Неуклонно растет число криопереносов в последние годы, увеличивая число живорождений, в частности, на фоне проведенного преимплантационного генетического тестирования. Сегментация цикла и использование витрифицированного эмбриона также позволяют снизить риск умеренного и тяжелого синдрома гиперстимуляции яичников (СГЯ) [1–3]. Это актуально и в связи с тем, что женщина, откладывая материнство, приходит в программу экстракорпорального оплодотворения (ЭКО) не только в старшем репродуктивном возрасте, но и с отягощением акушерско-гинекологического анамнеза. Для этой когорты пациенток клиник репродукции характерны наличие миомы матки, аденомиоза, фолликулярных кист яичников, гиперплазии эндометрия [4, 5]. Для этих пациенток протокол вспомогательных репродуктивных технологий (ВРТ) с агонистом гонадотропин-рилизинг-гормона (ГнРГ) перед криопереносом снижает негативное влияние перечисленных заболеваний на имплантацию, при этом следует учитывать, что только прием препаратов эстрогена позволяет в дальнейшем обеспечить состояние эндометрия, необходимое для успешной имплантации. Существует позиция, которая говорит о риске онкозаболеваний на фоне применения, особенно неоднократного, лекарственных препаратов, используемых при ЭКО. Некоторые из них повышают уровни лютеинизирующего (ЛГ) и фолликулостимулирующего гормонов, что, в свою очередь, повышает уровень эстрогена. Это внезапное повышение уровня эстрогена может увеличить экспрессию генов и, как следствие, риск рака молочной железы. Результаты исследований с большим размером выборки показывают, что женщины, получавшие терапию от бесплодия в течение длительного времени, особенно более года, более подвержены недостаткам препаратов для лечения бесплодия, о которых пациентку следует проинформировать [6]. В отношении профиля безопасности препаратов эстрогена по-прежнему сохраняется дискуссия, тогда как препараты прогестерона в ЭКО признаются безопасными, согласно метаанализу Katalinic и соавт. [7]. Между тем в программах ВРТ увеличивается период применения препаратов эстрогена с момента подготовки эндометрия до 8–9 недель наступившей беременности, что является дополнительной лекарственной нагрузкой пациента и требует дополнительного наблюдения и консультирования со стороны врача.
В ходе исследования метаболизма эстрогенов, связанного с работой фермента катехол-О-метилтрансферазы (СОМТ), было показано, что его полиморфизмы детерминируют более или менее высокую активность метилирования гидроксиформ эстрогенов с тем, чтобы перевести их в более безопасные метоксилированные метаболиты [8, 9]. Нами ранее показано, что уровень образования метоксилированных форм эстрогенов сопряжен с полиморфизмом гена COMT: с вариантами АА и GA ассоциирован более низкий уровень метоксиформ. При этом ни один из полиморфных вариантов не сопряжен с увеличением потенциально опасных продуктов метаболизма эстрогена у женщин, проходивших лечение в цикле гормональной терапии с предварительным назначением агониста ГнРГ [10]. В настоящем исследовании мы, оценивая уровень метилирования эстрогенов по соотношению гидрокси- и метоксиформ метаболитов эстрадиола в моче, искали ответа на вопрос: как влияет на молекулярный состав метаболитов эстрогенов в криопротоколе применяемый препарат эстрогена. С учетом генетического полиморфизма фермента, осуществляющего метилирование и детоксикацию метаболитов эстрогена, мы оценивали влияние на эти процессы двух наиболее часто используемых препаратов эстрогенов: 0,1% трансдермального геля с эстрадиолом и драже эстрадиола валерата, принимаемого перорально.
Цель исследования: сравнить содержание метаболитов эстрадиола в моче женщин с различными полиморфизмами гена COMT rs4680 и разными типами эстрогеновой поддержки в криопереносе в цикле гормональной терапии с предварительным назначением агониста ГнРГ.
Материалы и методы
В исследование включены 39 протоколов ЭКО с витрифицированным эмбрионом, все проведены с применением «длинного» протокола с агонистами ГнРГ и заместительной гормональной терапией (ЗГТ). Обоснованием этого типа протокола служили рецидивирующая гиперплазия эндометрия в анамнезе, аденомиоз 2–3 степени и ранние овуляции в естественном цикле.
В преддверии цикла криопереноса назначался агонист ГнРГ (Диферелин 3,75 мг) с 17–19-го дня цикла. Далее проводился мониторинг роста эндометрия и назначался препарат прогестерона (Дюфастон по 10 мг 3 раза в сутки или Утрожестан по 200 мг 3 раза во влагалище) при толщине эндометрия более 8 мм, перенос эмбриона проводили через 124–125 ч действия препарата.
