Treatment of infertility in women with multiple treatment failures using assisted reproductive technologies with co-culture of embryos with autologous follicular fluid-derived extracellular vesicles
Akhmedova Z.F., Sysoeva A.P., Gavrilov M.Yu., Zingerenko B.V., Shevtsova Yu.A., Silachev D.N., Nepsha O.S., Makarova N.P., Kulakova E.V., Kalinina E.A.
Relevance: The primary goal of infertility treatment using assisted reproductive technologies (ART) is to increase the rates of embryo implantation and the birth of healthy children. Scientific research has focused on enhancing culture conditions, particularly the co-culture of embryos at various developmental stages with somatic cells and extracellular vesicles (EVs). Experimental animal models have demonstrated that adding EVs derived from follicular fluid (FF) during in vitro culture can modulate embryo development and improve implantation rates. FF-derived EVs were specifically selected because of their proven roles in regulating oocyte quality and subsequent post-implantation embryo development.
Objective: To evaluate the clinical efficacy of ART for optimizing the embryological stage of infertility treatment by co-culturing FF-derived EVs with embryos in patients who have experienced multiple unsuccessful IVF attempts.
Materials and methods: This study involved married couples with a history of more than two unsuccessful IVF attempts, a normal karyotype, and mild pathozoospermia. A total of 76 women were included in this study. As part of the standard ART protocol, FF was collected on the day of transvaginal puncture from which EVs were isolated through sequential ultracentrifugation. After fertilization, oocytes were randomly divided into two groups: group 1 underwent co-culture of zygotes with FF-derived EVs for 24 h, while group 2 underwent classical culture. The addition of FF-derived EVs was performed as follows: in a well of a 4-well plate containing 0.3 ml of culture medium and post-ICSI oocytes, 2 μl of the medium with FF-derived EVs was added and incubated for 24 h. On the first day of culture, embryos at the zygote stage from group 1 were transferred to classical culture medium to continue developing to the blastocyst stage. Embryological and clinical outcomes were assessed, with the primary endpoint being pregnancy rate after transferring one embryo into the uterine cavity. The level of statistical significance was set at p<0.05.
Results: Assessment of early embryogenesis parameters revealed a statistically significant increase in fertilization rate for the co-culture group with FF-derived EVs: 88.1% compared to 77.3% in the classical culture group (p<0.001, chi-square test). Additionally, group 1 exhibited a significantly higher frequency of excellent- and good-quality blastocyst formation (55.0% vs. 42.6 %, p<0.01). When fertilized post-ICSI oocytes were co-cultured with FF-derived EVs, there were significantly more blastocysts suitable for transfer into the uterine cavity and cryopreservation, with a median of 1 [0; 1.25] in group 1 compared to 0 [0; 1] in group 2. Clinical data analysis indicated a pregnancy rate of 27.2% in group 2, while group 1 (EV FF) achieved clinical pregnancy in 14 of 45 transfers, with two biochemical pregnancies. The clinical pregnancy rate in group 1 was 31.1%. Notably, no statistically significant differences in clinical parameters were found across the overall cohort of women with a history of multiple IVF failures. However, there was a tendency for an increased pregnancy rate with co-culture of FF-derived EVs in younger women, in contrast to the near-complete absence of positive outcomes in patients of advanced reproductive age.
Conclusion: The results suggest that co-culture with FF-derived EVs may significantly enhance the embryological stage of infertility treatment programs in women with a history of multiple ART failures. Further studies are needed to establish statistically significant differences in clinical outcomes.
Authors' contributions: Akhmedova Z.F., Sysoeva A.P., Makarova N.P. – conception and design of the study; Akhmedova Z.F., Sysoeva A.P., Makarova N.P., Kulakova E.V. – drafting and editing of the manuscript; Akhmedova Z.F., Nepsha O.S., Gavrilov M.Yu. – statistical analysis; Akhmedova Z.F., Sysoeva A.P., Zingerenko B.V. – collection of biological material; Shevtsova Yu.A., Silachev D.N. – laboratory stage; Makarova N.P., Kulakova E.V., Kalinina E.A. – approval of the manuscript for submission.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: The study was conducted within the framework of the initiative scientific project "Study of the influence of extracellular vesicles of biological fluids of reproductive organs and tissues on gametes, the process of fertilization and early human embryogenesis and implantation" (2025-2027, supervisor N.P. Makarova) of V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P (Ref. No: 10 of October 20, 2022).
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Akhmedova Z.F., Sysoeva A.P., Gavrilov M.Yu., Zingerenko B.V., Shevtsova Yu.A., Silachev D.N., Nepsha O.S., Makarova N.P., Kulakova E.V., Kalinina E.A. Treatment of infertility in women with
multiple treatment failures using assisted reproductive technologies with co-culture of
embryos with autologous follicular fluid-derived extracellular vesicles.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (5): 66-77 (in Russian)
https://dx.doi.org/10.18565/aig.2025.85
Keywords
The goal of assisted reproductive technology (ART) in treating infertility is to increase the implantation rate, achieve pregnancy, and ultimately give birth to a healthy child. However, the low implantation rate – which is limited to no more than 40% – remains a major challenge in reproductive medicine [1, 2]. Furthermore, the process of culturing embryos from the zygote stage to the expanded blastocyst has proven to be highly inefficient; on average, only about 50% of cultured embryos reach the cleavage stage, and approximately 30% reach the blastocyst stage. These outcomes vary depending on factors such as the woman’s age, underlying causes of the couple’s infertility, and laboratory conditions. Consequently, efforts to improve the embryological stage of ART programs continue, with one promising approach being the co-culture of various cells from the woman's reproductive tract with embryos. These methods have shown excellent results in animal embryo cultures [3, 4]. However, co-culture techniques have not been widely adopted in clinical settings for humans, primarily because of ethical concerns and practical challenges, such as contamination and the difficulty of adapting the culture system. Currently, only a limited number of studies have explored the co-culture of endometrial cells, fallopian tubes, and cumulus cells with embryos [3, 5, 6]. These studies have demonstrated the feasibility of the procedure in a small sample of infertile women. Notably, a 2022 study by Asfarova G.R. et al. demonstrated the effectiveness of co-culturing embryos with autologous cumulus cells in clinical practice within ART programs, confirming the feasibility and safety of optimizing the embryological stage [7].
