Predictors of pregnancy rate in assisted reproductive technologies: results of the IRIS observational program in the population of Russia and Kazakhstan
Nazarenko T.A., Pestova T.I., Lokshin V.N., Dzhusubalieva T.M., Serov V.N., Baranov I.I., Bezhenar V.F., Gavisova A.A., Gorodnova E.A., Dolgushina N.V., Kalugina A.S., Kvashnina E.V., Kogan I.Yu., Koloda Yu.A., Korsak V.S., Krasnopolskaya K.V., Molchanova I.V., Sabirova V.L., Tapilskaya N.I., Sukhikh G.T.
Objective: To evaluate the predictors of in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) success in females receiving oral dydrogesterone for luteal phase support with subsequent evaluation of the relationship between influencing factors and pregnancy rates, and the creation for the first time of a prognostic table for clinical pregnancy in IVF and ICSI cycles, identifying the most influential predictors.
Materials and methods: Multicenter open-label observational program for assessing the probability of pregnancy in IVF cycles enrolled females who were prescribed dydrogesterone 30 mg a day for luteal phase support while undergoing assisted reproductive technology in routine setting of Russia and Kazakhstan according to the national guidelines. The primary objective of the study was to evaluate the predictors of pregnancy rate in IVF and ICSI, followed by the creation of a prognostic table of clinical pregnancy in IVF and ICSI cycles considering 4–5 most significant predictors. Secondary objectives assessed included biochemical and clinical pregnancy rates, convenience and overall satisfaction with therapy, live birth, and maternal and fetal safety were also assessed.
Results: 1150 patients were enrolled from 42 study sites in the Russian Federation and 2 study sites in the Republic of Kazakhstan.
1146/1150 (99.7%) patients were exposed to Duphaston and constituted the safety set, out of them 534 patients were from the IVF subgroup, and 612 patients were from the ICSI subgroup. 1143/1150 (99.4%) patients constituted the full analysis set (994 from Russia and 149 from Kazakhstan) with a median age of 34 years. Clinical pregnancy rate was 36.7% and the live birth rate was 30.1%. The collected data has been used to build a clinical pregnancy rate prediction model. According to the prediction model, the best pregnancy probability while taking dydrogesterone would be for females under 37 years of age with antral follicle count of ≤12 and top-quality embryos, for whom IVF would be used. The study confirmed a high level of satisfaction with dydrogesterone therapy. In the study population dydrogesterone showed a favorable safety profile for fetus/newborn and mothers.
Conclusion: Results of the study provide the practical a robust predictive model for IVF/ICSI success while demonstrating favorable oral dydrogesterone efficacy and safety profiles in routine practice.
Authors’ contributions: Nazarenko T.A., Pestova T.I., Lokshin V.N., Dzhusubalieva T.M., Serov V.N., Baranov I.I., Bezhenar V.F., Gavisova A.A., Gorodnova E.A., Dolgushina N.V., Kalugina A.S., Kvashnina E.V., Kogan I.Yu., Koloda Yu.A., Korsak V.S., Krasnopolskaya K.V., Molchanova I.V., Sabirova V.L., Tapilskaya N.I., Sukhikh G.T. – developing the conception and design of the study, collecting and processing the material, writing the text, editing the article.
Conflicts of interest: The authors declare no conflicts of interest.
Funding: The prospective data collection and medical writing support were provided with support by Abbott Laboratories LLC.
Acknowledgements: The authors would like to thank the researchers presented below:
D.P. Kamilova, T.V. Kaznacheeva, I.M. Zorina, L.B. Kindarova, L.R. Khechumyan, Yu.A. Fetisova, E.Sh. Ablyaeva, E.N. Gavrilina, N.V. Shilova (Moscow); O.E. Vasilyeva, A.G. Tkachuk, D.A. Gerculov, E.V. Zhigalova, S.V. Nikitin
(St. Petersburg); A.V. Mukhina (Volgograd); I.N. Korotkikh (Voronezh); N.V. Bashmakova (Yekaterinburg); S.S. Semenenko, Yu.N. Zholobov (Ivanovo); E.B. Druzhinina, A.V. Khoroshavina (Irkutsk); Yu.Yu. Borodina, A.I. Abitova (Kazan); V.A. Popandopulo, A.A. Baklakova, O.Yu. Kostrikova (Krasnodar); T.V. Gvozdikova (Lipetsk); E.V. Kuznetsova (Novosibirsk); M.M. Ovchinnikova (Odintsovo); O.V. Fotina (Perm); I.V. Moiseeva (Samara); K.V. Artemenko (Saratov); E.M. Kasparova (Stavropol); Zh.F. Gaifulina (Tomsk); E.E. Privalova, N.Yu. Likhacheva, E.V. Orekhova (Chelyabinsk); Sh.K. Karibaeva, A.T. Abshekenova, R.K. Valiev, V.A. Nekhorosheva, A.R. Onlas, E.V. Popova, A.N. Rybina (Almaty).
Assistance in the preparation of this publication was provided by the Medical Advisor group, represented by Yakov Pakhomov and Nikolai Tabakaev.
Ethical Approval: The study design and documents were reviewed and approved by national ethics committees of the clinical centres from the Russian Federation. In Kazakhstan, ethical review and approval was provided by the Central Bioethics Commission at the Salidat Kairbekova National Research Center for Health Development; the approval by national ethics committees was optional.
Patient Consent for Publication: The patients signed an informed consent form to participate in the study.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator (Nazarenko T.A.).
For citation: Nazarenko T.A., Pestova T.I., Lokshin V.N., Dzhusubalieva T.M., Serov V.N., Baranov I.I., Bezhenar V.F., Gavisova A.A., Gorodnova E.A., Dolgushina N.V., Kalugina A.S., Kvashnina E.V., Kogan I.Yu., Koloda Yu.A.,
Korsak V.S., Krasnopolskaya K.V., Molchanova I.V., Sabirova V.L., Tapilskaya N.I., Sukhikh G.T.
Predictors of pregnancy rate in assisted reproductive technologies: results of the IRIS observational program
in the population of Russia and Kazakhstan.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; 3: 144-158 (in Russian)
https://dx.doi.org/10.18565/aig.2025.82
Keywords
Infertility as a dynamically growing medical and social problem is raising concerns among national and international healthcare organizations. Systematic review and meta-analysis of epidemiological studies conducted by Zhu H. et al. (2023) pinpoints that the overall prevalence of infertility is around 13% in the world population, including 7% of primary and 6% of secondary infertility. [1]. Based on the literature review, Schmidt A.A. et al. (2019) concluded that infertility rates may vary across the Russian Federation and could be up to 20% of population in selected regions [2], while national statistics report approximately 255 thousand (1%) females with infertility in Russia among the population of women of reproductive age (18–49 years) in 2023 [3]. In Kazakhstan, between 12 and 15.5% of couples are struggling with infertility [4].
Modern assisted reproductive technologies have significantly increased the effectiveness of infertility treatment. According to the 2022 report from Russian assisted reproductive technology (ART) registry, of 159 thousand assisted reproductive technology cycles 42.7 thousand resulted in pregnancy and approximately 30 thousand led to delivery [5]. Based on the analysis of data from Australian fertility clinics, Lazzari E. et al. (2023) stated that ARTs significantly extend reproductive life for females with positive projected trend for growth in its use [6].
