 3,
elena.mayasina@gmail.com, https://orcid.org/0000-0002-3387-819X
Alexandra I. Kozhevnikova, embryologist assistant, Clinical Institute of Reproductive Medicine, 620000, Russia, Yekaterinburg, B. Yeltsin str., 3, kkozhhh@yandex.ru
Igor V. Ivanov, Dr. Med. Sci., General Director, All-Russian Research and Testing Institute of Medical Equipment of Roszdravnadzor, 115582, Russia, Moscow,
Kashirskoye Shosse, 24-16, ministr38@gmail.com, https://orcid.org/0000-0003-0971-053X
Oksana B. Fedoseeva, Lecturer at the Center for Scientific Research and Advanced Development, All-Russian Research and Testing Institute of Medical Equipment of Roszdravnadzor, 115582, Russia, Moscow, Kashirskoye Shosse, 24-16, fob18-fob18@rambler.ru, https://orcid.org/0000-0001-7882-8885
Emma V. Vartanyan, Dr. Med. Sci., Professor, Department of Obstetrics and Gynecology, N.I. Pirogov Russian National Research Medical University, Ministry of Health of Russia, 117513, Russia, Moscow, Ostrovityanov str., 1; President, SRO «Association of ART Clinics»; Director, ART Clinic «Test Tube Children», Moscow,
emma-vartanyan@mail.ru, https://orcid.org/0000-0003-0337-086X
Vitaly A. Levin, endocrinologist, specialist in the organization of health and public health, Ornament Health AG, Lucerne, Switzerland, vitalylevin0205@gmail.com,
https://orcid.org/0009-0000-8758-0842
Alexander M. Gzgzyan, Dr. Med. Sci., Professor, Head of the ART Department, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, 199034, Russia, St. Petersburg, Mendeleevskaya Line, 3, agzgzyan@mail.ru, https://orcid.org/0000-0003-3917-9493
Igor Yu. Kogan, Dr. Med. Sci., Professor, Corresponding Member of RAS, Director, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology,
199034, Russia, St. Petersburg, Mendeleevskaya Line, 3, ikogan@mail.ru, https://orcid.org/0000-0002-7351-6900
Corresponding author: Tatyana V. Lisovskaya, tv.lis@mail.ru