This study is a continuation of a previous study in which extracellular vesicles (EVs) derived from follicular fluid (FF) were used. In 2017, promising results were achieved with autologous co-culture of EVs with immature oocytes and embryos in cattle, which laid the groundwork for similar studies in humans undergoing ART [8]. The addition of FF-derived EVs during in vitro culture has been shown to modulate embryo development and increase implantation rates. These EVs were selected because of their established role in regulating oocyte quality and the subsequent post-implantation development of human embryos [9]. According to the current Russian and international scientific literature, EVs consist of the same molecules as the cells from which they originate, and contain corresponding nucleic acids (DNA, coding, and non-coding RNA), proteins (enzymes, cytokines, growth factors, antioxidants), and lipids. EVs are crucial for cellular communication, activating specific cell surface receptors with ligand proteins and bioactive lipids, or fusing their membrane with the cytoplasmic membrane of recipient cells, thereby transporting bioactive molecules involved in regulating essential biological processes [9–12].
In light of the above, this study aimed to evaluate the clinical efficacy of ART by optimizing the embryological stage of infertility treatment through the co-culture of FF-derived EVs with embryos in patients who have experienced multiple unsuccessful IVF attempts.
Materials and methods
Patient characteristics
Recruitment for the study was carried out at the Prof. B.V. Leonov Department of Assistive Technologies in Infertility Treatment (Head – Dr. Med. Sci., Professor, Kalinina E.A.) the V.I. Kulakov NMRC for OG&P (Director – Academician of the RAS, Dr. Med. Sci., Professor, Sukhikh G.T.) among married couples who sought infertility treatment using ART from 2022 to 2024.
The inclusion criteria for the study were normal karyotypes of both spouses, women aged 18–40 years inclusive, retrieval of at least two oocytes on the day of transvaginal follicle puncture, history of at least two failed embryo transfers (both native and thawed), and mild pathozoospermia in the spouse. The exclusion criteria were the use of donor gametes in the studied ART cycle, surrogacy, uterine factor of infertility, decreased ovarian reserve, obesity, cancellation of transfer into the uterine cavity, and lack of fertilization. All married couples were examined in accordance with the current regulatory legal acts (Order of the Ministry of Health of the Russian Federation No. 803n of July 31, 2020, "On the procedure for using assisted reproductive technologies, contraindications and restrictions on their use"; clinical guidelines "Female Infertility" (2022, 2024), clinical guidelines "Male Infertility" (2022)). No contraindications for the use of ART were identified in any married couple. Before joining the ART program, married couples provided informed voluntary consent. The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P (Ref. No: 10 of October 20, 2022). According to the inclusion/exclusion criteria, 76 married couples were recruited, of which 9 had a cancellation of the transfer into the uterine cavity due to endometrial failure. Thus, embryological parameters were analyzed in 76 married couples, and clinical parameters were assessed in 67 couples.
Infertility treatment using ART
Stimulation of ovarian function was performed according to a protocol using gonadotropin-releasing hormone antagonists. Ovulation trigger was administered in the presence of follicles with a diameter of 17 mm or more in the ovaries. Human chorionic gonadotropin (hCG) at a dosage of 10,000 IU or a gonadotropin-releasing hormone agonist (GnRH) 0.2 mg) was used as an ovulation trigger. Transvaginal puncture of the follicles was performed under general anesthesia under ultrasound control with atraumatic needles (Vitrolife, Sweden). The entire embryological stage was performed on GLOBAL culture media (CooperSurgical, Denmark), according to the manufacturer's instructions. All patients underwent one embryo transfer into the uterine cavity on the 5th day after fertilization. Post-transfer support was performed according to the standard protocol using micronized progesterone (600 mg/day). Clinical pregnancy was diagnosed on the 21st day after embryo transfer during pelvic ultrasound examination based on the presence of an ovum in the uterine cavity.
Isolation of EVs from FF
The laboratory part of the study was conducted by the staff of the Cell Technologies Laboratory (Head of the Laboratory – Dr. Bio. Sci., D.N. Silachev), V.I. Kulakov NMRC for OG&P.
EVs were isolated from FF via sequential ultracentrifugation using an Avanti JXN-30 high-speed centrifuge (Beckman Coulter Life Sciences). FF (5 ml of FF collected from the dominant follicle) was centrifuged at 4°C for 10 min at 400 × g to remove cellular debris. The FF supernatant was then transferred to a centrifuge cup and adjusted to 40 ml with PBS (Gibco, USA), followed by centrifugation for 30 min at 10,000 × g (4°C, acceleration 4, deceleration 4). The supernatant was transferred to new tubes and centrifuged again for 1.5 h at 108,000 g (temperature 4°C, acceleration 4, deceleration 4). The pellet was resuspended in PBS (Gibco, USA), the final volume was brought to 40 ml and centrifuged again for 1.5 h at 108,000 g (temperature 4°C, acceleration 4, deceleration 4). The pellet in the form of FF-derived EVs was diluted with GLOBAL culture medium (CooperSurgical, Denmark) to a final volume of 10 μl.
Experimental design using autologous FF-derived EVs
The design presented in the figure was developed to study the effect of FF-derived EVs on embryological parameters and outcomes of infertility treatment programs using ART methods.