There are various identified factors impacting on ART effectiveness. Non-modifiable factors include female and male baseline parameters such as age, duration of infertility, ethnicity, antral follicle count (AFC), prior pregnancies, infertility cause, and sperm parameters. At the same time levels of hormones, quality of transferred embryos, female body mass index (BMI), fertilization methods, stimulation of ovarian function, day of embryo transfer, number of transferred embryos, ect., can all be modified to improve ART success rate [7,8]. Simopoulou M. et al. (2018) suggested that good quality prediction model could significantly improve effectiveness of ART by allowing to better choose fertilization method and account for non-modifiable factors [9]. However, a convenient visual prognostic model in IVF cycles has not been developed yet. Thus, the primary objective of the study was to develop practical model for predicting the outcome of ART, with secondary objectives to determine biochemical, pregnancy rates, convenience and overall satisfaction with therapy, live birth rates, and safety for maternal and fetal in routine clinical practice.
Materials and methods
Study design
Prospective, multicenter, observational, non-interventional, non-randomized, post-marketing program for assessing the probability of pregnancy in ART programs using oral dydrogesterone for luteal phase support. The non-interventional study design allows the outcome of a given therapy to be assessed in real-life settings, while the prospective design allows the incidence of events to be assessed and eliminates the risk of some bias. Epidemiological methods were utilized for data collection, i.e., investigators used source medical documents to fill in event report forms and not a primary data collection from participant according to the study purposes.
The study was divided into two phases. The phase I, that lasted for 10 weeks after embryo transfer, comprised at least three interactions with participant.
At the enrollment on the day of single embryo transfer participants signed an informed consent form for study participation. Second interaction with patient occurred in 12–14 days after the embryo transfer via phone to collect data on the pregnancy test performed by participant. Participants with no confirmed pregnancy 14 days after embryo transfer were excluded from the study. Participants with confirmed pregnancy continued dydrogesterone treatment at dose of 30 mg/day for next 10 weeks according to the approved product label. For these 10 weeks participant could be contacted either via phone or during routine physician appointment to collect the data on the course of pregnancy.
In study phase II participant were contacted via phone 30 days after the planned delivery date to collect the data on delivery time (pregnancy week), pregnancy outcome (live birth, or stillbirth, etc.), mother and newborn safety and wellbeing including the number of delivered newborns, gender, height and weight, APGAR score at 1 and 5 minutes, as well as any malformations/abnormalities.
Prospective data were collected from March 2020 to February 2021 in Russia, from May 2021 to March 2022 in Kazakhstan.
Participants
The study enrolled females who were undergoing treatment using ART in the routine clinical practice according to the national clinical guidelines and had a single embryo transfer in stimulated (fresh) IVF cycle. Study participants were prescribed dydrogesterone (Duphaston) 10 mg three times daily (TID) to support the luteal phase according to international (ESHRE [10]) and national (Russian [3] and Kazakhstan [11], respectively) clinical guidelines, with the registered and approved by the Ministry of Health of the Russian Federation instructions for use of the medicine. Females with a history of gestational surrogacy, oocyte or embryo donation were not included in the study.
Overall, 42 sites in Russian Federation and 2 sites in Republic of Kazakhstan enrolled participants.
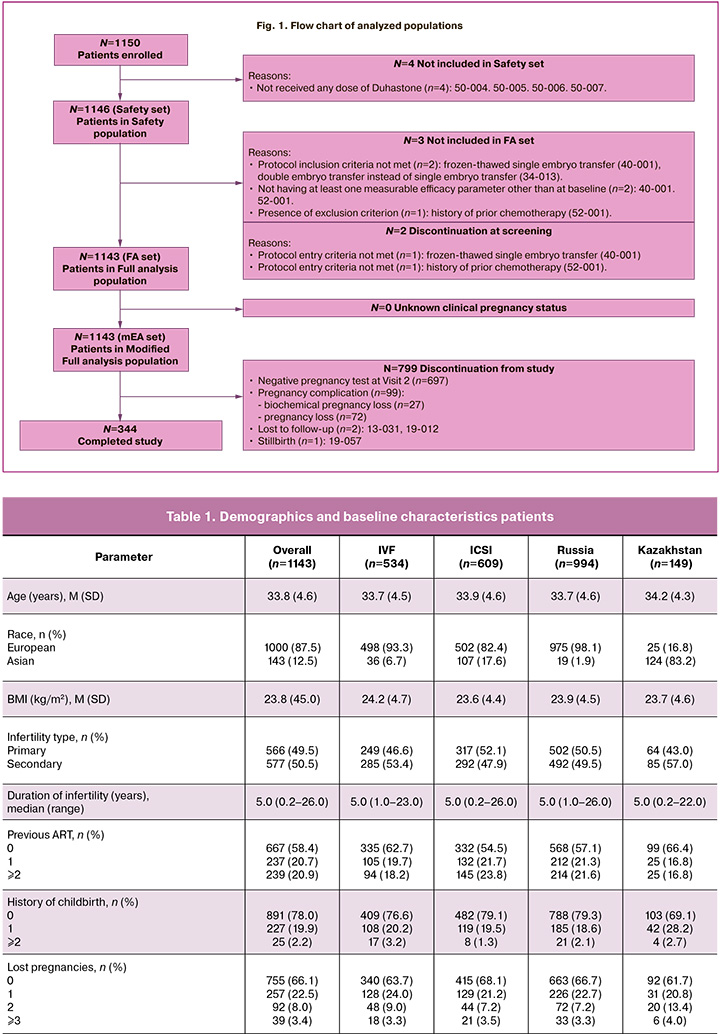
A total of 1150 patients were included, of which 1146 patients were in Safety population and 1143 patients were in the Full analysis population.
The Russian population included 1000 participants, with the safety evaluation sample consisting of 966 patients and 994 patients in the Full analysis population. Kazakhstan population included 150 patients, with the safety population consisting of 150 patients and 149 in the Full analysis population.
Study outcomes and endpoints
The primary objective of the study was to evaluate the predictors of pregnancy rate in in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) in a sample of Russian and Kazakhstan females taking oral dydrogesterone for luteal phase support during ART according to the national guidelines, followed by the creation of a prognostic table of clinical pregnancy in IVF and ICSI cycles considering 4–5 most significant predictors. For ART effectiveness, primary variable of interest was the clinical pregnancy rate defined as a pregnancy diagnosed by ultrasound visualization (in a period of 3–10 weeks after embryo transfer) of one or more gestational sacs [11–13]. Of note, the definition included ectopic pregnancy, multiple gestational sacs were counted as one clinical pregnancy. Clinical pregnancy could also be diagnosed with fetal heartbeat evaluation, which, by definition, included ectopic pregnancy.
To build a predictive model relationship between key factors expected to impact pregnancy rates were analyzed as further described below.
As secondary goals, the patient's convenience and overall satisfaction with the use of the medicine to support the luteal phase were assessed using the medicine satisfaction questionnaire (TSQM-9), the clinical pregnancy rate, and biochemical pregnancy in the study population.
