Activation of ovarian function in patients with diminished ovarian reserve through administration of extracellular vesicles
Martirosyan Ya.O., Nazarenko T.A., Kadaeva A.I., Goryunov K.V., Shevtsova Yu.A.
Relevance: Poor response to ovarian stimulation during an IVF program is a pressing issue that limits the effectiveness of assisted reproductive technology (ART). Currently, there are no verified and effective methods in routine clinical practice to improve the success rates of treatment in patients with a poor ovarian response. The use of cell-free therapy as a preparation for IVF/ICSI programs offers a promising alternative to donor oocytes, allowing this group of patients to have genetically related children.
Case report: This article presents three clinical observations that demonstrate the effectiveness of a technique involving the activation of ovarian function through intraovarian administration of mesenchymal stem cell-derived extracellular vesicles (MSC-EVs) in patients with a history of multiple IVF failures and poor response to ovarian stimulation.
Conclusion: The presented information on the efficacy of MSC-EVs, based on clinical cases of complex and challenging patients with significantly diminished ovarian function, provides hope for the development of a new direction in reproductive medicine based on the use of cellular technologies. Further research is needed to study the intraovarian regulation of folliculogenesis and the mechanisms by which extracellular vesicles influence these processes.
Authors' contributions: Martirosyan Ya.O., Nazarenko T.A., Kadaeva A.I., Goryunov K.V., Shevtsova Yu.A. – design of the study, data collection for analysis, reviewing relevant publications, data analysis, preparation of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: The study was supported by research project 121040600410-7 Nazarenko T.A. Solving the problem of infertility in modern conditions through the development of a clinical diagnostic model of infertile marriage and the use of innovative technologies in assisted reproduction programs.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Martirosyan Ya.O., Nazarenko T.A., Kadaeva A.I., Goryunov K.V., Shevtsova Yu.A.
Activation of ovarian function in patients with diminished ovarian reserve through administration of extracellular vesicles.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (6): 98-104 (in Russian)
https://dx.doi.org/10.18565/aig.2024.63
Keywords
Improving the effectiveness of in vitro fertilization (IVF) programs in patients with diminished ovarian reserves is currently the most pressing problem in reproductive medicine. The incidence of a "poor" ovarian response to gonadotropic stimulation in IVF programs is steadily increasing. According to experts, it ranges from 5–6% to 35.1% among all patients undergoing treatment [1, 2]. This increase is primarily due to the fact that more women are planning to have children after the age of 35, which is considered late reproductive age. Other factors that play a significant role in reducing ovarian function include ovarian resection, surgical interventions on reproductive organs, long-term therapy (especially gonadotoxic therapy), autoimmune diseases, and other somatic diseases [3]. In some cases, there are no visible reasons for the decrease in ovarian reserve, whereas in others, a decrease in reserve is often associated with infertility of unknown origin [4]. The reasons for this decrease lie in complex and poorly understood molecular genetic and epigenetic mechanisms that regulate intraovarian folliculogenesis and the entire reproductive system [5]. It is worth noting that a decrease in ovarian reserve and premature ovarian failure can be associated with fragile X syndrome [6]. However, recent attempts have been made to activate intraovarian folliculogenesis either mechanically or by introducing biologically active agents directly into the ovary with both systemic and local effects [7, 8]. Researchers believe that cell therapy may be the most promising approach for activating ovarian functions. Animal experimental studies have shown the effectiveness of mesenchymal stem cells (MSCs) in activating intraovarian folliculogenesis [9]. Clinical data are limited, but it has demonstrated a positive effect of autologous stem cell administration in cases of "poor" ovarian response [10]. Although the literature describes examples of using MSCs with low immunogenicity and high proliferative activity to treat premature ovarian failure, the safety of this type of therapy is still being discussed. The discovery of the mechanisms of therapeutic effects using extracellular vesicles (EVs) has caused a paradigm shift and introduced a new direction in cellular technologies such as decellularized drugs. EVs, first discovered in 1967 by Peter Wolf and initially referred to as "platelet junk," are structures secreted by tissues and organs that transmit information between cells. This transmitted information, including proteins, lipids, sugars, nucleic acids, microRNAs, and mitochondrial DNA, can have a positive effect on recipient cells. The spectrum of action of EVs was comparable to that of MSCs. For example, MSC-EVs derived from the bone marrow and amniotic fluid can protect granulosa cells from apoptosis using miRNAs such as miR-21, miR-644-5P, miR-145-5P, miR-10a, and miR-222. They also secrete vascular endothelial growth factor, transforming growth factor-β, and placental growth factor and play a role in oogenesis [11–15]. MSC-EVs are believed to activate the ovary by inducing the PI3K-AKT pathway, which stimulates the phosphorylation of FOXO3a and FOXO1 in primordial follicles and granulosa cells [16]. Furthermore, it is believed that MSC-EVs trigger the Hippo signaling cascade, which regulates the proliferation and apoptosis of granulosa cells [17]. Based on these proposed mechanisms of action, the use of EVs to activate ovaries with diminished ovarian reserve appears to be a promising direction.
