Analysis of treatment satisfaction in patients with premature ovarian failure
Objective: To analyze treatment satisfaction in patients with premature ovarian failure (POF) and to study the frequency and severity of symptoms of estrogen deficiency in patients with POF taking hormone replacement therapy (HRT). Materials and methods: A cross-sectional study which was carried out on the basis of the National Medical Research Center for Obstetrics, Gynecology and Perinatology, included 223 patients diagnosed with POF. Treatment satisfaction was assessed using a structured survey; the severity of estrogen deficiency symptoms was determined using a questionnaire (Greene Climacteric Scale). Results: Most of the study participants (86.3%) received HRT medications containing a standard dose of estradiol (E2): 2 mg were administered orally and 1 mg of 1% gel was administered via the transdermal route. Low-dose HRT was taken by 2.2%, combined oral contraceptives (COC) were received by 1.8%. Higher doses of E2 were taken by 2.2% of the participants. The survey of the women showed that 53.8% were satisfied and 41.3% were not satisfied with the therapy. The lowest indicators of treatment satisfaction were obtained by patients who took low-dose HRT (p=0.022) and COC (p=0.048). The patients who took higher doses of E2 as part of HRT were satisfied with the treatment in 100% of cases. The average severity of climacteric syndrome among the participants was 12 points according to the Greene Climacteric Scale. The ROC analysis of the dependence of the negative assessment of therapy on the total score on the Greene Scale showed that the area under the ROC curve was 0.809 (0.031) with 95% CI: 0.748–0.870 (p <0.001). The threshold value of the total score on the Greene Scale at the cut-off point was 14 points; the cut-off point corresponded to the highest value of the Yuden index. Conclusion: The high percentage of patients dissatisfied with therapy as well as persistent symptoms of estrogen deficiency associated with HRT administration indicate the need to revise approaches to the management of patients with POF.Averkova V.G., Yureneva S.V.
Keywords
Premature ovarian failure (POF) is a clinical syndrome characterized by hypergonadotropic amenorrhea and a progressive decrease in the level of sex hormones due to the loss of functional activity of the ovaries in women under 40 years of age [1, 2]. The diagnosis of POF is associated with early loss of fertility, emotional and sexual disorders, the consequences of estrogen deficiency at the systemic level and an increased risk of age-associated diseases in quite young women [2].
The clinical manifestations of estrogen deficiency characteristic of POF are specific and are not identical to the symptoms typical of the classic climacteric syndrome [2, 3]. According to the literature, the intensity of most symptoms does not decrease with age unlike natural menopause [4]. Young women with POF experience chronic stress for a long time and it is largely due to the stressful effect of the diagnosis and unwillingness to accept it. All this significantly affects the quality of patients’ lives. According to the results of a recent meta-analysis, the quality of life associated with health in women with POF is lower than in women of the same age with preserved ovarian function [5]. It is worth noting that a decrease in the quality of life was detected even among patients who regularly took hormone replacement therapy (HRT).
In 2014, American scientists made a conclusion that women with POF experience a significant range of menopausal symptoms and have a potential dissatisfaction with the treatment [6]. Ideally, this therapy should adequately compensate for estrogen deficiency, completely control the symptoms associated with it, prevent or significantly slow down the processes of premature aging and provide satisfaction to the patients with the treatment.
Nowadays, special attention of medical specialists is paid to the issues of patients’ subjective assessment of the medical care provided to them, as well as the issues of satisfaction with therapy. This indicator is the degree when patients believe that the treatment meets their health needs [8]. It indirectly reflects the effectiveness of therapy and can significantly influence the degree of adherence to it.
The results of the study published in 2016 showed that 52% of patients with POF had suboptimal indicators of adherence to treatment: women either never took HRT, or started therapy a long time after diagnosis, or refused to continue therapy as they were younger than 45 years old [9]. This study did not provide the analysis of the causes of the results, however, summarizing the conclusions of recent studies on the treatment of patients with POF, it is possible to identify the following major unresolved problems in this area: delayed diagnosis, unsatisfactory indicators of adherence to therapy, persistent symptoms of estrogen deficiency in a number of patients and low quality of life indicators, despite receiving HRT.
To date, the question of the optimal doses of HRT that should be recommended to patients with POF remains open. According to several guidelines of different medical communities, higher doses of hormonal preparations are necessary to meet the health needs of young women with POF in comparison with women in peri- and post-menopause stages [2, 7, 10].
In our opinion, the study of indicators of treatment satisfaction in patients with POF is of particular importance. Firstly, it is an integral part of the concept of a patient-centered approach in medicine. Secondly, it can help doctors with the right choice of adequate hormone therapy to ensure a high quality of life and successful prevention of age-associated diseases in this cohort of patients.