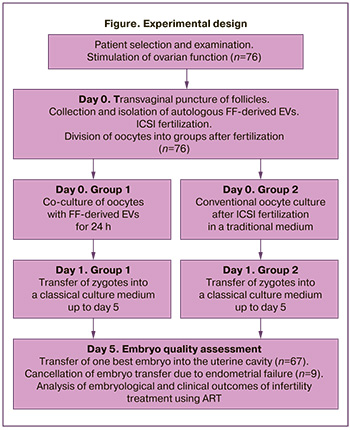
The oocytes after fertilization by intracytoplasmic sperm injection (ICSI) on the day of transvaginal puncture were divided into two groups: group 1, co-culture of zygotes with FF-derived EVs for 24 h; group 2, classical culture (Global, CooperSurgical, Denmark). The addition of FF-derived EVs was performed according to the following scheme: Two microliters of the medium with FF-derived EVs was added to a well of a 4-well plate (NUNC, USA) with 0.3 ml of culture medium (Global, CooperSurgical, Denmark) and oocytes after ICSI and left for 24 hours. On the 1st day of culture, embryos at the zygote stage (2PN2PB) of group 1 were transferred to classical culture medium, where they continued their development to the blastocyst stage. Morphological assessment of the embryos at the cleavage stage was not performed. On the 5th day of culture, the embryos were analyzed and ranked according to quality. One best embryo was transferred to the uterine cavity under ultrasound guidance.
Evaluation of the quality of embryos and the method of their ranking by quality
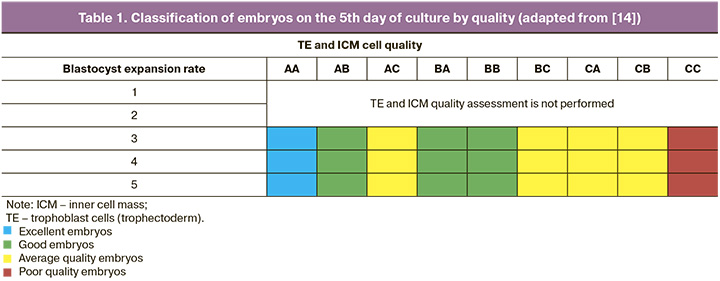
Embryo quality assessment at the embryological stage of the infertility treatment program using ART methods was performed according to the recommendations of the Russian Association of Fertility Reproduction (2021) [13]. On the 5th day of culture, morphological analysis of the degree of expansion of the blastocyst, trophoblast cells, and inner cell mass was performed. The classification of Sallem et al. was used for subsequent analysis and stratification of embryos into groups. [14], as listed in Table 1.
Data analysis
The primary study endpoints were the fertilization rate (amount of zygotes/number of mature MII oocytes, in %) and the frequency of formation of excellent- and good-quality blastocysts depending on the type of culture (amount of blastocysts/number of zygotes, in %). The study endpoint was the clinical pregnancy rate per embryo transfer (number of pregnant women/number of transfers, in %).
Statistical analysis
Statistical analyses were performed using Microsoft Excel and IBM SPSS Statistics v. 27.0.1.0 statistical software (USA). The normality of the distribution of continuous variables was tested using the Shapiro-Wilk test. Counts (n) and percentages (%) were used to describe categorical binary data. Continuous variables showing normal distribution were expressed as mean (M) and standard deviation (SD) and presented as M (SD); otherwise, the median (Me) with interquartile range (Q25; Q75) was reported. The nonparametric Mann–Whitney U test was used to compare continuous variables. Categorical data were analyzed using Pearson’s χ² test. The threshold significance level (p) was set to 0.05.