Случайным образом пациентки были распределены в группы в зависимости от применяемого препарата эстрогена, причем в работу взяты 2 самых распространенных клинических протокола. Старт приема эстрогенов (Дивигель по 1 г 2 раза на кожу в группе 1 и Прогинова по 2 мг 2 раза в день в группе 2) проводился индивидуально по УЗИ-оценке после менструальноподобной реакции после введения агониста ГнРГ, что составило 21±5 дней. В обеих группах прием препаратов эстрогена продолжался без снижения до УЗИ по беременности – это 3 недели от переноса, потом – индивидуальная скорость отмены с контролем эстрадиола крови к 8–9-й неделе беременности. Прием препаратов прогестерона продолжался до 12–20 недель беременности.
Все пациентки получали прегравидарную подготовку согласно рекомендациям клинического протокола МАРС 2021 и были обследованы по приказу № 803н. Проводился гормональный мониторинг цикла ЭКО. Контрольной точкой 1 был день назначения препарата прогестерона, что соответствовало максимальному уровню эстрадиола в цикле при достигнутой оптимальной толщине эндометрия, и под контролем за стартом приема прогестерона. Поскольку целью исследования была оценка безопасности различных форм препаратов эстрогена в протоколе криопереноса, проводили анализ утренней порции мочи на метаболиты эстрогенов с использованием метода высокоэффективной жидкостной хроматографии с тандемным масс-спектрометрическим детектированием (лаборатория «Хеликс»). В дальнейшем производили расчет соотношений метилированных и гидроксилированных форм эстрона и эстрадиола. В день переноса (контрольная точка 2) повторно проводился анализ крови на гормоны. Всем пациенткам проводилась оценка полиморфизмов rs 4680 гена СОМТ в лаборатории «Геномед», определяли аллели AA, AG или GG.
Перенос эмбриона осуществлялся с использованием УЗ-контроля катетером Kitazato #213325 или TDT. Пациентки сразу вставали после проведенного переноса. Диагностика наступления беременности проводилась через 14 дней анализом крови на хорионический гонадотропин человека (ХГЧ) и визуализацией плодного яйца в полости матки через 21 день после переноса эмбриона. Для каждой женщины производили расчет частоты имплантации, вычисляя его как отношение числа плодных яиц к числу перенесенных эмбрионов, выраженное в процентах [11].
Статистический анализ
Статистический анализ полученных результатов проводился при помощи пакета программ Statistica 6.0 (Statsoft, США). Для количественных признаков рассчитывались медианное значение и интерквартильный разброс (Ме (Q25%– Q75%)), для качественных – абсолютная величина (n) и доля, выраженная в процентах. При межгрупповом сравнении показателей (их распределение отличалось от нормального, согласно значению критерия Шапиро–Уилка) использовали критерий Манна–Уитни. Различия между качественными признаками проверялись при помощи теста χ2. Если достигнутый уровень различий не превышал 0,05, их считали статистически значимыми [12].
Результаты
Клиническая характеристика пациенток, чьи криопротоколы были включены в исследование, приведена в таблице 1. Из нее следует, что пациентки с разной эстрогеновой поддержкой в криопротоколе не имели статистически значимых различий в основных показателях анамнеза, гормонального статуса, а также продолжительности применения препарата эстрогена. Полученное различие в дозировке получаемого эстрогенного препарата следует рассматривать с учетом особенности распределения эстрадиола валерата, 50% которого в организме связывается с белками плазмы.