We present a series of clinical observations on the use of mesenchymal stem cell-derived extracellular vesicles (MSC-EVs) to activate ovarian function in reproductive-aged patients with a diminished ovarian reserve. These patients showed poor responses to IVF programs and failed to achieve pregnancy. Fifteen patients underwent intraovarian EV injections. In this study, we focus on three of the most illustrative cases. Prior to entering the IVF program, all patients underwent standard examinations as required by the Order of the Ministry of Health of Russia dated July 31, 2020, No. 803n “On the procedure for using assisted reproductive technologies, contraindications and restrictions on their use.”
Description of the cell product
Isolation and phenotyping of primary cultures of MSC-EVs from postpartum placentas of women after normal delivery were performed at the Laboratory of Cell Technologies of the V.I. Kulakov NMRC for OG&P of Minzrdav, Russia.
Primary cell cultures were isolated from placental tissue obtained after normal delivery at term. Informed consent was obtained from all postpartum women. The placental bioexplant was washed with saline, mechanically crushed, and incubated with type II collagenase (0.05%) for 2–3 h. The resulting primary cell culture was pelleted by centrifugation and seeded onto a culture dish containing growth medium. DMEM/F12 supplemented with 10% fetal bovine serum, penicillin, and streptomycin was used as growth medium. The cell culture was passaged on days 4–5 after reaching 80% confluence. To analyze the immunophenotype of human MSC-EVs, a commercial set of antibodies against specific surface antigens CD90, CD73, CD105, CD20, CD14, CD45, and CD34 (MSC Phenotyping Kit Human, Miltenyi Biotec, Germany) was used in accordance with the manufacturer’s recommendations. After incubation with the antibodies, the cells were resuspended in 500 μl of phosphate-buffered saline and transferred into tubes for flow cytometry. Using the S3e cell sorter (BioRad, USA), histograms of the distribution of the number of cells depending on the signal level of the fluorescent antibodies were obtained. The resulting MSC-EV cultures had a phenotype with a predominance of CD90, CD73, and CD105 above 95%.
EVs were obtained from the conditioned culture medium of MSC-EVs at passages 3–4. MSC-EVs were incubated for 24 h with culture medium, which was previously purified from fetal bovine serum EVs by differential centrifugation using an Avanti JXN-30 ultracentrifuge (Beckman, USA) at ×108,000 g. The conditioned medium was then collected, and EVs were isolated by differential centrifugation, as described previously [18]. The number of EVs and their sizes were assessed by analyzing the trajectories of nanoparticles on a NanoSight NTA LM10 device (Malvern Panalytical, England), as well as by the total amount of protein determined by absorption photometry at a wavelength of 280 nm. EVs were then resuspended in 1 ml saline and stored at −80°C until use. One ovarian therapeutic dose contained an average of 5.2–5.6×1012 particles per ml (corresponding to 0.3–0.25 mg total EV protein per ml) obtained from a daily conditioned medium of 11×107 MSC-EVs.