Results
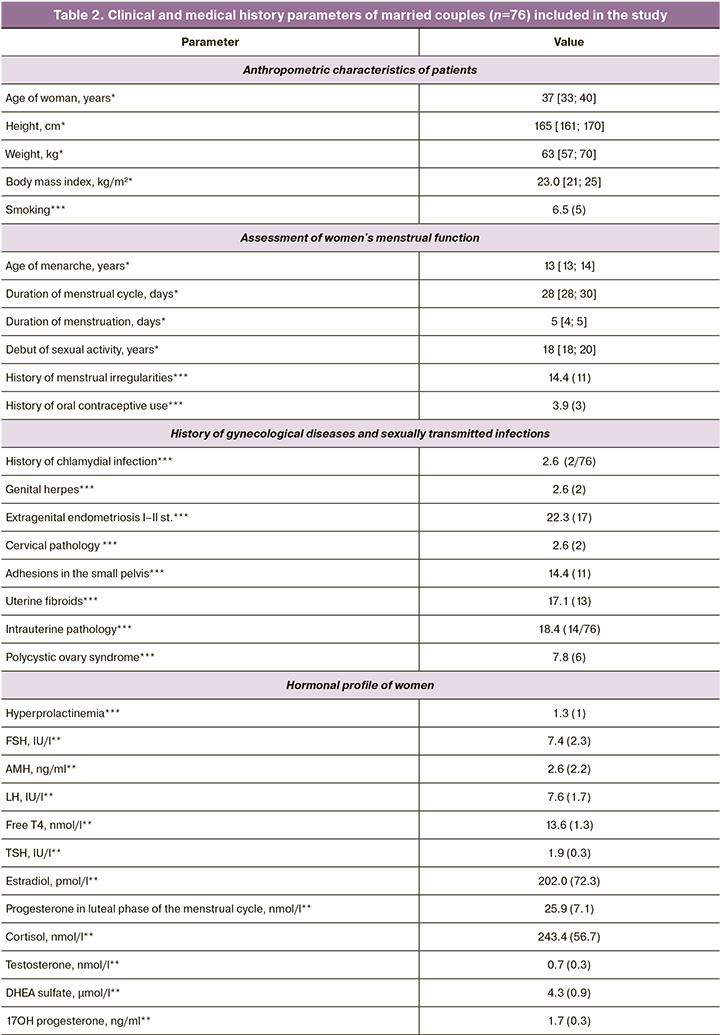
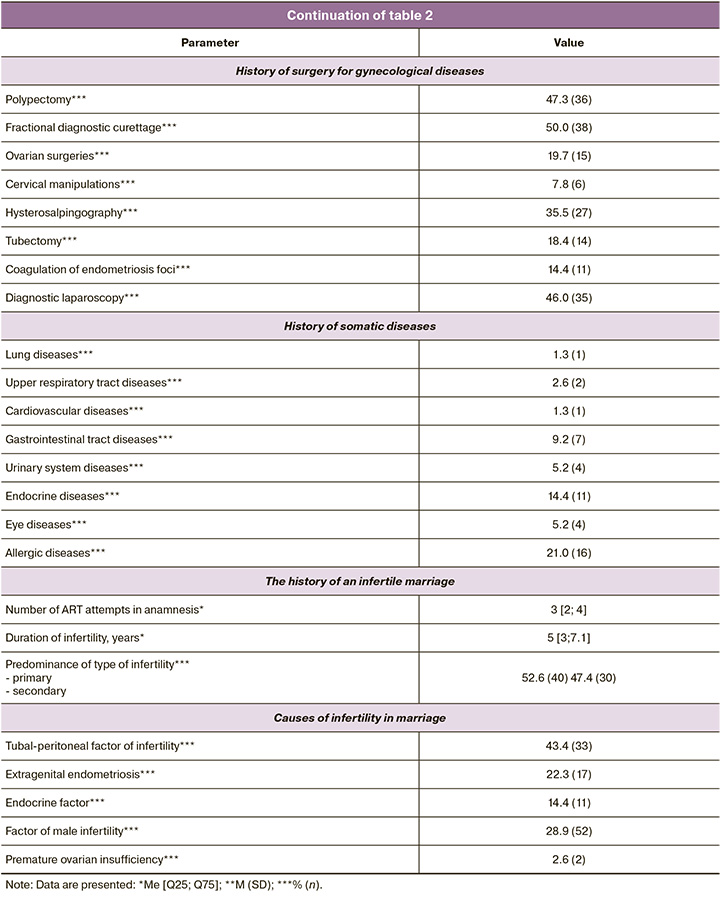
In the first stage of the study, the couples’ clinical and medical history data were assessed. Table 2 presents the results. As shown in the table, the mean age of the women was 37 years, and each couple had an average of three failed embryo transfers. Notably, among gynecological diseases, there was the predominance of extragenital endometriosis with stage I and II dissemination detected during diagnostic laparoscopy for adhesion, small uterine fibroids, or ectopic pregnancy. A history of sexually transmitted infections was present in only two patients (20.6%), while polycystic ovary syndrome was noted in 7.8%. Additionally, 50% of the women had a history of fractional diagnostic curettage, 35.5% underwent hysterosalpingography, 47.3% underwent polypectomy, and 46.0% underwent diagnostic laparoscopy. It is evident that the women recruited for this study had a history of multiple unsuccessful attempts and had undergone numerous surgical interventions for various gynecological diseases. Hormonal investigations of the women are also presented in Table 1, which shows that all hormonal levels were within the reference values. Somatic diseases were present in 21.0% of the cases, with allergic diseases being the most common. Endocrine diseases occurred in 14.4% of the patients and were managed by specialists at the pre-pregnancy stage of infertility treatment. All patients underwent standard ovarian stimulation, the details of which are presented in Table 3. The mean total dose of gonadotropins administered was 1689.83 (571.8) IU, and the mean duration of stimulation was 9.1 days. An average of nine oocyte-cumulus complexes were obtained from the study participants, seven of which were suitable for fertilization by ICSI and were subsequently divided into groups based on the type of culture. Spermatogenesis parameters on the day of fertilization are also presented in Table 3, with an average sperm concentration of 45.5 million/ml, progressively motile PR% at 54%, and morphologically normal sperm forms averaging 2%.
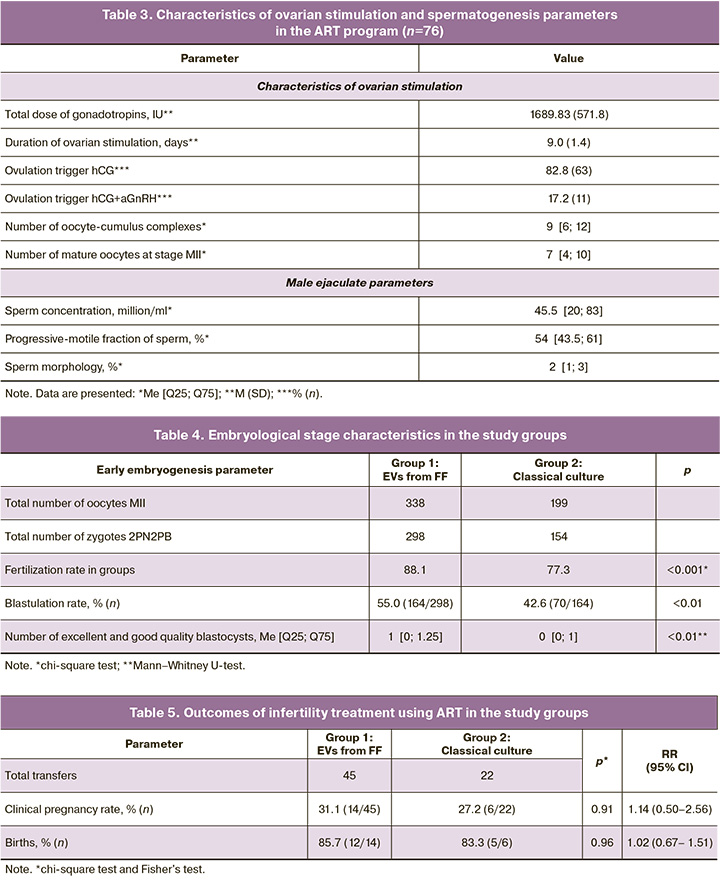
According to the study design, after fertilization by ICSI, all oocytes of 76 patients were randomly divided into two groups: group 1, addition of autologous FF-derived EVs for 24 h; group 2, classical oocyte culture. On the 5th day, the embryological stage parameters were assessed in the selected groups. Table 4 presents the results.