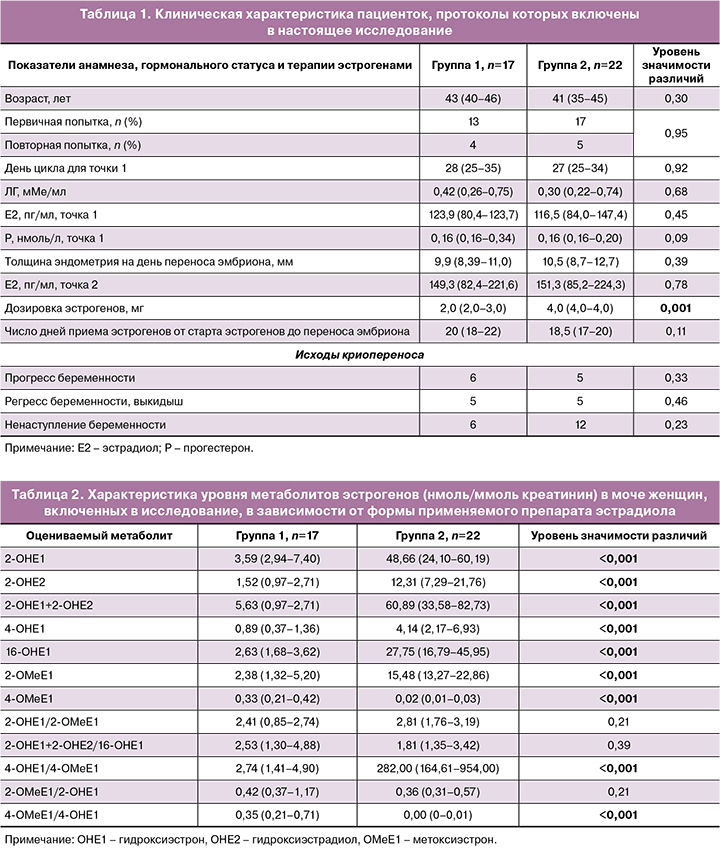
Оценив уровень гидроксилированных форм эстрогенов, пришли к заключению: эстрогены, принимаемые внутрь, ассоциируются с более высоким содержанием в моче гидроксилированных форм метаболитов, нежели при использовании трансдермального геля. В группе пациенток, принимавших пероральную форму эстрогеновой поддержки (эстрадиола валерат), уровень гидроксиформ метаболитов эстрадиола был достоверно выше (по некоторым позициям в 10 и более раз, табл. 2).
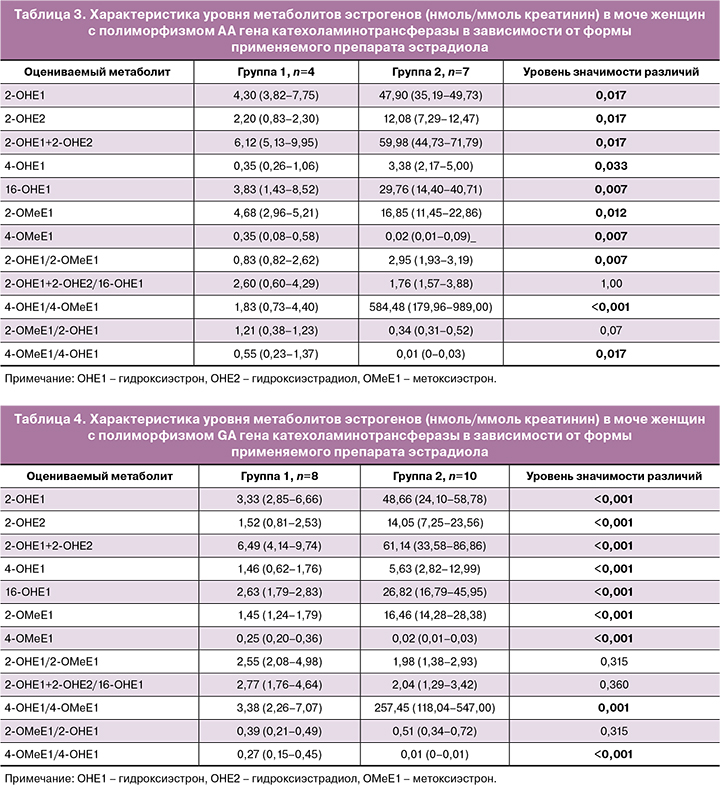
Рассматривая, как влияет полиморфизм гена COMT на метаболизм различных препаратов эстрогена, выявили: прием пероральной формы эстрогена при любом из вариантов гена COMT сопровождается более высоким уровнем гидроксилированных форм, нежели при использовании трансдермальной формы (табл. 3–5).

Метоксилирование 2- и 16-гидроксиэстрадиола и 2- и 16- гидроксиэстрона при этом сохраняется на уровне, сравнимом с таковым на фоне применения трансдермального геля эстрадиола, тогда как содержание 4-метоксиэстрона и 4-метоксиэстрадиола в моче на фоне приема пероральной формы статистически значимо ниже, чем на фоне трансдермальной. Следовательно, пул 4-гидроксилированных метаболитов эстрогенов на фоне приема пероральной формы превышает таковой на фоне трансдермальной.