The exploratory objective of phase I was to evaluate clinical pregnancy with fetal cardiac activity (in the study population and subgroups), phase II was to evaluate pregnancy outcomes, including live birth data, and to evaluate maternal and neonatal safety and well-being 30 days after delivery.
Ethical approval
The study design and documents were reviewed and approved by national ethics committees of study sites from Russian Federation. For Kazakhstan, ethical review and approval were provided by the Central Bioethics Commission at the Salidat Kairbekova National Scientific Center for Health Development, so ethical approval from local ethics committees was not required.
Statistical analysis
To create a predictive table of clinical pregnancy, the multiple regression model was constructed using the SAS (SAS Stat 15.2). The baseline variables list consisted 13 parameters, including participant’s age, body mass index (BMI), antral follicle count (AFC), ovarian sensitivity index (OSI), number of top-quality embryos, type of ART, and type of infertility. Likelihood Ratio Test was used for model comparisons. Final predictors (up to 5 factors and covariates) were selected during statistical analysis.
For creation of a predictive table of clinical pregnancy, was supposed to use the 4–5 most influent factors for creating a multiple regression model. The multiple regression model was constructed using the SAS (SAS Stat 15.2). The predictive primary model was created on FA set. Patients with unknown clinical pregnancy were considered as “not pregnant”. For sensitivity analysis the model was applied to mFA set. Baseline covariates list comprised 13 variables including participant age, body mass index (BMI), antral follicle count (AFC), ovarian sensitivity index (OSI), number of top-quality embryos, type of ART, and type of infertility. For model comparisons likelihood ratio test was used. During statistical analysis final predictors (up to 5 factors and covariates) were selected.
For population characteristics, effectiveness, and safety analyses the descriptive statistics methods were applied.
Quantitative data are presented as mean (M) and standard deviation (SD) in format M (SD), qualitative data are presented as number (and percentage) in each category.
The study was registered at ClinicalTrials.gov (NCT04249297).
Results
Study population
A total of 1150 patients were included, of which 1146 (99.7%) receiving Duphaston were in the Safety population. Among them, 534 patients were from the IVF subgroup and 612 patients were from ICSI subgroup.
The Full analysis population consisted of 1143/1150 (99.4%) patients, with 534 (46.7%) and 609 (53.3%) patients from the IVF and ICSI subgroups, respectively.
Flow chart of analyzed populations is presented in Figure 1.
Demographic characteristic
Median age (M (SD)) of the participants was 33.8 (4.6) years.
Most females 1000/1143 were European (87.5%), 143/1143 females were Asian (12.5%).
In the general population, the average BMI was 23.8 kg/m2.
History of infertility
In the general group, the median duration (min-max) of infertility was 5 years (0.2–26.0 years). Primary and secondary infertility were equally presented in the study sample.
Primary infertility was observed in 566/1143 patients (49.5%), secondary infertility in 577 patients (50.5%).
Tubal factor and male factor were the most common causes of infertility in the total group and was reported for 486/1143 (42.5%) patients and 316/1143 (27.6%) patients, correspondingly. Infertility due to anovulation, endometriosis and idiopathic/unexplained infertility occurred in 106/1143 (9.3%) patients, 95/1143 (8.3%) patients, and in 128/1143 (11.2%) patients, respectively.
Demographic characteristics and selected baseline variables according to indicators of previous ART attempts, previously born children, and history of pregnancy loss in the total group are included in Table 1.
Ovarian reserve and ovarian stimulation
The mean (SD) Antral Follicle Count (AFC) was 10.9 (6.1), number of oocytes was 8.9 (5.4), ovarian sensitivity index (OSI) was 1.380 (0.872) in patients from the total group in the FA set.
In the general group, ovarian stimulation protocols included protocols with a Gonadotropin-releasing hormone (GnRH) antagonist in 912/1143 patients (79.8%), long protocols with a GnRH agonist in 124/1143 patients (10.8%), and short protocols with a GnRH agonist in 106/1143 patients (9.3%).
Clinical outcome
A total of 1143/1150 (99.4%) patients eligible for FA set were included in the primary analysis, out of them 534 and 609 patients were in the IVF and ICSI subgroups, respectively. In the total group, a subset of patients had a confirmed clinical pregnancy rate of 36.7% (419/ 1143 participants, 95% Cl: 33.86–39.53), resulting in a confirmed clinical pregnancy rate of 37.2% in Russian and 32.9% in Kazakhstan populations: out of them there were 212 and 207 patients in the IVF and ICSI subgroups, respectively. These data were used for building a predictive model based on the observed data and analysis of predictive factors.
Pregnancy prediction model
To select factors for prediction model, a cluster analysis of 24 baseline factors was performed resulting in selection of 5 most impactful factors for a final predictive model. These factors included age, score of transferred embryos, AFC, ART type and infertility type with the last 2 variables intersected. The final model described 68.3% of variability in the model. As a random factor, study site has been added to the model. For visual representation, continuing variables were divided into ranges and a heatmap was created (Fig. 2). The colors range from dark red to bright green, indicating the increasing probability of clinical pregnancy according to the model.
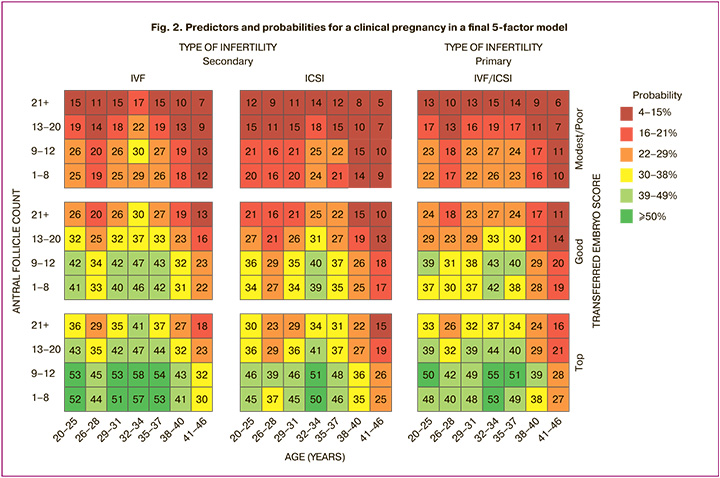
According to the said model, for females aged ≤37 years old with primary infertility clinical pregnancy rate of ≥50% is expected with top‑quality embryos transferred, AFC ≤12 for whom IVF was used. For ICSI in primary infertility, ≥50% pregnancy rate would be achieved for females with the same parameters except the narrower age range of 32–34 years. In secondary infertility regardless of ART method pregnancy rate of ≥39% would be achieved in females aged ≤37 years with an AFC of ≤20 and top-quality embryos transferred.
Secondary endpoints (study phase I)
The study results on the secondary endpoint (convenience score and overall satisfaction score of TSQM-9 questionnaire) demonstrated that convenience for patients of using Duphaston was high throughout the entire study, and overall satisfaction with dydrogesterone for supporting the luteal phase was also high. It can be assumed that patient’s overall satisfaction was maintained at a high level also due to the convenience of taking oral medication throughout the duration of luteal support.