Requirements for a donor of biological material (postpartum placenta and umbilical cord)
Age 18–35 years, spontaneous onset of pregnancy, examination during pregnancy at prescribed times for a complex of infections (first trimester, or upon registration at the antenatal clinic) including blood tests for HIV, hepatitis B and C, syphilis, TORCH infections, smear by polymerase chain reaction for infections, microbiological culture of urine for sterility, smear for vaginal flora, culture of the cervix or femoflor 16. Clinical blood tests, blood tests for infection, and cervical canal cultures were performed prior to delivery. In case of negative results of the investigations for the presence of an infectious process, postpartum placental material was considered suitable for further use.
EV injection technique
Transplantation of EVs in an amount of 5.2–5.6×1012 particles per ml (corresponding to 0.3–0.25 mg of total EV protein per ml), obtained from a daily conditioned medium of 11×107 MSC-EVs, into both ovaries by injection using ovarian transvaginal puncture.
Description
Clinical observation 1: Patient N.
Medical history and examination results
A 34-year-old female patient was referred for IVF. She reported no pregnancy after 8 years of regular sexual activity without contraception. There were no pregnancies. History: She was born from the 2nd pregnancy, parents' age at the time of birth was 32 and 36 years, respectively. She had one older brother who was unmarried. The patient showed normal growth and development. The age at menarche was 14 years. Menstrual cycle length was 26–27 days. Height – 162 cm; weight – 56 kg; body mass index – 21.3 kg/m2. Sexual life from the age of 21. The examination was carried out in accordance with the current clinical recommendations; the cause of infertility has not been determined, the menstrual cycle is regular, ovulatory, the fallopian tubes are patent, and the partner's sperm is fertile. Reduced parameters of ovarian reserve were recorded: FSH – 12 IU/l; AMH – 0.67 ng/ml, total antral follicle count <5, which was the basis for achieving pregnancy by IVF.
Results of previous IVF attempts
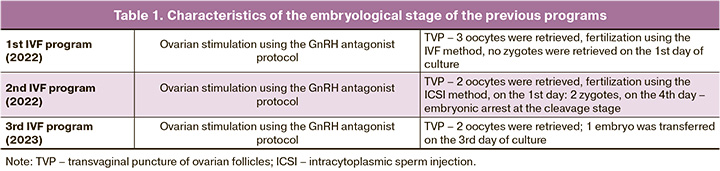
Three attempts were made to stimulate the ovaries using a gonadotropin-releasing hormone antagonist (GnRH antagonist). The daily dose of gonadotropin ranged from 225 IU to 300 IU, and the total dose for the course of treatment ranged from 2550 IU to 2775 IU; 2–3 oocytes were obtained in each program. A detailed description of the embryological stages of previous programs is provided in Table 1. It is worth noting that, in one of the programs, two oocytes were obtained, but no fertilization of the oocytes occurred. In another program, there was no embryo fragmentation. In another program, on the 3rd day of culture, an embryo with six blastomeres was transferred, but pregnancy did not occur. Thus, there was a typical situation of diminished ovarian reserve, poor response to ovarian stimulation, and impaired embryo development.
In preparation for the IVF program, the patient underwent EVs transplantation. EVs were injected into the right ovary via transvaginal puncture at a dose of 5.2–5.6×1012 particles per 1 ml (corresponding to 0.3–0.25 mg total EV protein per ml), derived from a daily conditioned medium of 11×107 MSC-EVs.
The table shows an analysis of the patients’ ovarian reserve status at baseline and 30 days after EVs administration. Data were collected on the 2nd day of the menstrual cycle.
The data obtained showed an increase in the antral follicle count of the right ovary, where EVs were injected, while it did not change in the left ovary. The estradiol level naturally increased and the FSH value decreased, while the AMH concentration did not change.