Отмечено, что полиморфизмы гена COMT AA и GA ассоциированы с более высоким содержанием гидроксилированных продуктов обмена эстрогенов вне зависимости от того, какой тип препарата эстрогенов использован в протоколе. Так, у женщин с полиморфизмом АА на фоне трансдермального эстрадиола уровень гидроксиформ был выше в 1,5 раза по сравнению с таковым у женщин с полиморфизмом GG (p=0,014). У женщин с полиморфизмом GA уровень указанных метаболитов в моче превышал таковой у женщин с GG вариантом гена в 1,6 раза (р=0,010).
При анализе содержания гидроксиформ в моче на фоне приема пероральной формы у пациенток с полиморфизмами АА и GA по сравнению с аналогичным показателем у пациенток с полиморфизмом GG статистически значимой разницы выявлено не было.
Обсуждение
Тема безопасности применения препаратов в рамках ВРТ, а число последних растет с каждым годом, обсуждается в России и за рубежом, поскольку вопрос связи между назначением препаратов, применяемых для лечения бесплодия, и риском развития онкологических заболеваний остается малоизученным. Как показал проведенный российскими исследователями метаанализ, результаты применения различных подходов к лечению бесплодия и протоколов ВРТ свидетельствуют о наличии определенных факторов риска у данной категории пациенток, причем статус сниженной фертильности сам ассоциирован с повышенным риском развития злокачественных новообразований в матке [13]. Не случайным является внимание к уровню и обмену эстрогенов: их гидроксилированные формы метаболитов ассоциированы с раком молочной железы [14], хотя возможность прогнозирования развития онкопатологии на основании анализа продуктов метаболизма эстрадиола и полиморфных вариантов генов, детерминирующих обменные процессы, является дискутабельной. Между тем описана ассоциация мутантных форм гена rs4680 COMT с риском такой патологии [15, 16].
Нами ранее было показано, что носители различных аллелей гена, детерминирующего фермент метаболизма эстрогенов, могут неодинаково накапливать гидроксилированные и метоксилированные формы эстрона и эстрадиола [10]. Как показал сравнительный анализ, вне зависимости от типа препаратов эстрогенов в программах ВРТ сохраняется меньшая активность метоксилирования у пациенток с полиморфизмом АА и GА. Показано, что прием пероральной формы эстрогенов ассоциирован с более высоким содержанием гидроксилированных форм метаболитов по сравнению с таковым на фоне приема трансдермальной формы эстрогена. Вместе с тем метаболизм 2-гидроксиэстрона и 2-гидроксиэстрадиола является компенсированным, судя по соотношению уровня этих молекул к их метоксилированным метаболитам. Этого не наблюдается для реакции метоксилирования гидроксигруппы в четвертом положении как в случае 4-гидроксиэстрона, так и в случае 4-гидроксиэстрадиола. Следовательно, если принимать во внимание меньший уровень метоксиформ в моче у женщин с полиморфизмами АА и GА, следует рекомендовать им применение трансдермальной формы препарата эстрогена как лекарственного агента, не повышающего накопление проонкогенных продуктов обмена эстрогенов.
Пока не достигнут консенсус в вопросе безопасности применения препаратов эстрогенов и риска возможного развития рака разных локализаций, ассоциированного с ним [17, 18], следует отдавать предпочтение тому методу фармакотерапии, который сопряжен с минимальным риском для пациента. В настоящем исследовании показано, что трансдермальная форма эстрогенов является более предпочтительной в криопротоколе с ЗГТ на агонистах ГнРГ для пациенток старшей возрастной группы с отягощенным анамнезом, нежели пероральная форма эстрогена.
Выводы
1. Уровень образования метоксилированных форм эстрогенов вне зависимости от типа примененного препарата эстрогена оказался более низким у женщин полиморфными вариантами АА и GA гена COMT.
2. Криопротокол с агонистами ГнРГ в сочетании с применением 0,1% трансдермального геля эстрадиола характеризуется более высоким уровнем метоксиформ метаболитов эстрогенов, нежели с применением драже эстрадиола валерата.
3. Криопротокол с агонистами ГнРГ в сочетании с применением эстрадиола валерата сопровождается статистически значимым снижением уровня метоксилирования 4-гидроксиэстрона и 4-гидроксиэстрадиола по сравнению с аналогичным протоколом, в котором использовали трансдермальный гель эстрадиола у пациенток вне зависимости от полиморфизма гена COMT.
4. У пациенток с полиморфизмом СОМТ AG и GG в «длинном» протоколе целесообразным является применение трансдермальной формы эстрогена как препарата, не повышающего накопление гидроксилированных форм метаболитов эстрогенов.