As can be seen from the assessed parameters of early embryogenesis, the fertilization rate was significantly higher in the group with a co-culture of oocytes with FF-derived EVs (88.1% vs. 77.3%, p<0.001, chi-square test). In group 1, the frequency of formation of excellent- and good-quality blastocysts was significantly higher (55.0% vs. 42.6%, p<0.01). Co-culture of fertilized oocytes with FF-derived EVs after ICSI resulted in significantly more blastocysts suitable for transfer and cryopreservation: 1 [0; 1.25] in group 1 and 0 [0; 1] in group 2.
Thus, we can conclude that co-culture with FF-derived EVs in women with a history of multiple unsuccessful ART attempts can significantly enhance the embryological stage of infertility treatment.
Among the patients recruited for the study, embryo transfer was canceled in 9 patients due to unsatisfactory endometrial quality, leaving clinical parameters calculated for 67 married couples.
In group 2 (classical culture), embryo transfer occurred in 22 patients; of these, clinical pregnancy was achieved in 6 women and biochemical pregnancy in 1. The pregnancy rate in group 2 was 27.2%. In group 1 (EV FF), clinical pregnancy was diagnosed in 14 women after 45 transfers, with two experiencing biochemical pregnancies. The clinical pregnancy rate in group 1 was 31.1%. The results are presented in Table 5. As indicated by the data, no statistically significant differences in clinical parameters were found among the overall cohort of recruited women with a history of multiple IVF failures.
When analyzing clinical outcomes, a trend was noted towards an increased pregnancy rate with co-culture of FF-derived EVs in younger women, while there was an almost complete absence of positive outcomes in patients of late reproductive age. Consequently, pregnant women (n=20) were further stratified by age, and the proportion of young patients in groups 1 and 2 was calculated. It was found that the proportion of women aged 25–34 years among those who became pregnant from embryos co-cultured with FF-derived EVs was 71.4% (10/14), compared to 33.3% (2/6) in group 2 with classical culture. Although no statistical difference was found (p=0.11), it can be concluded that treatment programs involving the co-culture of oocytes with FF-derived EVs in young patients have more favorable outcomes.
Discussion
Ovarian follicle growth is accompanied by somatic cell proliferation and the accumulation of FF, which is secreted by granulosa cells in antral follicles. FF plays a critical role in ovarian steroidogenesis and oogenesis. The main components of FF include electrolytes, steroid hormones, metabolites, proteins, polysaccharides, reactive oxygen species, antioxidant enzymes, and various molecules including non-coding RNAs. These components facilitate autocrine and paracrine communication between theca, mural, and cumulus cells, as well as the maturing oocyte. FF has several oocyte-related functions, such as protection against proteolysis and aiding in the development and maturation of oocytes and cumulus cells [15]. Cross-communication between the oocyte and granulosa cells occurs via gap junctions; however, in the last decade, EVs in FF have emerged as a novel mode of communication within the follicle [16–18].
As previously reported, EVs play important roles in the major phases of the reproductive process, including gametogenesis, fertilization, implantation, and embryo development. Understanding the various mechanisms involved in these steps is crucial not only for basic research but also for clarifying cases of unexplained infertility, thereby improving reproductive success [19, 20]. EVs promote cumulus cell expansion, stimulate granulosa cell proliferation, enhance oocyte development, and may also accelerate oocyte maturation. Additionally, they can alter gene expression in tubal epithelial cells, potentially improving sperm survival and fertilization [21]. In our study, we found that co-culturing oocytes from patients with multiple unsuccessful IVF attempts with FF-derived EVs increased the frequency of fertilization and quality of blastocysts suitable for transfer to the uterine cavity and cryopreservation. This supports the notion that EVs influence embryo blastocyst formation and survival, thereby contributing to in vitro embryo development [21]. Moreover, our analysis of clinical outcomes revealed an increase in pregnancy rates with the co-culture of FF-derived EVs in younger women, whereas in patients of late reproductive age, there was an almost complete absence of positive outcomes.
The age of a woman is a critical factor for assessing the functional state of the reproductive system. Although individual variations exist, particularly in the late reproductive age owing to human genetics, general biological patterns are inevitable. With age, the number of follicles in the ovaries of women decreases and the quality of oocytes declines. Ovarian aging begins as early as 27 years of age, with a sharp acceleration in follicle elimination after 37 years. By the time of menopause (45–50 years), follicles are no longer detectable [22].
Compared with other body systems, the female reproductive system exhibits a higher rate of aging, leading to an earlier decline in reproductive capacity. Age-related changes primarily affect oocyte quality and the composition of EVs in FF, affecting the interaction between female gametes and sperm during natural fertilization [23].
In previous studies comparing the size and number of EVs in FF in women of early and advanced reproductive age, contradictory results were obtained. Thus, in our previous study, an increase in the number of large EVs in the FF of women of late reproductive age was observed, and there were no significant differences in the concentration and size of EVs between age groups [23]. In a study by Battaglia R. et al., it was found that the FF of women of late reproductive age contains two times more small EVs than the FF of women of early reproductive age [24]. In another study, a greater number of EVs were found in the FF of women of late reproductive age than in younger women [25]. In a study by Gu Y. et al., the concentration of exosomes and exosomal proteins from FF in women of late reproductive age was slightly lower than that in women of early reproductive age [26]. Furthermore, circulating exosomes from women of late reproductive age were more readily internalized by B cells and had higher major histocompatibility complex II (MHC-II) expression on monocytes than exosomes from women of late reproductive age, suggesting that the decrease in exosome concentrations with age may be partly due to increased internalization [26].