IVF Program Results
Ovarian stimulation was performed in the IVF program for nine days in a protocol with GnRH antagonists, and the dose of gonadotropin was 300 IU per day and 2700 IU per treatment course. Four mature oocytes were obtained from the right ovary and no oocytes were obtained from the left ovary. Fertilization by ICSI involved three zygotes; on the 5th day of culture, blastocyst 4AB was transferred into the uterine cavity, and embryo 4BB was cryopreserved. Singleton pregnancies have been established and are progressing.
Clinical observation 2: Patient Sh.
Medical history and examination results
The patient, aged 37 years, had a history of secondary infertility associated with absolute tubal factor and advanced reproductive age. Her mother's first child was stillborn (a boy); the cause was unknown, and her third pregnancy was ectopic. A hysterectomy was performed at the age of 40 years for uterine fibroids. She had a brother (41 years old) with no children.
She had a history of infertility for six years during first marriage. In 2019, the patient underwent laparotomy with tubectomy for ectopic pregnancy, and in 2021, laparoscopy with left tubectomy for hydrosalpinx.
The patient underwent eight IVF attempts. Table 3 presents the results of the last four attempts.
According to the spermogram of the spouse, normozoospermia.
Results from previous IVF attempts
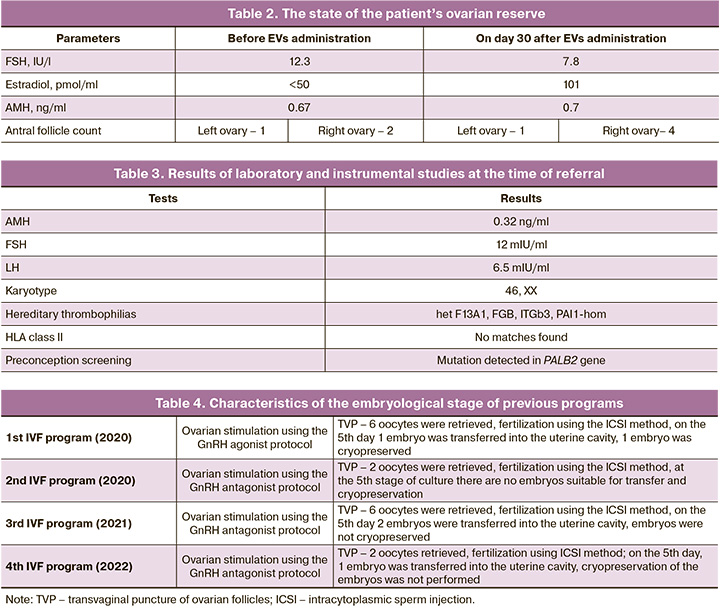
Four attempts were made to stimulate the ovaries in protocols with GnRH antagonists and agonists. Four embryo transfers were performed; however, pregnancy did not occur. The detailed characteristics of the embryological stages of the previous programs are presented in Table 4.
The patient underwent EVs transplantation during the preparation for the IVF program. In total, EVs were injected into both ovaries using transvaginal puncture in the amount of 5.2–5.6×1012 particles per 1 ml (corresponding to 0.3–0.25 mg of total EV protein per ml), obtained from a daily conditioned medium 11×107 MSC-EVS.
IVF Program Results
Ovarian stimulation was performed in the IVF program for eight days according to the protocol with GnRH antagonists, and ovulation was induced with follitropin alfa at a dose of 150–300 IU/day and human menopausal gonadotropin at a dose of 75 IU/day and 2325 IU for one treatment course. Four mature oocytes were retrieved from the right and left ovary. Fertilization by ICSI involved three zygotes; on the 5th day of culture, a 3AB blastocyst was transferred into the uterine cavity, and two other embryos (2AA) were cryopreserved. A singleton pregnancy was achieved, and the pregnancy progressed.
Clinical observation 3: Patient S.
Medical history and examination results
A 33-year-old female patient with a 5-year history of infertility was referred for IVF. The patient was evaluated according to the clinical recommendations. The patient was diagnosed with infertility of unknown origin.
Medical history: The patient was born from the 1st pregnancy. Her father had acute coronary stenosis at 56 years of age, and her maternal grandmother had diabetes mellitus.