Materials and methods
This was a cross-sectional study of women who were examined and/or treated at the Department of Gynecological Endocrinology at the Academician V.I. Kulakov National Medical Research Centre for Obstetrics, Gynecology and Perinatology in Moscow in the period from 2016 to 2021. The study was approved by the Ethics Committee of the Center. All participants gave a written (in the case of a face-to-face interview) or oral (in the case of a telephone interview) consent to participate in the survey.
The study included patients aged 18 to 45 years with a diagnosis of POF made at least 12 months ago and who received HRT constantly for minimum 3 last months. The diagnostic criteria of POF corresponded to those adopted in 2016 by the European Society of Human Reproduction and Embryology; the criteria included oligo/amenorrhea for at least 4 months, follicle-stimulating hormone (FSH) levels >25 mMU/L confirmed twice with an interval of 4-6 weeks, the age of patients up to 40 years [11]. The study did not include patients with iatrogenic POF, karyotype changes, a previous history of cancer, severe somatic pathology, thyroid and adrenal gland dysfunction and the presence of contraindications to HRT.
At the first stage, the study included women whose data were in the medical database of the Academician V.I. Kulakov National Medical Research Centre for Obstetrics, Gynecology and Perinatology, Moscow, Russia. A total of 350 patients were selected in the database, 296 of them potentially met the criteria for inclusion in the study. The women were contacted by the phone number indicated in the medical record. The consent to answer the interview questions by telephone was obtained from 165 patients. After consulting a gynecologist at the Department of Gynecological Endocrinology of the Center, 60 more patients were included in the study.
During the study, treatment satisfaction of the patients was assessed using a structured survey. The severity of menopausal symptoms was determined using a questionnaire Greene Climacteric Scale (GCS) [12]. This questionnaire is characterized by high validity and it is widely used both in scientific research and in clinical practice in various countries of the world [13, 14]. The severity of symptoms is scored on a four-point scale (from 0 points referring to the absence of a symptom to 3 points referring to a severe manifestation of the symptom). When evaluating the results, the total number of points is calculated; therefore, mild (11 or less points), moderate (from 12 to 19 points) and severe (20 or more points) degrees of climacteric syndrome are identified. Demographic parameters and clinical data, as well as information about diagnostic studies and current pharmacotherapy, were obtained from primary medical documentation (outpatient medical records) and during communication with the patients.
In order to get a general subjective assessment of treatment satisfaction, the patients were asked to answer the question: “How well are you satisfied with your current hormone replacement therapy?”. The patients were offered a multiple choice of answers: “completely satisfied”, “rather satisfied”, “rather not satisfied”, “not satisfied”, “cannot say”.
The primary endpoint in this study was the indicator of satisfaction in POF patients with hormone replacement therapy. Secondary endpoints were indicators of satisfaction with the quality of medical care, the level of adherence to treatment, the frequency and reasons for changing therapy, the frequency and severity of symptoms of estrogen deficiency that persisted while the patient was taking HRT.
Statistical analysis
Statistical analysis was carried out using the Microsoft Office Excel 2010 software package, IBM SPSS Statistics 25 HC IMAGO 5.0, license No. 5725-A54 and StatTech v. 2.0.0 (StatTech LLC, Russia).
The normality of the data distribution was assessed using the Shapiro–Wilk test (if the number of subjects was less than 50) or the Kolmogorov–Smirnov test (if the number of subjects was more than 50). In the case of a normal distribution, quantitative data were described using the mean (M) and standard deviation (SD). If there was no normal distribution, quantitative data were described using the median (Me) and the lower and upper quartiles (Q1–Q3). Categorical data were described as absolute numbers and percentages.
The comparison of the two groups using a quantitative indicator, the distribution of which corresponded to the normal one, was performed by means of the Student’s t-test. The comparison of the two groups using a quantitative indicator, the distribution of which differed from the normal one, was performed by means of the Mann-Whitney U test. The comparison of percentages was performed using the Pearson chi-square test (with values of the expected phenomenon greater than 10) during the analysis of fourfold contingency tables. The comparison of percentages was performed using the Pearson’s chi-square (χ2) test during the analysis of multipole contingency tables.
The method of analysis of ROC curves was used to assess the diagnostic significance of quantitative signs in predicting a certain outcome. The threshold value of the quantitative variable at the cut-off point was determined by the highest value of the Youden Index.
Results
A total of 225 patients were selected to participate in the study and 223 of them (99.1%) answered all survey questions, filled out questionnaires; their data were carefully analyzed. Clinical, anamnestic and demographic data of the study participants are presented in Table 1.