Previously, we found that total levels of cholesterol esters, lysophosphatidylcholines, and phosphatidylcholines were higher in vesicles obtained from women of early reproductive age, whereas levels of di- and triglycerides, monogalactosyldiacylglycerols, oxidized forms of phosphatidylcholines, and ceramides were higher in vesicles obtained from women of late reproductive age [23]. A study by Chinese authors showed that, in a group of women of late reproductive age, there was a significant increase in the levels of inosine, cytidine, and taurine [26]. Cytidine plays an important role in phosphoinositide signaling and lipid synthesis. In addition to its incorporation into nucleic acids, circulating pyrimidine, also known as cytidine, can act as a substrate for the pyrimidine nucleotide salvage pathway and as a precursor of cytidine triphosphate, which is required for the biosynthesis of phosphatidylcholine and phosphatidylethanolamine. Elevated levels of inosine and cytidine in women of late reproductive age may reflect altered nucleotide stress expression and the synthesis of more transfer ribonucleic acids (tRNA) and lipids that support the expression of stress response proteins and energy substrates. The effects of taurine on female fertility may primarily be achieved by regulating the activity of hormones associated with the hypothalamic-pituitary-ovarian axis. Increased taurine in FF exosome concentration in women of late reproductive age may indicate a compensatory increase in ovarian endocrine function with age.
Furthermore, in a study by Gu Y. et al., elevated levels of oleic acid, alpha-lactose, and d-maltose were observed in a group of late reproductive-age women compared to a group of early reproductive-age women, which may partly reflect age-related changes in ovarian glycolysis and fatty acid metabolism in late reproductive-age women [26]. Follicles predominantly use a glycolytic route to produce adenosine triphosphate (ATP). Oleic acid is a long-chain unsaturated omega-9 fatty acid that is the major monounsaturated fatty acid in the lipid extracts of bovine, ovine, porcine, and human oocytes. Other studies have shown that oleic acid has beneficial effects on lipid accumulation, oocyte maturation, and subsequent embryonic development in cattle, thereby overcoming the detrimental effects of the other two major saturated fatty acids (palmitic and stearic). Several in vitro studies have also shown that elevated oleic acid concentration in FF from women of late reproductive age may reflect decreased lipid accumulation, inadequate energy storage, and lower cellular metabolism. Furthermore, elevated alpha-lactose and d-maltose concentrations in FF exosomes from women of late reproductive age may reflect enhanced glycolysis, including increased glycogen breakdown and lactose conversion to glucose to provide energy for follicular development. Elevated cholesterol and cholesterol ester levels support the hypothesis that age-related metabolic changes affect both oocyte quality and its ability to attract and activate sperm. Cholesterol is involved in many regulatory and energetic processes and is a substrate in the preparation and process of fertilization. With age, the success of these processes and the reproductive potential of an individual decrease.
In our previous study, we examined the effect of FF-derived EVs from a young donor on the expression of various genes (MKI67, MYBL2, CCNB1, CCND1, CCNE1, CALM2, BAX, NDRG1, TP53I3, VEGF, VCAN, HAS2, CTSL2, PIBF1, RPL37, PFKP, GPX3, and AQP3) in embryos of different age groups [20]. When FF-derived EVs were co-cultured with blastocysts from young women, we observed a significant increase in the expression of CTSL2, CCND1, CCNE1, and VEGF mRNA and a decrease in the expression of BAX mRNA compared to embryos from women of late reproductive age. We hypothesized that adding FF-derived EVs from young donor oocytes to embryo culture medium may slow the apoptosis process typical of blastocyst cells in women over 36 years of age.
The data from studies published in the scientific literature on both humans and animal models convincingly demonstrate that the effect of co-culturing FF-derived EVs with oocytes in ART programs is not incidental, but has a scientific basis. Although the number of patients treated in our study did not allow us to obtain statistically significant clinical results, additional data are required. Nonetheless, even the preliminary findings of this study suggest the potential for improving the embryological stage of infertility treatment programs through ART methods by co-cultivating oocytes with FF-derived EVs.
Conclusion
The results presented indicate that co-culture with EVs isolated from ovarian FF is a promising approach to enhance embryo quality in women with a history of multiple unsuccessful ART attempts. Therefore, optimizing the conditions that affect embryo development is essential, and the addition of EVs to in vitro culture media may represent a valuable strategy for improving embryo development, potentially allowing for personalized treatment in ART protocols to achieve better clinical outcomes.
References
- Назаренко Т.А., ред. Бесплодный брак: клинические задачи и их решение. Пособие для врачей. М.: МЕДпресс-информ; 2024. 144 с. [Nazarenko T.A., ed. Infertile marriage: clinical problems and their solution. Manual for doctors. Moscow: MEDpress-inform; 2024. 144 p. (in Russian)].