She grew and developed normally. The patient had moderate myopia and chronic gastroduodenitis in remission. The age of menarche was 13 years. Menstruation every 26–28 days for 4 days. Sexual life from the age of 21. She was married once. Spermogram showed normozoospermia.
Results from previous IVF attempts
Four attempts were made to stimulate the ovaries in protocols with GnRH antagonists and agonists. Four embryos were transferred; however, pregnancy did not occur. The detailed characteristics of the embryological stages of the previous programs are presented in Table 5.
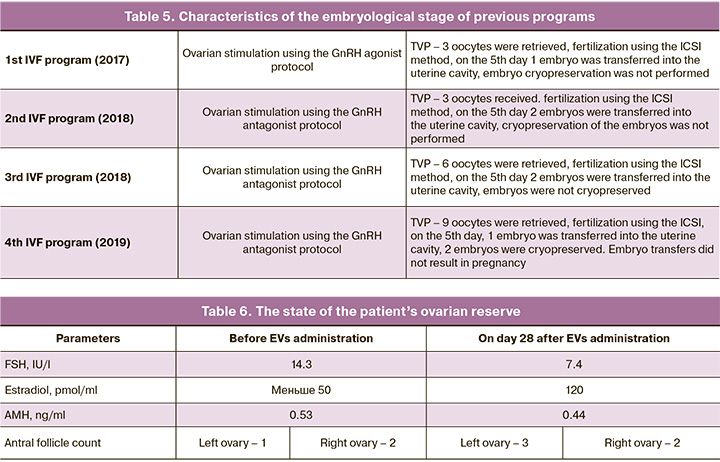
At the time of referral, a decrease in the ovarian reserve was observed (Table 6).
In preparation for the IVF program, the patient underwent EVs transplantation. EVs were injected into the left ovary by transvaginal puncture at a dose of 5.2-5.6×1012 particles per 1 ml (corresponding to 0.3-0.25 mg total EV protein per ml), derived from a daily conditioned medium of 11×107 MSC-EVS.
The table shows an analysis of the patients’ ovarian reserve status at baseline and 30 days after EVs administration. Data were collected on the 2nd day of the menstrual cycle.
The data obtained demonstrated an increase in the antral follicle count in the left ovary, where EVs were injected, while it did not change in the right ovary. The estradiol level naturally increased and the FSH value decreased, while the AMH concentration did not change.
IVF Program Results
Ovarian stimulation was performed in the IVF program for 8 days in a protocol with GnRH antagonist at a gonadotropin dose of 225–300 IU per day and 2100 IU per treatment course; three mature oocytes were obtained from the left ovary and one from the right ovary. Three zygotes were fertilized by ICSI; on the 5th day of culture, a 4AB blastocyst was transferred into the uterine cavity, and embryo cryopreservation was not performed. A singleton pregnancy was achieved and the pregnancy progressed.
Discussion
The present observations demonstrate clinical success in complex and unpromising situations of diminished ovarian reserve, poor ovarian response, and previous failed IVF attempts when specialists suggested the use of donor oocytes. Although we have already administered EVs to 15 patients with a similar clinical picture, we have not yet obtained evidence for the effectiveness of this method. Objective criteria for evaluating the action of EVs can include a decrease in FSH concentrations, a corresponding increase in estradiol levels, and an increase in the antral follicle count. However, it remains to be seen whether this information can be considered objective or falls within the limits of cycle-to-cycle variability in ovarian function. A positive finding was the visualization of an increase in the number of antral follicles in the ovary into which EVs were injected compared to the contralateral ovary. In the first stage, we deliberately injected EVs into only one ovary to observe their effects on intraovarian folliculogenesis. However, the mechanism of action of EVs is still speculative, as supported by literature data that hypothetically suggests the possible involvement of various factors in the processes of follicle growth. It is worth noting that, in our observations, the AMH level did not change, and the clinical effect was recorded 30 days after EVs administration. It can be assumed that activation occurs at later stages of folliculogenesis, specifically at the level of preantral follicles, as the maturation of follicles from primordial to antral follicles takes at least 120 days. Based on this assumption, clinical groups of patients can be formed in which intraovarian administration of EVs may be an effective way to activate the ovaries.