In the total group, positive biochemical pregnancy test rate was established in 446/1143 (39.0%; 95% CI: 36.18–41.92) patients, clinical pregnancy was confirmed in 419/1143 (36.7%; 95% CI: 33.86–39.53) patients, clinical pregnancy rate with fetal heartbeat was confirmed in 376/1143 (32.9%) patients.
Exploratory endpoint (study phase II)
Live birth rate was 30.1% (30.7% in Russian and 26.2% in Kazakhstan populations) – 344/1143 in patients from the FA set.
Out of 344 patients with live births, 340 patients had a singleton delivery, and 4 patients had a multiple delivery with twins. Delivery method was vaginal delivery in 156/344 patients and caesarean section in 188/344 patients. Depending on the term of delivery, delivery at term was in 310/419 patients, pre-term delivery (≤22 and <37 weeks of pregnancy) was in 34/419 patients, of which 1/419 patient had pre-term delivery at 22–27 weeks of pregnancy, 10/419 patients had pre-term delivery at 28–32 weeks of pregnancy and 24 (24/419) patients had pre-term delivery at 33–36 completed weeks of pregnancy.
Out of 348 newborns in most events of the total group (89.7%, 312/348), no abnormal findings were found on physical examination of newborns at birth; abnormalities on physical examination of newborns at birth were found in 10.3% (36/348) newborns.
The mean (SD) weight of newborns was 3245.2 (610.0) g, the mean (SD) height was 51.1 (3.8) cm, and the mean (SD) head circumference was 34.0 (1.9) cm. The mean (SD) APGAR scores at 1 min postpartal and 5 min postpartal were 7.7 (0.9) and 8.5 (0.8), respectively.
Safety
Overview of treatment emergent adverse events and treatment emergent serios adverse events (TEAEs and TESAEs)
As expected, there was good tolerability of dydrogesterone in this patient population, which is consistent with the known safety and tolerability profile of this medicine.
Events with pharmacovigilance-relevant information in patients
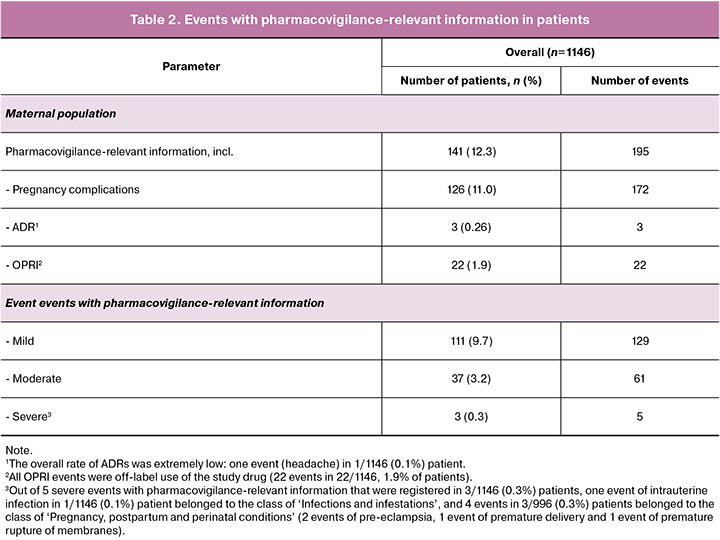
Out of 1146 patients that were included in safety set, a total of 195 events of pharmacovigilance (PV) relevant information were reported in 141/1146 (12.3%) patients: 172 events of pregnancy complications in 126/1146 (11.0%) patients, 3 adverse drug reaction (ADRs) in 3/1146 (0.26%) patients, and 22 reports of other relevant information (OPRI) in 22/1146 (1.9%) patients.
The overall rate of ADRs was extremely low (3/1146, 0.26%): one event of headache, one OPRI event (product administration error), and one event of pregnancy complication (biochemical pregnancy) were considered to be the ADR events.
Among 126/1146 patients with 172 events of pregnancy complications, 99 events in 99/1146 (8.6%) patients led to pregnancy loss and to discontinuation from study; other 27 patients had pregnancy complications that did not lead to pregnancy loss.
All OPRI events were related to the off-label use of the study medicine - 22 events in 22/1146, 1.9% of patients.
A total of 95 events with pharmacovigilance-relevant information were serious in 56/1146 (4.9%) patients; none of these serious events were related to study medicine or led to patient death.
Adverse Events in Fetuses/Newborns
A total of 98 adverse events (AEs) were reported for 41/354 fetuses/newborns, 94 AEs were serious in 39 fetuses/newborns, one AE led to patient’s discontinuation from study, and 2 AEs led to fetal death in 1 fetus. None of AEs had reasonable possibility of causal relationship with study medicine based on causal relationship assessment by Investigator.
The majority of serious AEs reported in fetuses/newborns belonged to the class of ‘Pregnancy, puerperium and perinatal conditions’ and were presented by 34 events in 29/354 (8.1%) fetuses/newborns: out of them there were 24 events of premature baby in 23/354 (6.5%) fetuses/newborns, 6 events of neonatal jaundice (6/354), 1 event of fetal distress syndrome (1/354), 1 event of low-birth weight baby (1/354), 1 event of placental insufficiency (1/354), and 1 event of small for dates baby (1/354).
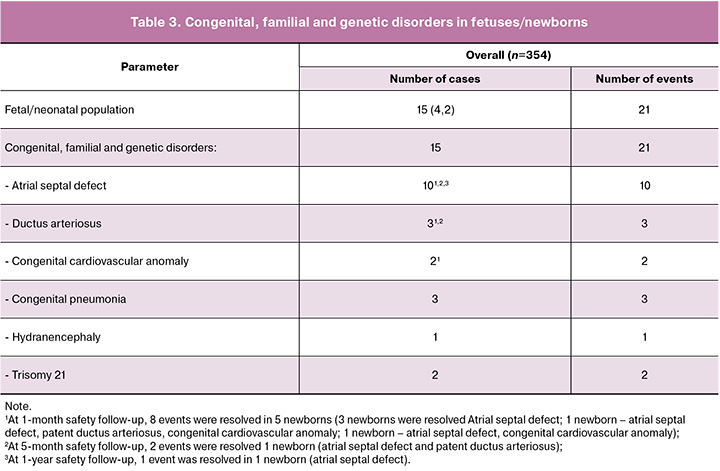
Among serious AEs were registered 21 events from ‘Congenital, familial and genetic disorders’ in 15/354 (4.2%) fetuses/newborns. Out of them, malformations and developmental abnormalities were registered in 13 (3.7%) fetuses/newborns: 2 fetuses and 11 newborns, and 3 reports of congenital pneumonia in 3 newborns. During the first year, all cardiac abnormalities resolved in 9 newborns spontaneously; 1 event was resolved by surgical treatment. In total, during the first year, all cardiac abnormalities were resolved (in 10 children).
The number of congenital diseases and developmental anomalies identified in the study does not exceed population data on the prevalence of congenital defects in children undergoing ART [14, 15].
This study confirmed the positive safety profile of dydrogesterone used for luteal phase support as a part of ART treatment in real clinical practice. Neither maternal serious ADRs, nor neonatal AEs with reasonable possibility of causal relationship with the study medicine were reported during the study.