- Shingshetty L., Cameron N.J., Mclernon D.J., Bhattacharya S. Predictors of success after in vitro fertilization. Fertil. Steril. 2024; 121(5): 742-51. https://dx.doi.org/10.1016/j.fertnstert.2024.03.003
- Wu C., Cai H., Pu Q., Yu L., Goswami A., Mo Z. Investigating the role of oviductal mucosa-endometrial co-culture in modulating factors relevant to embryo implantation. Open Med. (Wars.). 2024; 19(1): 20241077. https://dx.doi.org/10.1515/med-2024-1077
- Lorenzo M.S., Teplitz G.M., Luchetti C.G., Cruzans P.R., Bertonazzi A., Lombardo D.M. The coculture of in vitro produced porcine embryos and oviductal epithelial cells improves blastocyst formation and modify embryo quality. Theriogenology. 2024; 226: 141-50. https://dx.doi.org/10.1016/j.theriogenology.2024.06.007
- Le Saint C., Crespo K., Bourdiec A., Bissonnette F., Buzaglo K., Couturier B. et al. Autologous endometrial cell co-culture improves human embryo development to high-quality blastocysts: a randomized controlled trial. Reprod. Biomed. Online. 2019; 38(3): 321-9. https://dx.doi.org/10.1016/j.rbmo.2018.12.039
- Carles M., Lefranc E., Bosquet D., Capelle S., Scheffler F., Copin H. et al. In vitro maturation of oocytes from stimulated IVF-ICSI cycles using autologous cumulus cell co-culture: A preliminary study. Morphologie. 2023; 107(356): 28-37. https://dx.doi.org/10.1016/j.morpho.2022.02.002
- Асфарова Г.Р., Смольникова В.Ю., Макарова Н.П., Бобров М.Ю., Эльдаров Ч.М., Зингеренко Б.В., Калинина Е.А. Клинические и молекулярные аспекты аутологичного сокультивирования эмбрионов с клетками кумулюса в программах экстракорпорального оплодотворения. Акушерство и гинекология. 2023; 4: 97-110. [Asfarova G.R., Smolnikova V.Yu., Makarova N.P., Bobrov M.Yu., Eldarov Ch.M., Zingerenko B.V., Kalinina E.A. Clinical and molecular aspects of autologous embryo-cumulus cells co-culture in IVF programs. Obstetrics and Gynecology. 2023; (4): 97-110 (in Russian)]. https://dx.doi.org/10.18565/aig.2022.306
- da Silveira J.C., Andrade G.M., Del Collado M., Sampaio R.V., Sangalli J.R., Silva L.A. et al. Supplementation with small-extracellular vesicles from ovarian follicular fluid during in vitro production modulates bovine embryo development. PLoS One. 2017; 12(6): e0179451. https://dx.doi.org/10.1371/journal.pone.0179451
- Довгань А.А., Ахмедова З.Ф., Сысоева А.П., Зингеренко Б.В., Романов Е.А., Силачев Д.Н., Макарова Н.П., Калинина Е.А. Внеклеточные везикулы фолликулярной жидкости: клинические аспекты и молекулярная биология. Акушерство и гинекология. 2023; 6: 38-43. [Dovgan A.A., Akhmedova Z.F., Sysoeva A.P., Zingerenko B.V., Romanov E.A., Silachev D.N., Makarova N.P., Kalinina E.A. Extracellular vesicles in follicular fluid: clinical aspects and molecular biology. Obstetrics and Gynecology. 2023; (6): 38-43 (in Russian)]. https://dx.doi.org/10.18565/aig.2022.320
- Duval C., Wyse B.A., Tsang B.K., Librach C.L. Extracellular vesicles and their content in the context of polycystic ovarian syndrome and endometriosis: a review. J. Ovarian. Res. 2024; 17(1): 160. https://dx.doi.org/10.1186/s13048-024-01480-7
- Aoki S., Inoue Y., Hara S., Itou J., Shirasuna K., Iwata H. microRNAs associated with the quality of follicular fluids affect oocyte and early embryonic development. Reprod. Med. Biol. 2024; 23(1): e12559. https://dx.doi.org/10.1002/rmb2.12559.
- Nepsha O.S., Burmenskaya O.V., Akhmedova Z.F., Romanov E.A., Sysoeva A.P., Goryunov K.V. et al. Changes in the transcription of proliferation- and apoptosis-related genes in embryos in women of different ages under the influence of extracellular vesicles from donor follicular fluid in vitro. Bull. Exp. Biol. Med. 2024; 176(5): 658-65. https://dx.doi.org/10.1007/s10517-024-06087-y
- Российская Ассоциация Репродукции Человека. Секция «Клиническая эмбриология». Оценка ооцитов и эмбрионов в лаборатории ВРТ. Методические рекомендации. 2021. [Russian Association of Human Reproduction. Clinical Embryology Section. Evaluation of oocytes and embryos in the ART laboratory. Guidelines. 2021. (in Russian)].