Conclusion
In our opinion, the administration of EVs is a promising method for activating ovarian function. It is probably the only promising way to overcome infertility in the most complex group of patients with diminished ovarian reserves and low pregnancy rates after IVF. It is necessary to accumulate and summarize clinical data and formulate a pathogenic rationale for the use of cell therapy in clinical practice.
References
- Abu-Musa A., Haahr T., Humaidan P. Novel physiology and definition of poor ovarian response; Clinical Recommendations. Int. J. Mol. Sci. 2020; 21(6): 2110. https://dx.doi.org/10.3390/ijms21062110.
- Giannelou P., Simopoulou M., Grigoriadis S., Makrakis E., Kontogeorgi A., Pantou A. et al. The conundrum of poor ovarian response: from diagnosis to treatment. Diagnostics (Basel). 2020; 10(9): 687. https://dx.doi.org/10.3390/diagnostics10090687.
- Seok J., Park H., Choi J.H., Lim J.Y., Kim K.G., Kim G.J. Placenta-derived mesenchymal stem cells restore the ovary function in an ovariectomized rat model via an antioxidant effect. Antioxidants (Basel). 2020; 9(7): 591. https://dx.doi.org/10.3390/antiox9070591.
- Jankowska K. Premature ovarian failure. Prz. Menopauzalny. 2017; 16(2): 51-6. https://dx.doi.org/10.5114/pm.2017.68592.
- Jaillard S., Bell K., Akloul L., Walton K., McElreavy K., Stocker W.A. et al. New insights into the genetic basis of premature ovarian insufficiency: novel causative variants and candidate genes revealed by genomic sequencing. Maturitas. 2020; 141: 9-19. https://dx.doi.org/10.1016/j.maturitas.2020.06.004.
- Gersak K., Meden-Vrtovec H., Peterlin B. Fragile X premutation in women with sporadic premature ovarian failure in Slovenia. Hum. Reprod. 2003; 18(8): 1637-40. https://dx.doi.org/10.1093/humrep/deg327.
- Zheng Q., Fu X., Jiang J., Zhang N., Zou L., Wang W. et al. Umbilical cord mesenchymal stem cell transplantation prevents chemotherapy-induced ovarian failure via the NGF/TrkA pathway in rats. Biomed. Res. Int. 2019; 2019: 6539294. https://dx.doi.org/10.1155/2019/6539294.
- Zhang H., Luo Q., Lu X., Yin N., Zhou D., Zhang L. et al. Effects of hPMSCs on granulosa cell apoptosis and AMH expression and their role in the restoration of ovary function in premature ovarian failure mice. Stem Cell Res. Ther. 2018; 9(1): 20. https://dx.doi.org/10.1186/s13287-017-0745-5.
- Liu R., Zhang X., Fan Z., Wang Y., Yao G., Wan X. et al. Human amniotic mesenchymal stem cells improve the follicular microenvironment to recover ovarian function in premature ovarian failure mice. Stem Cell Res. Ther. 2019; 10(1): 299. https://dx.doi.org/10.1186/s13287-019-1315-9.
- Buigues A., Ramírez-Martin N., Martínez J., Pellicer N., Meseguer M., Pellicer A. et al. Systemic changes induced by autologous stem cell ovarian transplant in plasma proteome of women with impaired ovarian reserves. Aging (Albany NY). 2023; 15(24): 14553-73. https://dx.doi.org/10.18632/aging.205400.
- Lu Y., Wei Y., Shen X., Tong Y., Lu J., Zhang Y. et al. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles improve ovarian function in rats with primary ovarian insufficiency by carrying miR-145-5p. J. Reprod. Immunol. 2023; 158: 103971. https://dx.doi.org/10.1016/j.jri.2023.103971.