Subgroup of patients from Russian Federation
The Russian population included 1000 participants, the sample of safety assessment was 996 patients, 994 patients – in Full analysis population.
A total of 994 participants from Russia were included in the analysis. Mean (SD) age of participants was 37.7 (4.6) years and 98.4% were European. IVF was used for 503 (50.3%) patients and ICSI for 491 (49.4%), 50.5% of participants had primary infertility, while 49.5% had secondary infertility.
Clinical pregnancy was confirmed in 370 of 994 participants resulting in clinical pregnancy rate of 37.2%. Mean (SD) age of participants with confirmed pregnancy was 33.2 (4.2) years. Live birth of 308 children (including 3 pairs of twins) to 305 patients – 30.7%.
The prediction model was built as described above with the same five selected predictive factors. Results of modeling for Russian population were comparable with those obtained for overall population Figure 3.

Discussion
Our study presents a practical prediction model for clinical pregnancy after the ART in populations of Russian Federation and Kazakhstan. Multiple authors suggested different approaches to ART effectiveness prediction. Gao H. et al. (2021) suggested a model for ART prediction based on nine factors, including maternal age and education, number of prior ART treatments, prior abortions, endometrial thickness prior to transfer, number and quality of transferred embryos [16].
Kothandaraman R. et al. (2021) suggested a machine learning model for prediction of IVF/ICSI success based on medical history [8]. Henderson I. et al. (2021) conducted systematic review studies suggesting predictive models for ART success. Overall, of 69 studies, authors subtracted 7 main groups of predictors: demographic characteristics (including age), medical history, pathology related to female and male partner, ovarian reserve variables, stimulation, variables related to embryo transfer and other factors such as ICSI and donor sperm use [17]. We used demographic factors (maternal age), pathology (type of infertility), ovarian reserve (AFC), post-stimulation (embryo quality) and fertilization method (ICSI). Considering this systematic review the main limitation of our model is a lack of external validation. Shingshetty L. et al. (2024) in a recent publication noted that there are 11 main predictive factors for IVF success models, including age, infertility duration and cause, ethnicity, BMI, AFC, and prior pregnancies [7].
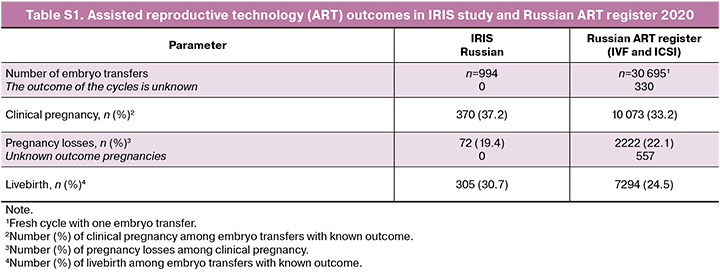
Reported confirmed clinical pregnancy rate in our study was 36.7% (37.2% in Russian population) which is slightly higher compared to the numbers reported in Russian ART registry (33.2%) with similar time period [5, 18]. In our study live birth rate was 30.1% (30.7% in Russia) which is slightly higher compared to the numbers reported in Russian ART registry (24.5%) [18]. Of note, the main difference between ART cycles in present studies and Russian ART registry is the approach to luteal phase support: for IRIS study 100% of cycles were performed with dydrogesterone (Duphaston) for luteal phase support while in ART registry dydrogesterone use rate attributed to 32% of cycles [18] due to adding proposed clinical use to the product label of dydrogesterone of in Russia in 2017. Refer to Supplementary Material for detailed comparison of data from Russian ART registry and present study (Supplementary Table S1). Noncomparative analysis of data suggest that dydrogesterone may improve ART outcomes due to greater effectiveness in achieving clinical pregnancy and reducing number of pregnancy losses.
Impact of oral dydrogesterone on ART outcomes has been studied by several authors. Tournaye H. et al. (2017) in Phase 3 study demonstrated non-inferiority of oral dydrogesterone versus micronized vaginal progesterone (MVP) for IVF in 973 females with respective pregnancy rates of 37.6 and 33.1% and live birth rates of 34,6 and 29.8%, respectively [19]. Comparable results were later reported in another Phase 3 study with similar design published by Griesinger G. et al. (2018) [20]. The results of a systematic review and meta-analysis of nine studies (n=1957) by Griesinger G. et al. (2020) showed that dydrogesterone provided superior pregnancy rates (odds ratio [OR] = 1.32 [95% CI 1.08–1.61]) an live birth (OR = 1.28 [95% CI 1.04–1.57]) [21] compared to MVP for luteal phase support during IVF.
The use of oral dydrogesterone in LF support of IVF cycles is associated with reduced late pregnancy complications such as preeclampsia [22]. In this study, the incidence of preeclampsia was 0.6% – 2/344 patients. It is important to note that the incidence of extremely early preterm birth was very low and amounted to 0.3% – 1/344 patients, which is significantly lower than to the population data.
In our study, a high level of satisfaction with oral dydrogesterone therapy was observed, which is important for treatment adherence and its results. The results of a previously published large-scale survey indicate that oral forms of drugs for luteal phase support are the most preferred by doctors and patients due to higher compliance [23].
In addition, a number of studies have shown that the use of dydrogesterone was associated with a lower risk of adverse events from the genital organs compared to MVP [24, 25].
The safety issues of ART and the drugs used in the programs are regularly discussed around the world, including regarding dydrogesterone. In this regard, much attention is focused on studying issues related to the safety of the effect of both the procedure itself and the hormonal drugs used on the body of the pregnant woman and the fetus.
In our study, malformations and anomalies were detected in 3.2% of newborns. The number of detected deviations and their structure correspond to both population data (for the period 2020–2023, according to Rosstat, congenital anomalies occurred among 3.3–3.6% of newborns [26], according to statistics of Kazakhstan among 3.0–3.7% [27]) and previously published results of randomized clinical trials [19, 20, 21]. However, the topic of safety of the entire ART procedure and the drugs used, including dydrogesterone, is regularly discussed. Thus, in 2024, Katalinic A. et al. published a systematic review and meta-analysis assessing the relationship between dydrogesterone intake in early pregnancy and the risk of developing congenital anomalies in the fetus [28]. This work represents the most comprehensive and reliable analysis of the peer-reviewed published literature on the safety of dydrogesterone in relation to the fetus when administered in the first trimester of pregnancy [28]. Based on the conducted meta-analysis of randomized controlled studies, the authors concluded that dydrogesterone does not increase the risk of congenital anomalies above the level expected under the influence of environmental and genetic factors [28]. Also, in 2024, Russian scientists conducted an expert analysis of data on the safety of hormonal therapy during pregnancy [29]. Based on international and domestic data, dydrogesterone has a well-known and favorable safety profile when used as luteal phase support, therapy for threatened/habitual miscarriage [29].
In 2025, an analysis of the VigiBase database was published, which revealed a disproportionate frequency of reports of congenital malformations with the use of dydrogesterone [30]. However, VigiBase registered only 145 reports of adverse events associated with the use of dydrogesterone over more than 50 years [30]. Of the 145 AE reports, 60 events (including 68 cases) belonged to the class of congenital defects, and therefore the ratio of congenital defects to other AEs (77) was 1.13. Based on this, the authors concluded that there is a possible safety signal for further study of the safety profile of dydrogesterone for the fetus [30].