- Sallem A., Santulli P., Barraud-Lange V., Le Foll N., Ferreux L., Maignien C. et al. Extended culture of poor-quality supernumerary embryos improves ART outcomes. J. Assist. Reprod. Genet. 2018; 35(2): 311-9. https://dx.doi.org/10.1007/s10815-017-1063-7
- Gabryś J., Gurgul A., Szmatoła T., Kij-Mitka B., Andronowska A., Karnas E. et al. Follicular fluid-derived extracellular vesicles influence on in vitro maturation of equine oocyte: impact on cumulus cell viability, expansion and transcriptome. Int. J. Mol. Sci. 2024; 25(6): 3262. https://dx.doi.org/10.3390/ijms25063262
- Soares M., Pinto M.M., Nobre R.J., de Almeida L.P., da Graça Rasteiro M., Almeida-Santos T. et al. Isolation of extracellular vesicles from human follicular fluid: size-exclusion chromatography versus ultracentrifugation. Biomolecules. 2023; 13(2): 278. https://dx.doi.org/10.3390/biom13020278
- Neyroud A.S., Chiechio R.M., Moulin G., Ducarre S., Heichette C., Dupont A. et al. Diversity of extracellular vesicles in human follicular fluid: morphological analysis and quantification. Int. J. Mol. Sci. 2022; 23(19): 11676. https://dx.doi.org/10.3390/ijms231911676
- Simon C., Greening D.W., Bolumar D., Balaguer N., Salamonsen L.A., Vilella F. Extracellular vesicles in human reproduction in health and disease. Endocr. Rev. 2018; 39(3): 292-32. https://dx.doi.org/10.1210/er.2017-00229
- Andronico F., Battaglia R., Ragusa M., Barbagallo D., Purrello M., Di Pietro C. Extracellular vesicles in human oogenesis and implantation. Int. J. Mol. Sci. 2019; 20(9): 2162. https://dx.doi.org/10.3390/ijms20092162
- Зингеренко Б.В., Бурменская О.В., Сысоева А.П., Шевцова Ю.А., Силачев Д.Н., Макарова Н.П., Калинина Е.А. Изменение транскрипции генов в клетках кумулюса возрастных женщин под действием внеклеточных везикул фолликулярной жидкости молодых доноров. Бюллетень экспериментальной биологии и медицины. 2024; 178(12): 767-73. [Zingerenko B.V., Burmenskaya O.V., Sysoeva A.P., Shevtsova Yu.A., Silachev D.N., Makarova N.P., Kalinina E.A. Gene transcription changes in advanced maternal aged women cumulus cells under influence of young donors follicular fluid extracellular vesicles. Bulletin of Experimental Biology and Medicine. 2024; 178(12): 767-73. (in Russian)]. https://dx.doi.org/10.47056/0365-9615-2024-178-12-767-773
- Fan W., Qi Y., Wang Y., Yan H., Li X., Zhang Y. Messenger roles of extracellular vesicles during fertilization of gametes, development and implantation: Recent advances. Front. Cell Dev. Biol. 2023; 10: 1079387. https://dx.doi.org/10.3389/fcell.2022.1079387
- Назаренко Т.А. Стимуляция функции яичников. 7-е изд. М.: МЕДпресс-информ; 2023. 268 с. [Nazarenko T.A. Stimulation of ovarian function. 7th ed. Moscow: MEDpress-inform; 2023. 268 p. (in Russian)].
- Sysoeva A., Akhmedova Z., Nepsha O., Makarova N., Silachev D., Shevtsova Y. et al. Characteristics of the follicular fluid extracellular vesicle molecular profile in women in different age groups in ART programs. Life (Basel). 2024; 14(5): 541. https://dx.doi.org/10.3390/life14050541
- Battaglia R., Musumeci P., Ragusa M., Barbagallo D., Scalia M., Zimbone M. et al. Ovarian aging increases small extracellular vesicle CD81+ release in human follicular fluid and influences miRNA profiles. Aging (Albany NY). 2020; 12(12): 12324-41. https://dx.doi.org/10.18632/aging.103441
- Zhang H., Lin S., McElroy C.L., Wang B., Jin D., Uteshev V.V. et al. Circulating pro-inflammatory exosomes worsen stroke outcomes in aging. Circ. Res. 2021; 129(7): e121-e140. https://dx.doi.org/10.1161/CIRCRESAHA.121.318897
- Gu Y., Zhang X., Wang R., Wei Y., Peng H., Wang K. et al. Metabolomic profiling of exosomes reveals age-related changes in ovarian follicular fluid. Eur. J. Med. Res. 2024; 29(1): 4. https://dx.doi.org/10.1186/s40001-023-01586-6
Received 25.03.2025
Accepted 21.04.2025
About the Authors
Zumriiat F. Akhmedova, PhD student at the Prof. B.V. Leonov Department for Assisted Technologies in Infertility Treatment, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, zyuka-1997@mail.ru,https://orcid.org/0000-0002-4483-8820
Anastasia P. Sysoeva, PhD, Clinical Embryologist at the Prof. B.V. Leonov Department for Assisted Technologies in Infertility Treatment, Academician V.I. Kulakov
National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4,
a_sysoeva@oparina4.ru, https://orcid.org/0000-0002-6502-4498
Maxim Yu. Gavrilov, Junior Researcher at the Prof. B.V. Leonov Department of Assistive Technologies in Infertility Treatment, Academician V.I. Kulakov National
Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, maxgavr67@gmail.com,
https://orcid.org/0000-0001-6189-0287
Boris V. Zingerenko, Junior Researcher at the Prof. B.V. Leonov Department of Assistive Technologies in Infertility Treatment, Academician V.I. Kulakov National
Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, b_zingerenko@oparina4.ru,
https://orcid.org/0000-0002-8784-5502
Yulia A. Shevtsova, Junior Researcher at the Cell Technologies Laboratory, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, u_shevtsova@oparina4.ru
Denis N. Silachev, Dr. Bio. Sci., Head of the Cell Technologies Laboratory, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, d_silachev@oparina4.ru, https://orcid.org/0000-0003-0581-9755
Oksana S. Nepsha, PhD, Researcher at the Prof. B.V. Leonov Department of Assistive Technologies in Infertility Treatment, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, o_nepsha@oparina4.ru,
https://orcid.org/0000-0002-9988-2810
Natalya P. Makarova, Dr. Bio. Sci., Leading Researcher at the Prof. B.V. Leonov Department of Assistive Technologies in Infertility Treatment, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4,
np_makarova@oparina4.ru, https://orcid.org/0000-0003-1396-7272
Elena V. Kulakova, Dr. Med. Sci., Senior Researcher at the Prof. B.V. Leonov Department of Assistive Technologies in Infertility Treatment, Academician V.I. Kulakov
National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4,
e_kulakova@oparina4.ru, https://orcid.org/0000-0002-4433-4163
Elena A. Kalinina, Dr. Med. Sci., Professor, Head of the Prof. B.V. Leonov Department of Assistive Technologies in Infertility Treatment, Academician V.I. Kulakov
National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4,
e_kalinina@oparina4.ru, https://orcid.org/0000-0002-8922-2878