All participants were asked about their experience of receiving HRT. The median duration of HRT was 4 years (2–6 years), the median age of patients at the time of treatment initiation was 33 years (28–37 years). There were 17 (7.6%) study participants who had at least one break from taking HRT. The median duration of breaks was 4 months (2–12 months) with a minimum period of 1 month and a maximum of 4 years. The periods of pregnancy and lactation when patients did not take HRT were not taken into account. The participants were asked about the reasons why they interrupted the treatment. Ten women (58.8%) said that they stopped treatment due to unwillingness to take HRT and concerns about its negative health consequences (in particular, the onset of oncological diseases), four women (23.5%) interrupted therapy “for a body to have a rest” either on the doctor’s advice or on their own, three women (17.6%) took a break for 1–2 months due to a difficult financial situation or the lack of necessary medicines in the pharmacy.
Formulations of HRT preparations taken by the patients during the time of participation in the study are shown in Figure 1.
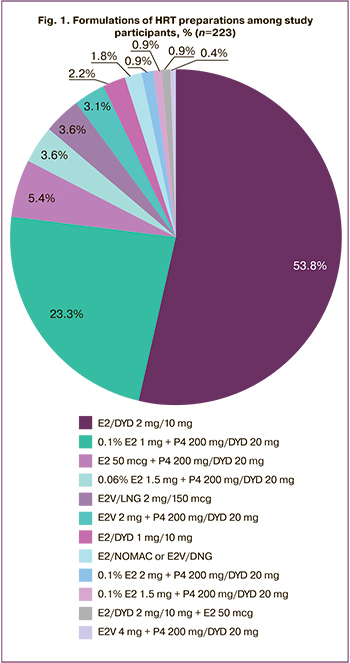
Most of the patients received preparations containing a standard dose of estradiol (E2) as part of cyclic HRT (combined preparations or estrogen mono-preparations in combination with preparations of progestogens): 120 (53.8%) women received an oral preparation containing 2 mg of E2 in combination with 10 mg of dydrogesterone (E2/DYD 2mg/10mg), 52 (23.3%) patients constantly took estradiol hemihydrate 0.1% transdermal gel as an estrogenic component of HRT, at the dose of 1 gram per day (0.1% E2 1 mg).
Five women (2.2%) took orally HRT preparations containing a low dose of E2: 1 mg E2 + 10 mg of dydrogesterone (E2/DYD 1 mg/10 mg). Four women (1.8%) took combined oral contraceptives (COC) with E2: 1.5 mg E2 + 2.5mg nomegestrol acetate (E2/NOMAC 1.5mg/2.5mg) in the 24/4 regimen and COC with the dynamic dosing regimen of estradiol valerate and dienogest (E2V/DNG) in the 26/2 regimen.
During the study, four respondents (1.8%) received high dosages of transdermal 0.1% gel with E2 (0.1% E2); among them there were two (0.9%) patients who took 2 g/day (0.1% E2 – 2 mg) and two patients (0.9%) who took 1.5 g/day (0.1% E2 – 2 mg). One patient (0.4%) reported taking a double dose of an oral drug containing estradiol valerate 2.0 mg (E2V 2 mg 2 tablets per day). It should be noted that two patients (0.9%) said that they had previously taken a combination of HRT drugs: E2/DYD 2 mg /10 mg + E2 50 mcg and E2/DYD 2 mg/10 mg + gel with testosterone 25 mg (T 25 mg). The doses of HRT drugs were increased by three women (1.4%) on their own; in other cases, the dose was increased due to the doctor’s recommendation.
There were 81 (36.3%) patients who received constantly transdermal estrogen or E2V orally; 52 patients (64.2%) received dydrogesterone, 20 mg/day (DYD 20 mg); 29 patients (35.8%) took micronized progesterone, 200 mg/day (P4 200 mg), and among them there were 24 women (82.7%) who received the preparation P4 vaginally and 5 women who received it orally (17.2%). Progestogens were used in a cyclic regimen for 12 or 14 days monthly, after these cycles the patients stopped taking progestogens.
Satisfaction with the treatment of patients with POF was accepted as the main relevant variable in this study. During the survey, 223 women answered the question “How well are you satisfied with your current hormone replacement therapy?”.
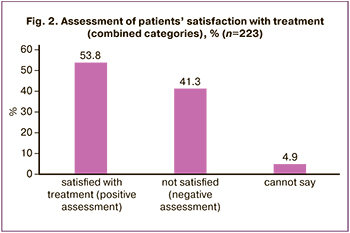
The answer “completely satisfied” was chosen by 19 respondents (8.5%), “rather satisfied” by 101 women (45.3%). Other 53 (23.8%) women answered that they were “rather not satisfied”, and 39 (17.5%) survey participants were completely “not satisfied” with therapy; 11 patients (4.9%) chose “cannot say” option to answer this question.