The secondary endpoints of our study were a detailed examination of neonatal health. Importantly, there were 182 reports in the patients and fetuses/newborn AEs during the study period, of which 15 were birth defects. The ratio of other AEs (167) to birth defects in the IRIS study was 11.1, which is closer to real-life clinical practice data and provides a more substantial evidence base for the safety of dydrogesterone.
In the previously mentioned Lotus I and Lotus II studies, there were 602 reports of AEs in the dydrogesterone group, which is 4 times higher than the VigiBase data [19, 20]. And among a larger sample of high-quality studies, no increase in the risk of birth defects was found after dydrogesterone use compared with vaginal progesterone, confirming the absence of evidence of a negative impact on the health of the offspring [19, 20].
Our study data make a special contribution to the existing data from real-life clinical practice, randomized clinical trials, and studies by Russian scientists, where the safety profile of dydrogesterone in ART programs was studied.
Due to the demonstrated efficacy and safety of dydrogesterone for luteal phase support in IVF cycles, based on numerous published clinical studies, Patki A. et al. (2024) in a review of the available data concluded that dydrogesterone has the potential to become the drug of choice for luteal phase support in millions of women undergoing ART [31].
The introduction of a new method in the form of a developed prediction model can improve the effectiveness of ART, reduce economic costs and the time required to obtain a positive result.
Conclusion
The developed model for predicting the effectiveness of ART corresponds to the trends and methods used by other groups of scientists and can be recommended for routine use. The results of the study present a practical model for predicting the success of IVF/ICSI and demonstrate a favorable profile of the effectiveness and safety of oral dydrogesterone in routine practice. Dydrogesterone has proven its effectiveness for luteal phase support in routine clinical practice in Russia and Kazakhstan.
Supplementary Material
References
- Zhu H., Zhou X., Li R., Gao Q., Wang X., Cheng P. et al. Global prevalence of infertility: a systematic review and meta-analysis of Community-based studies. Authorea. September 22, 2023. https://dx.doi.org/10.22541/au.169535894.43892783/v1.
- Шмидт А.А., Замятнин С.А., Гончар И.С., Коровин А.Е., Городнюк И.О., Коцур А.В. Эпидемиология бесплодия в России и за рубежом. Клиническая патофизиология. 2019; 25(1): 9-12. [Shmidt A.A., Zamyatnin S.A., Gonchar I.S., Korovin A.E., Gorodnyuk I.O., Kotsur A.V. Epidemiology of infertility in Russia and abroad. Clinical Pathophysiology. 2019; 25(1): 9-12. (in Russian)].
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Женское бесплодие. 2021. [Ministry of Health of the Russian Federation. Clinical guidelines. Female infertility. 2021. (in Russian)].
- Suleimenova M., Lokshin V., Glushkova N., Karibayeva S., Terzic M. Quality-of-life assessment of women undergoing in vitro fertilization in Kazakhstan. Int. J. Environ. Res. Public Health. 2022; 19(20): 13568. https://dx.doi.org/10.3390/ijerph192013568
- Российская Ассоциация Репродукции Человека (РАРЧ). Регистр ВРТ. Отчет за 2022 год. Доступно по: https://www.rahr.ru/d_registr_otchet/RegistrVRT_2022.pdf [Russian Association for Human Reproduction (RAHR). ART register. 2022 report. Available at: https://www.rahr.ru/d_registr_otchet/RegistrVRT_2022.pdf (in Russian)].
- Lazzari E., Potančoková M., Sobotka T., Gray E., Chambers G.M. Projecting the contribution of assisted reproductive technology to completed cohort fertility. Popul. Res. Policy Rev. 2023; 42(1): 6. https://dx.doi.org/10.1007/s11113-023-09765-3.
- Shingshetty L., Cameron N.J., Mclernon D.J., Bhattacharya S. Predictors of success after in vitro fertilization. Fertil. Steril. 2024; 121(5): 742-51. https://dx.doi.org/10.1016/j.fertnstert.2024.03.003.
- Kothandaraman R., Andavar S., Raj R.S.P. Dynamic model for assisted reproductive technology outcome prediction. Brazilian Archives of Biology and Technology. 2021; 64: e21200758. https://dx.doi.org/10.1590/1678-4324-2021200758.
- Simopoulou M., Sfakianoudis K., Antoniou N., Maziotis E., Rapani A., Bakas P. et al. Making IVF more effective through the evolution of prediction models: is prognosis the missing piece of the puzzle? Syst. Biol. Reprod. Med. 2018; 64(5): 305-23. https://dx.doi.org/10.1080/19396368.2018.1504347.
- ESHRE. Guideline on Ovarian Stimulation for IVF/ICSI. Available at: https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Ovarian-Stimulation-in-IVF-ICSI
- Приказ Министра здравоохранения Республики Казахстан от 15.12.2020 № КР ДСМ-272/2020 "Об утверждении правил и условий проведения вспомогательных репродуктивных методов и технологий". Зарегистрировано в Министерстве юстиции Республики Казахстан 20 декабря 2020 г. № 21816. [Order of the Ministry of Health of the Republic of Kazakhstan of 15.12.2020 No. KR DSM-272/2020 "On approval of the rules and conditions for carrying out assisted reproductive methods and technologies". Registered with the Ministry of Justice of the Republic of Kazakhstan on December 20, 2020 No. 21816. (in Russian)].
- Приказ Министерства здравоохранения Российской Федерации №107н от 30.08.2012 "О порядке использования вспомогательных репродуктивных технологий, противопоказаниях и ограничениях к их применению" (утратил силу с 01.01.2021). [Order of the Ministry of Health of the Russian Federation No. 107n dated 30.08.2012 "On the procedure for using assisted reproductive technologies, contraindications and restrictions on their use" (expired since 01.01.2021). (in Russian)].
- Приказ Министерства здравоохранения Российской Федерации №803н от 31.07.2020 "О порядке использования вспомогательных репродуктивных технологий, противопоказаниях и ограничениях к их применению" (вступил в силу с 01.01.2021). [Order of the Ministry of Health of the Russian Federation No. 803n dated 31.07.2020 "On the procedure for using assisted reproductive technologies, contraindications and restrictions on their use" (entered into force on 01.01.2021). (in Russian)].
- Hansen M., Kurinczuk J.J., Milne E., de Klerk N., Bower C. Assisted reproductive technology and birth defects: a systematic review and meta-analysis. Hum. Reprod. Update. 2013; 19(4): 330-53. https://dx.doi.org/10.1093/humupd/dmt006.
- Gullo G., Scaglione M., Laganà A.S., Perino A., Andrisani A., Chiantera V. et al. Assisted reproductive techniques and risk of congenital heart diseases in children: a systematic review and meta-analysis. Reprod. Sci. 2023; 30(10): 2896-906. https://dx.doi.org/10.1007/s43032-023-01252-6.