- Cai J.H., Sun Y.T., Bao S. HucMSCs-exosomes containing miR-21 promoted estrogen production in ovarian granulosa cells via LATS1-mediated phosphorylation of LOXL2 and YAP. Gen. Comp. Endocrinol. 2022; 321-322: 114015. https://dx.doi.org/10.1016/j.ygcen.2022.114015.
- Sun B., Ma Y., Wang F., Hu L., Sun Y. miR-644-5p carried by bone mesenchymal stem cell-derived exosomes targets regulation of p53 to inhibit ovarian granulosa cell apoptosis. Stem Cell Res. Ther. 2019; 10(1): 360. https://dx.doi.org/10.1186/s13287-019-1442-3.
- Xiao G.Y., Cheng C.C., Chiang Y.S., Cheng W.T., Liu I.H., Wu S.C. Exosomal miR-10a derived from amniotic fluid stem cells preserves ovarian follicles after chemotherapy. Sci. Rep. 2016; 6: 23120. https://dx.doi.org/10.1038/srep23120.
- Zhu W., Yang M., Shang J., Xu Y., Wang Y., Tao Q. et al. MiR-222 inhibits apoptosis in porcine follicular granulosa cells by targeting the THBS1 gene. Anim. Sci. J. 2019; 90(6): 719-27. https://dx.doi.org/10.1111/asj.13208.
- Yang Z., Du X., Wang C., Zhang J., Liu C., Li Y. et al. Therapeutic effects of human umbilical cord mesenchymal stem cell-derived microvesicles on premature ovarian insufficiency in mice. Stem Cell Res. Ther. 2019; 10(1): 250. https://dx.doi.org/10.1186/s13287-019-1327-5.
- Li Z., Zhang M., Zheng J., Tian Y., Zhang H., Tan Y. et al. Human umbilical cord mesenchymal stem cell-derived exosomes improve ovarian function and proliferation of premature ovarian insufficiency by regulating the Hippo signaling pathway. Front. Endocrinol. (Lausanne). 2021; 12: 711902. https://dx.doi.org/10.3389/fendo.2021.711902.
- Мартиросян Я.О., Назаренко Т.А., Кадаева А.И., Краснова В.Г., Бирюкова А.М., Погосян М.Т. Новые подходы к изучению регуляции преимплантационного развития эмбрионов. Акушерство и гинекология. 2023; 6: 29-37. [Martirosyan Ya.O., Nazarenko T.A., Kadaeva A.I., Krasnova V.G., Biryukova A.M., Pogosyan M.T. New approaches to studying the regulation of preimplantation embryonic development. Obstetrics and Gynecology. 2023; (6): 29-37. (in Russian)]. https://dx.doi.org/10.18565/aig.2023.10.
Received 20.03.2024
Accepted 19.06.2024
About the Authors
Yana O. Martirosyan, Junior Researcher at the F. Paulsen Research and Educational Center for ART, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,117997, Russia, Moscow, Ac. Oparin str., 4, +7(925)124-99-99, marti-yana@yandex.ru, https://orcid.org/0000-0002-9304-4410
Tatiana A. Nazarenko, Dr. Med. Sci., Head of the Institute of Reproductive Technologies, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, Ac. Oparin str., 4, +7(915)322-08-79, t.nazarenko@mail.ru, 117997, Russia, Moscow, Ac. Oparin str., 4, https://orcid.org/0000-0002-5823-1667
Albina I. Kadaeva, PhD student, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, +7(917)762-82-11,
albina.karimovai@mail.ru
Kirill V. Goryunov, PhD (Bio), Researcher at the Laboratory of Cell Technologies, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow,
Ac. Oparin str., 4, +7(916)410-88-44, k_gorunov@oparina4.ru, https://orcid.org 0000-0002-8776-7196
Yulia A. Shevtsova, Junior Researcher at the Laboratory of Cell Technologies, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow,
Ac. Oparin str., 4, +7(925)673-24-27, yu_shevtsova@oparina4.ru