In order to simplify further calculations and obtain more generalized data, the patients’ responses were combined into two categories regarding their satisfaction with therapy: the answers “completely satisfied” and “rather satisfied” were considered as a positive assessment of therapy, the answers “not satisfied” or “rather not satisfied” were considered as a negative (negative) assessment (Fig. 2). For the same purpose, the HRT preparations taken by the study participants were divided into three groups depending on the dose of the estrogenic component in its formulation: HRT with a low, standard and high dose of E2 [2, 10]. COC was considered separately.
Satisfaction with therapy was analyzed depending on the HRT preparations taken by the participants during the study. The results of this analysis are presented in Table 2.
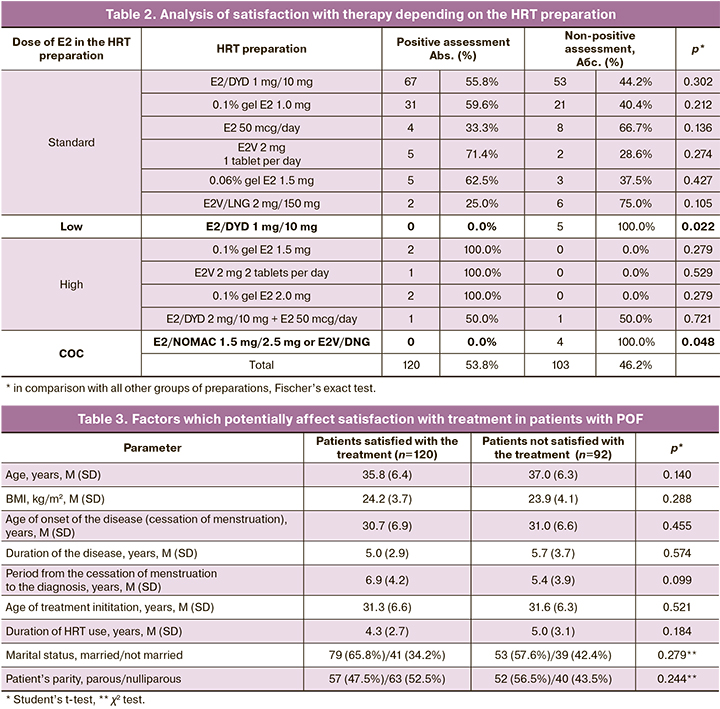
The most common preparations containing the standard dose of E2 were assessed by the patients the following way: 30 (57.7%) of 52 patients gave a positive assessment of 0.1% E2 1.0 mg therapy, and 66 (55%) of 120 patients gave a positive assessment of E2/DYD 2 mg/10 mg therapy. There was no positive evaluation of therapy from patients who took low-dose HRT 1 mg/10 mg E2/DYD (0%) and COC (E2/NOMAC 1.5 mg/2.5 mg or E2V/DNG in a dynamic dosing regimen (0%)). A positive assessment of therapy (100% of cases) was given by patients who received HRT with E2 exceeding the standard dose (0.1% E2 1.5 mg, 0.1% E2 2.0 mg, E2V 2 mg 2 tablets per day in combination with progestogens).
After evaluating the factors potentially affecting satisfaction with treatment (positive and negative evaluation of therapy), we obtained the following results (Table 3).
Non-satisfactory assessment of therapy did not depend on the age of the patients, BMI, age of onset of the disease, duration of the disease, period from the cessation of menstruation to diagnosis, age of treatment initiation, duration of HRT, marital status, parity (p>0.05).
One of the stages of the study was to assess the satisfaction of its participants with the quality of medical care. The patients were asked questions about the timing of diagnosis, gynecologist’s care, and experience of HRT use. A negative assessment of the quality of medical care was given by 72 women (32.3%). The patients identified the following reasons for the negative assessment of medical care: delayed diagnosis, lack of doctor’s empathy, constant use of the word “menopause” by the doctor, lack of the detailed explanation of the causes of the disease, lack of the explanation why it is necessary to take HRT, incorrect administration of HRT in patients’ opinion.
All study participants were asked to fill out the GCS questionnaire. According to GCS, the gradation of the climacteric scale severity is assessed by summing up the scores for each of the points in the questionnaire. The median of total GCS scores among all women who filled out the questionnaire was equal to 12 scores (6-18 points), which corresponded to the average severity of climacteric scale; the minimum number was 0 score, which was assessed as the absence of menopausal symptoms, and the maximum number was 40 scores.