- Gao H., Liu D.E., Li Y., Wu X., Tan H. Early prediction of live birth for assisted reproductive technology patients: a convenient and practical prediction model. Sci. Rep. 2021; 11(1): 331. https://dx.doi.org/10.1038/s41598-020-79308-9.
- Henderson I., Rimmer M.P., Keay S.D., Sutcliffe P., Khan K.S., Yasmin E. et al. Predicting the outcomes of assisted reproductive technology treatments: a systematic review and quality assessment of prediction models. F&S Reviews. 2021; 2(1): 1-10. https://dx.doi.org/10.1016/j.xfnr.2020.11.002.
- Российская Ассоциация Репродукции Человека (РАРЧ). Регистр ВРТ. Отчет за 2020 год. Доступно по: https://www.rahr.ru/d_registr_otchet/RegistrVRT_2021.pdf [Russian Association for Human Reproduction (RAHR). ART register. 2020 report. Available at: https://www.rahr.ru/d_registr_otchet/RegistrVRT_2021.pdf (in Russian)].
- Tournaye H., Sukhikh G.T., Kahler E., Griesinger G. A Phase III randomized controlled trial comparing the efficacy, safety and tolerability of oral dydrogesterone versus micronized vaginal progesterone for luteal support in in vitro fertilization. Hum. Reprod. 2017; 32(5): 1019-27. https://dx.doi.org/10.1093/humrep/dex023.
- Griesinger G., Blockeel C., Sukhikh G.T., Patki A., Dhorepatil B., Yang D.Z. et al. Oral dydrogesterone versus intravaginal micronized progesterone gel for luteal phase support in IVF: a randomized clinical trial. Hum. Reprod. 2018; 33(12): 2212-21. https://dx.doi.org/10.1093/humrep/dey306.
- Griesinger G., Blockeel C., Kahler E., Pexman-Fieth C., Olofsson J.I., Driessen S. et al. Dydrogesterone as an oral alternative to vaginal progesterone for IVF luteal phase support: A systematic review and individual participant data meta-analysis. PLoS One. 2020; 15(11): e0241044. https://dx.doi.org/10.1371/journal.pone.0241044.
- Храмцова А.Ю., Башмакова Н.В., Семенов Ю.А., Карибаева Ш.К., Мелкозерова О.А. Сравнительный анализ исходов беременности после переноса эмбрионов в стимулированном цикле экстракорпорального оплодотворения в зависимости от типа прогестагена, используемого для посттрансферной поддержки. Акушерство и гинекология. 2024; 10: 130-7. [Khramtsova A.Yu., Bashmakova N.V., Semenov Yu.A., Karibaeva Sh.K., Melkozerova O.A. Comparative analysis of pregnancy outcomes after embryo transfer in a stimulated in vitro fertilisation cycle depending on the progestogen type used for post-transfer support. Obstetrics and Gynecology. 2024; (10): 130-7 (in Russian)]. https://dx.doi.org/10.18565/aig.2024.236.
- Shoham G., Leong M., Weissman A. A 10-year follow-up on the practice of luteal phase support using worldwide web-based surveys. Reprod. Biol. Endocrinol. 2021; 19(1): 15. https://dx.doi.org/10.1186/s12958-021-00696-2.
- Сабирова В.Л., Курбатина М.М., Миннуллина Ф.Ф., Филюшина А.В. Сравнительный анализ эффективности и безопасности гестагенов для лютеиновой фазыподдержка в свежих циклах ЭКО/ИКСИ с переносом одного эмбриона. Вопросы гинекологии, акушерства и перинатологии. 2023; 22(6): 28-35. [Sabirova V.L., Kurbatina M.M., Minnullina F.F., Filyushina A.V. Comparative analysis of the efficacy and safety of gestagens for luteal phase support in fresh IVF/ICSI cycles with single embryo transfer. Gynecology, Obstetrics and Perinatology. 2023; 22(6): 28-35. (in Russian)]. https://dx.doi.org/10.20953/ 1726-1678-2023-6-28-35.
- Беспалова О.Н., Бутенко М.Г., Баклейчева М.О., Косякова О.В., Саркисян Г.С., Коган И.Ю. Эффективность прогестагенов в лечении угрозы выкидыша у женщин с многоплодной беременностью, наступившей в результате вспомогательных репродуктивных технологий. Вопросы гинекологии, акушерства и перинатологии. 2021; 20(1): 47-54. [Bespalova O.N., Butenko M.G., Bakleycheva M.O., Kosyakova O.V., Sargsyan G.S., Kogan I.Yu. Efficacy of progestogens in the management of threatened miscarriage in women with multiple pregnancies resulting from assisted reproductive technologies. Gynecology, Obstetrics and Perinatology. 2021; 20(1): 47-54. (in Russian)]. https://dx.doi.org/10.20953/1726-1678-2021-1-47-54.
- Федеральная служба государственной статистики (Росстат). Здравоохранение в России 2023. Статистический сборник. М.: Росстат; 2023. 179 с. [Federal State Statistics Service (Rosstat). Healthcare in Russia 2023. Statistical compilation. Moscow: Rosstat; 2023. 179 p. (in Russian)].
- Агентство по стратегическому планированию и реформам Республики Казахстан. Бюро национальной статистики. Дети Казахстана 2018-2022. Статистический сборник. Астана; 2023. 126 с. [Agency for Strategic Planning and Reforms of the Republic of Kazakhstan. Bureau of National Statistics. Children of Kazakhstan 2018-2022. Statistical compilation. Astana; 2023. 126 p. (in Russian)].
- Katalinic A., Noftz M.R., Garcia-Velasco J.A., Shulman L.P., van den Anker J.N., Strauss Iii J.F. No additional risk of congenital anomalies after first-trimester dydrogesterone use: a systematic review and meta-analysis. Hum. Reprod. Open. 2024; 2024(1): hoae004. https://dx.doi.org/10.1093/hropen/hoae004.
- Сухих Г.Т., Серов В.Н., Андреева М.Д., Артымук Н.В., Базина М.И., Баранов И.И., Башмакова Н.В., Беженарь В.Ф., Белоцерковцева Л.Д., Геппе Н.А., Долгушина Н.В., Зарецкая Н.Б., Захарова И.Н., Зубков В.В., Енькова Е.В., Есаян Р.М., Каткова Н.Ю., Квашнина Е.В., Коган И.Ю., Корсак В.С., Краснопольская К.В., Кукарская И.И., Молчанова И.В., Назаренко Т.А., Пестова Т.И., Подзолкова Н.М., Савельева И.В., Сазонова А.И., Семенов Ю.А., Тапильская Н.И., Тетруашвили Н.К., Тиселько А.В., Фадеев В.В., Шамугия Н.Л., Шахова М.А., Ших Е.В., Ярмолинская М.И. Безопасность гормональной терапии во время беременности. Совместная позиция экспертов в области репродуктивной медицины, акушерства и гинекологии, эндокринологии, клинической фармакологии, неонатологии, педиатрии и репродуктивной генетики. Акушерство и гинекология. 2024; 8: 196-206. [Sukhikh G.T., Serov V.N., Artymuk N.V., Andreeva M.D., Bazina M.I., Baranov I.I., Bashmakova N.V., Bezhenar V.F., Belotserkovtseva L.D., Geppe N.A., Dolgushina N.V., Zaretskaya N.V., Zakharova I.N., Zubkov V.V., Enkova E.V., Yesayan R.M., Katkova N.Yu., Kvashnina E.V., Kogan I.Yu., Korsak V.S., Krasnopolskaya K.V., Kukarskaya I.I., Molchanova I.V., Nazarenko T.A., Pestova T.I., Podzolkova N.M., Saveljeva I.V., Sazonova A.I., Semenov Yu.A., Tapilskaya N.I., Tetruashvili N.K., Tiselko A.V., Fadeev V.V., Shamugia N.L., Shakhova M.A., Shikh E.V., Yarmolinskaya M.I. The safety of hormone therapy during pregnancy. Joint statement by experts in reproductive medicine, obstetrics and gynecology, endocrinology, clinical pharmacology, neonatology and pediatrics. Obstetrics and Gynecology. 2024; (8): 196-206 (in Russian)]. https://dx.doi.org/10.18565/aig.2024.201.