Depending on the severity of climacteric symptoms, the patients were distributed as follows: 89 women (39.9%) had mild estrogen deficiency symptoms at the time of filling out the questionnaire, 87 women (39.0%) had moderate symptoms, 43 women (19.3%) had severe clinical manifestations of estrogen deficiency. Only 4 patients (1.8%) did not show the presence of any of the symptoms indicated in the questionnaire.
The next stage of the study was the analysis of the negative assessment of therapy depending on the total GCS scores. The results of the analysis are presented in Table 4.
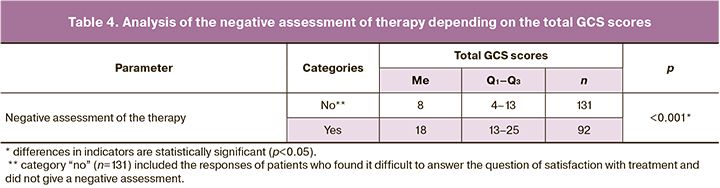
There were statistically significant differences (p<0.001) in the assessment of dissatisfaction with therapy depending on the total GCS scores (Mann–Whitney U test). After evaluating the dependence of the probability of a negative assessment of therapy on the total GCS scores with the help of ROC analysis, we obtained the following curve (Fig. 3).
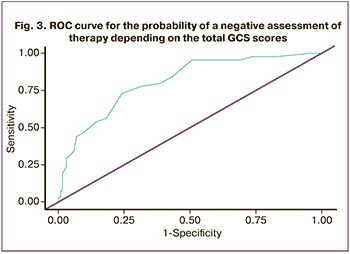
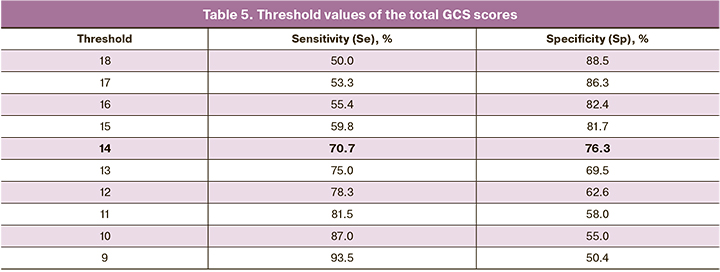
The area under the ROC curve was 0.802 (0.031) with 95% CI: 0.740–0.863. The obtained model was statistically significant (p<0.001). The threshold value of the total GCS scores at the cut-off point was 14 and it corresponded to the highest value of the Youden Index (Table 5). Dissatisfaction with therapy was predicted when the total GCS scores were higher or equal to this value. Sensitivity and specificity of the model were 70.7% and 76.3%, respectively.
The study participants who scored 14 or more points in the GCS questionnaire but were satisfied with the treatment (26 patients (11.7%)) were asked why they gave a positive assessment of therapy in the presence of existing symptoms. There were the following most common answers: “if I do not experience hot flashes, I am already satisfied with the treatment” (8 patients (30.8%)), “I do not believe that my condition can improve” (6 patients (23.1%)), “other therapy did not improve my condition” (5 patients (19.2%)), “if I cancel therapy, I will feel very bad” (3 patients (11.5%)).
Discussion
It is the first time that the analysis of satisfaction with the treatment of patients with POF has been carried out in the Russian Federation. Moreover, the data on the frequency and severity of symptoms of estrogen deficiency that persisted when patients received HRT are presented.
According to the literature, a global medical problem is the poor quality of communication between an obstetrician-gynecologist and a patient with POF [2]. A high percentage of women remain dissatisfied with the information received from the doctor about their disease, its long-term health consequences, the goals and benefits of using HRT, and optimal duration of receiving HRT [15–17]. The data of this study confirm the following conclusions: more than 30% of the patients stated dissatisfaction with the quality of communication with the doctor, the delay in making the correct diagnosis, the lack of complete information about the disease and the low level of psychological support of the doctor during the consultation.
The survey of the study participants showed that they used various forms and names of estradiol containing preparations. It should be noted that a large number of preparations are registered in the Russian Federation and can be used as replacement (or menopausal) hormone therapy by women. The main indication for the use of these preparations is the relief of menopausal symptoms and the prevention of osteoporosis. As symptoms of POF are similar to natural menopause, the same therapy regimens are prescribed to women with POF in most cases, without taking into account patients’ age and their needs. Almost half of the women in our study (53.8%) received HRT preparations containing a standard dose of E2 (E2/DYD 2 mg/10 mg) orally. Another 23.3% of women received a standard dose of transdermal E2 gel (0.1% E2 1.0 mg) in combination with progestogens. Other therapy regimens including HRT with different doses of E2 were used by patients much less frequently (in 5% or less cases).
The analysis of satisfaction with treatment showed that only 19 patients (8.5%) were completely satisfied with therapy. On the other hand, the number of women who were completely dissatisfied with the administered HRT was twice as much (39 (17.5%)). The total percentage of patients dissatisfied with therapy (41.3%) did not exceed the percentage of patients satisfied with treatment (53.8%), however, their number is too high to give a certain positive assessment of the doses and treatment regimens prescribed to patients with POF today.
Patient’s satisfaction with treatment is largely a subjective parameter. Two groups of factors which could influence this indicator have been identified in the research on determinants of satisfaction with the quality of medical care and treatment. The first group includes the socio-cultural and psychological characteristics of the patient; the second group includes the characteristics of the medical organization, the actions and behavior of the doctor, as well as the effectiveness of the treatment prescribed by him, which to some extent corresponds to the expectations of the patient [18, 19].
In our study, we selected factors (age of patients, BMI, duration of the disease and HRT use, marital status, parity, etc.), which, in our opinion, affect the satisfaction with treatment, however, there were no positive correlations between these indicators. Thus, it is possible to assume that among the objective indicators, clinical effectiveness had the greatest impact on the satisfaction with therapy; this effectiveness was assessed by the patients on the basis of their condition.
The lowest parameters of satisfaction with treatment were found among the study participants who took HRT preparations with a low dose of E2 (E2/DYD 1 mg/10 mg), as well as COC (E2/NOMAC and E2V/LNG). There are limited data in the literature regarding the use of COC in patients with POF. In the studies where the use of COC gave worse results for skeletal health and cardiovascular system in comparison with the use of HRT, COC was taken with synthetic estrogens in a 21/7 regimen [20]. COC which was taken by the participants of this study, in combination with E2 and a shortened hormone-free interval has a better safety profile and provides a constant level of endogenous E2 in the blood, however, the dose of the estrogenic component may not be sufficient to compensate for estrogen deficiency in a number of patients [21]. The regimen of using E2V/LNG preparation implies a seven-day break, during which menstrual-like bleeding occurs. The absence of estrogen use during a one-week break causes sharp fluctuations in estrogen level every month and may be associated with the return of symptoms, and thus may lead to low satisfaction with treatment with this preparation. As for the use of preparations with a low dose of E2 in the formulation, they are not recommended as HRT for patients with POF [2, 7, 10, 11].
The patients who received HRT preparations containing standard doses of E2 (E2/DYD 2 mg/10 mg, 0.1% E2 1.0 mg, 0.06% E2 1.5 mg) had comparable indicators of satisfaction with treatment (50–60%). The detailed analysis of these results, as well as the description of the remaining symptoms of estrogen deficiency, was carried out at the second stage of the study, and its data will be presented in the next article.
Only a third of patients (33.3%) who used a skin patch with E2 50 mcg/day as HRT were satisfied with therapy. In our country, the dose 50 mcg/day of E2 for a skin patch is considered to be standard, but in foreign studies, the doses 75–100 mcg/day are most often used. It has also been proved that a dose of E2 of at least 100 mcg/day can effectively block the symptoms of POF and achieve average serum levels of E2 in the range typical for young women with preserved ovarian function [21].
It is also interesting that some patients received HRT with high doses of E2 (0.1% E2 1.5 mg and 2.0 mg, E2V 2 mg 2 tablets per day) and added preparations to the standard HRT regimen (E2/DYD 2 mg/10 mg + patch with E2 50 mcg/day and E2/DYD 2/10 mg + T 25 mg. The analysis of the above results suggests that HRT, which contains low and in standard doses of E2, is not able to stop the symptoms of estrogen deficiency in women with POF and provide a high level of quality of life. Indicators of 100% satisfaction with treatment can be found only among patients who took HRT with an excess of the standard dose of E2.
One of the stages of the study was the evaluation of the indicators obtained after filling in GCS questionnaires. Only four women (1.8%) did not report any symptoms of concern presented in the questionnaire. The percentage of women who answered that they were completely satisfied (8.5%) or rather satisfied (45.3%) with the treatment exceeds the percentage of women who did not get a single GCS score. Therefore, one can assume that even those participants who had certain clinical manifestations of estrogen deficiency at the time of the survey gave a positive assessment of the therapy.