- Henry A., Santulli P., Bourdon M., Maignien C., Chapron C., Treluyer J.M. et al. Birth defects reporting and the use of dydrogesterone: a disproportionality analysis from the World Health Organization pharmacovigilance database (VigiBase). Hum. Reprod. Open. 2025; 2025(1): hoae072. https://dx.doi.org/10.1093/hropen/hoae072.
- Patki A. Role of dydrogesterone for luteal phase support in assisted reproduction. Reprod. Sci. 2024; 31(1): 17-29. https://dx.doi.org/10.1007/s43032-023-01302-z.
Received 21.03.2025
Accepted 27.03.2025
About the Authors
Tatiana A. Nazarenko, Dr. Med. Sci., Professor, Director of the Institute of Reproductive Medicine, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia (Moscow), +7(495)531-44-44, t_nazarenko@oparina4.ruTatiana I. Pestova, PhD, Head of the Department of Assisted Reproductive Technologies, Regional Perinatal Center (Chelyabinsk); Chief Freelance Specialist in Reproductive Health of the Ministry of Health of the Chelyabinsk Region, +7(912)790-88-24, glav_vrach6767@mail.ru
Vyacheslav N. Lokshin, Academician of the National Academy of Sciences of the Republic of Kazakhstan, Professor, Chief Reproductive Specialist of the Ministry of Health of the Republic of Kazakhstan; President of the Kazakhstan Association of Reproductive Medicine; President of the International Academy of Reproductology; General Director of the PERSONA International Clinical Center for Reproductology (Almaty), +7(701)755-82-09, v_lokshin@persona-ivf.kz, https://orcid.org/0000-0002-4792-5380
Tamara M. Dzhusubalieva, PhD, CEO of the IRM Clinic, obstetrician-gynecologist of the highest category, WHO expert on reproductive health (Almaty),
+7(701)111-34-37, tamara_kmpa@mail.ru
Vladimir N. Serov, Dr. Med. Sci., Professor, Academician of RAS, President of the Russian Society of Obstetricians and Gynecologists, Chief Scientific Consultant, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia (Moscow), +7(495)531-44-44, v_serov@oparina4.ru
Igor I. Baranov, Dr. Med. Sci., Professor, Head of the Department of Scientific and Educational Programs, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia (Moscow), +7(495)438-94-92, i_baranov@oparina4.ru
Vitaly F. Bezhenar, Dr. Med. Sci., Professor, Head of the Department of Obstetrics, Gynecology and Neonatology, Head of the Department of Obstetrics, Gynecology and Reproductology, Head of the Clinic of Obstetrics and Gynecology, Academician I.P. Pavlov First St. Petersburg State Medical University, Ministry of Health of Russia;
Chief Freelance Specialist in Obstetrics and Gynecology of the Health Committee of St. Petersburg (St. Petersburg), +7(812)338-67-44, bez-vitaly@yandex.ru
Alla A. Gavisova, Dr. Med. Sci., Head of the 1st Gynecological Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia (Moscow), +7(495)531-44-44, a_gavisova@oparina4.ru
Elena A. Gorodnova, PhD, Head of the Center for Scientific and Clinical Research, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology
and Perinatology, Ministry of Health of Russia (Moscow), +7(495)438-94-92, e_gorodnova@oparina4.ru
Natalia V. Dolgushina, Dr. Med. Sci., Professor, Deputy Director for Research, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia (Moscow), +7(495)438-49-77 (1362), n_dolgushina@oparina4.ru
Alla S. Kalugina, Dr. Med. Sci., Professor, Professor at the Department of Obstetrics, Gynecology and Neonatology, Academician I.P. Pavlov First St. Petersburg State Medical University, Ministry of Health of Russia; Chief Freelance Specialist in Women’s Reproductive Health of the Ministry of Health of Russia in the North-Western Federal
District (St. Petersburg), alla19021962@gmail.com
Elena V. Kvashnina, PhD, Reproductologist, Deputy Director for Medical Affairs, IVF Center Partus Clinic (Yekaterinburg), +7(343)385-57-38, centreko@ivf-partus.ru
Igor Yu. Kogan, Dr. Med. Sci., Professor, Corresponding Member of RAS, Director of the D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology
(St. Petersburg), +7(812)679-55-51, iagmail@ott.ru
Yulia A. Koloda, PhD, Associate Professor, Department of Obstetrics and Gynecology, Russian Medical Academy of Continuing Professional Education; Obstetrician-Gynecologist, Medical Director of the «Life Line» Reproduction Center; Member of ESHRE and Chairman of the RAHR Committee (Moscow), julkol@yandex.ru
Vladislav S. Korsak, Dr. Med. Sci., Professor, President of the RAHR; General Director of International Center for Reproductive Medicine JSC (St. Petersburg),
+7(812)385-69-85, ivf@mcrm.ru
Ksenia V. Krasnopolskaya, Dr. Med. Sci., Corresponding Member of RAS, Professor, Head of the Department of Assisted Reproductive Technologies,
Academician V.S. Krasnopolsky Moscow Regional Research Institute of Obstetrics and Gynecology; Medical Director, Clinic Prior LLC (Moscow), guzmoniiag@gmail.com
Irina V. Molchanova, PhD, Chief Physician of the Altai Regional Clinical Perinatal Center; Chief Freelance Specialist in Obstetrics and Gynecology in the Altai Territory (Barnaul), +7(903)949-10-64, molcanova2008@yandex.ru
Venera L. Sabirova, PhD, Head of the IVF Department, «Scandinavia (AVA-Kazan» Reproduction Clinic; Senior Lecturer, Institute of Fundamental Medicine and Biology (Kazan), sabirova-vl@avaclinic.ru
Natalya I. Tapilskaya, Dr. Med. Sci., Professor, Head of the Department of Reproductology, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology
(St. Petersburg), +7(921)933-61-26, tapnatalia@yandex.ru
Gennady T. Sukhikh, Dr. Med. Sci., Professor, Academician of RAS, Director of the Academician V.I. Kulakov National Medical Research Center for Obstetrics,
Gynecology and Perinatology, Ministry of Health of Russia (Moscow), +7(495)438-18-00, g_sukhikh@oparina4.ru