The average GCS scores among the study participants was 12 points and it corresponds to the average severity of climacteric scale manifestations. When constructing a prognostic model of the probability of dissatisfaction with treatment, depending on the degree of manifestation of symptoms of estrogen deficiency, we obtained a threshold value of the total GCS score equal to 14. The obtained result indicates that women with POF have to experience significant changes in their well-being in order to assess them as dissatisfaction with treatment. The results of the study showed that about 10% of patients who scored more than 14 points did not make any active complaints when they filled in the GCS questionnaire. This may be due to a lack of awareness about their disease and the existing unsuccessful experience of changing the preparation in search of optimal HRT. The situation is aggravated by the fact that doctors do not actively ask patients about the presence or absence of certain symptoms. As a result, most women with persistent symptoms of estrogen deficiency in spite of taking HRT do not receive adequate care.
Our study has a number of limitations related to its design where there is a chance of obtaining unreliable or incomplete data. The registration of symptoms of estrogen deficiency was carried out using a questionnaire designed for menopausal women where clinical symptoms may differ to one degree or another from the symptoms characteristic of POF. This study did not take into account the potential effect of the gestagenic component of HRT on the symptoms of estrogen deficiency, which could affect the indicators of satisfaction with treatment.
The main advantage of the study is the data obtained directly from patients in real clinical practice, which provide valuable information for clinicians and researchers.
Conclusion
Women with POF need a special care and approach to medical communication, which implies psychological support, clarification of general and particular issues related to the disease, possible long-term consequences of estrogen deficiency for health, goals and duration of treatment. It is also necessary to identify, study and thoroughly analyze complaints that may indicate insufficient compensation for estrogen deficiency during HRT administration.
The high percentage of patients dissatisfied with therapy as well as persistent symptoms of estrogen deficiency associated with HRT administration indicate the need to revise approaches to the management of patients with POF.
References
- Табеева Г.И., Шамилова Н.Н., Жахур Н.А., Позднякова А.А. Марченко Л.А. Преждевременная недостаточность яичников – загадка XXI века. Акушерство и гинекология. 2013; 12: 16-21. [Tabeyeva G.I., Shamilova N.N., Zhakhur N.A., Pozdnyakova A.A., Marchenko L.A. Premature ovarian failure is an enigma of the 21st century. Obstetrics and Gynecology. 2013; 12: 16-21. (in Russian)].
- Panay N., Anderson R.A., Nappi R.E., Vincent A.J., Vujovic S., Webber L., Wolfman W. Premature ovarian insufficiency: an International Menopause Society White Paper. Climacteric. 2020; 23(5): 426-46. https://dx.doi.org/10.1080/13697137.2020.1804547.
- Torrealday S., Kodaman P., Pal L. Premature ovarian insufficiency – an update on recent advances in understanding and management. F1000Res. 2017; 6: 2069. https://dx.doi.org/10.12688/f1000research.11948.1.
- Allshouse A.A., Semple A.L., Santoro N.F. Evidence for prolonged and unique amenorrhea-related symptoms in women with premature ovarian failure/primary ovarian insufficiency. Menopause. 2015; 22(2): 166-74.https://dx.doi.org/10.1097/GME.0000000000000286.
- Li X.T., Li P.Y., Liu Y., Yang H.S., He L.Y., Fang Y.G. et al. Health-related quality-of-life among patients with premature ovarian insufficiency: a systematic review and meta-analysis. Qual. Life Res. 2020; 29(1): 19-36.https://dx.doi.org/10.1007/s11136-019-02326-2.
- Gibson-Helm M., Teede H., Vincent A. Symptoms, health behavior and understanding of menopause therapy in women with premature menopause. Climacteric. 2014; 17(6): 666-73. https://dx.doi.org/10.3109/13697137.2014.913284.
- Baber R.J., Panay N., Fenton A., IMS Writing Group. 2016 IMS Recommendations on women's midlife health and menopause hormone therapy. Climacteric. 2016; 19(2): 109-50. https://dx.doi.org/10.3109/13697137.2015.1129166.
- Shikiar R., Rentz A.M. Satisfaction with medication: an overview of conceptual, methodologic, and regulatory issues. Value Health. 2004; 7(2): 204-5.https://dx.doi.org/10.1111/j.1524-4733.2004.72252.x.
- Hipp H.S., Charen K.H., Spencer J.B., Allen E.G., Sherman S.L. Reproductive and gynecologic care of women with fragile X primary ovarian insufficiency (FXPOI). Menopause. 2016; 23(9): 993-9. https://dx.doi.org/10.1097/GME.0000000000000658.
- Российское общество акушеров-гинекологов. Менопауза и климактерическое состояние у женщины. Клинические рекомендации. М.: 2021. 86c. [Russian Society of Obstetricians and Gynecologists. Clinical guidelines "Menopause and menopausal condition in women". Moscow: 2021. (in Russian)].
- Webber L., Davies M., Anderson R., Bartlett J., Braat D., Cartwright B. et al.; European Society for Human Reproduction and Embryology (ESHRE) Guideline Group on POI. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum. Reprod. 2016; 31(5): 926-37.https://dx.doi.org/10.1093/humrep/dew027.
- Greene J.G. Constructing a standard climacteric scale. Maturitas. 1998; 29(1): 25-31. https://dx.doi.org/10.1016/s0378-5122(98)00025-5.
- Green S.M., Donegan E., McCabe R.E., Fedorkow D.M., Streiner D.L., Frey B.N. Objective and subjective vasomotor symptom outcomes in the CBT-Meno randomized controlled trial. Climacteric. 2020; 23(5): 482-8. https://dx.doi.org/10.1080/13697137.2020.1737929.
- Travers C., O'Neill S.M., King R., Battistutta D., Khoo S.K. Greene Climacteric Scale: norms in an Australian population in relation to age and menopausal status. Climacteric. 2005; 8(1): 56-62. https://dx.doi.org/10.1080/13697130400013443.
- Singer D., Mann E., Hunter M.S., Pitkin J., Panay N. The silent grief: psychosocial aspects of premature ovarian failure. Climacteric. 2011; 14(4):428-37. https://dx.doi.org/10.3109/13697137.2011.571320.
- Mann E., Singer D., Pitkin J., Panay N., Hunter M.S. Psychosocial adjustment in women with premature menopause: a cross-sectional survey. Climacteric. 2012; 15(5): 481-9. https://dx.doi.org/10.3109/13697137.2011.647841.
- Groff A.A., Covington S.N., Halverson L.R., Fitzgerald O.R., Vanderhoof V., Calis K., Nelson L.M. Assessing the emotional needs of women with spontaneous premature ovarian failure. Fertil. Steril. 2005; 83(6): 1734-41. https://dx.doi.org/10.1016/j.fertnstert.2004.11.067.
- Morgan M.W., Salzman J.G., LeFevere R.C., Thomas A.J., Isenberger K.M. Demographic, оperational, and нealthcare utilization factors associated with emergency department patient satisfaction. West J. Emerg. Med. 2015; 16(4): 516-26. https://dx.doi.org/10.5811/west-jem.2015.4.25074.
- Ku J.H., Danve A., Panq H., Choi D., Rosenbaum J.T. Determinants of рatient satisfaction in an academic rheumatology practice. J. Clin. Rheumatol. 2015; 21(5): 256-62. https://dx.doi.org/10.1097/RHU.0000000000000263.
- Webber L., Anderson R.A., Davies M., Janse F., Vermeulen N. HRT for women with premature ovarian insufficiency: a comprehensive review. Hum. Reprod. Open. 2017; 2017(2): hox007. https://dx.doi.org/10.1093/hropen/hox007.
- Sullivan S.D., Sarrel P.M., Nelson L.M. Hormone replacement therapy in young women with primary ovarian insufficiency and early menopause. Fertil. Steril. 2016; 106(7): 1588-99. https://dx.doi.org/10.1016/j.fertnstert.2016.09.046.
Received 14.09.2022
Accepted 12.10.2022
About the Authors
Svetlana V. Yureneva, Dr. Med. Sci., Professor at the Department of Obstetrics and Gynecology of the Department of Vocational Education, Deputy Director for Science, Institute of Oncology and Mammology, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia,syureneva@gmail.com, 117997, Russia, Moscow, Ac. Oparina str., 4.
Victoria G. Averkova, Ph.D. student at the Department of Gynecological Endocrinology, V.I. Kulakov National Medical Research Center for Obstetrics,
Gynecology and Perinatology, Ministry of Health of Russia, buch1202@mail.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Corresponding author: Victoria G. Averkova, buch1202@mail.ru
Authors’ contributions. Yureneva S.V., Averkova V.G. – developing the concept and design of the study; Averkova V.G.,
Yureneva S.V. – obtaining the data for analysis, writing the text; Averkova V.G. – statistical data processing; Yureneva S.V. – editing the article.
Conflict of interest: The authors declare that there are no conflicts of interest.
Funding: The article was prepared without sponsorship.
Ethical Approval: The study was approved by the Ethical Review Board of Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Averkova V.G., Yureneva S.V.
Analysis of treatment satisfaction in patients with premature ovarian failure.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 10: 83-92 (in Russian)
https://dx.doi.org/10.18565/aig.2022.10.83-92



