Experience of myo-inositol use in patients of advanced reproductive age undergoing assisted reproduction
Saraeva N.V., Tugushev M.T., Shurygina O.V., Montanino Oliva M., Levin V.A., Devyatov I.M.
The effectiveness of infertility treatment using assisted reproductive technologies (ART) is lower in patients of advanced reproductive age compared to women under 35 years of age.
Objective: To evaluate the outcomes of in vitro fertilization (IVF) programs after administration of the combination of myo-inositol (MI) + α-lactalbumin (α-LA) in the preconception period in patients of advanced reproductive age.
Materials and methods: A total of 167 patients were selected for the study according to the inclusion criteria. Group 1 consisted of 68 patients who received a combination of MI 600 mg + α-LA + folic acid 200 mcg
(Inofert Forte) 1 capsule twice a day, orally, 3 months before the IVF program. Group 2 included 99 patients who received folic acid 400 mcg per day, orally, for 3 months. Controlled ovarian stimulation was performed according to the standard protocol in both groups.
Results: The average total dose of gonadotropins per treatment cycle of ovarian stimulation was lower in the main group and it was 2000 (1575; 2450) IU versus 2150 (1900; 2500) IU in the control group (p=0.037). The percentage of embryos reaching the blastocyst stage in the main group was significantly higher than in the control group and it was 69.1% versus 50.1% (p<0.001); the average number of obtained blastocysts was also significantly higher in the main group, namely 2.82 (2.8) (95% CI: 2.15–3.5) versus 2.01 (1.9) (95% CI: 1.63–2.39), respectively (p=0.026). The clinical pregnancy rate in the frozen-thawn embryo transfer cycles was significantly higher in the main group and it was 13/18 (72.2%) versus 7/22 (31.8%) in the control group (p=0.028). The outcome measure for achieving clinical pregnancy was 56.3% in the main group and 38.3% in the control group (p=0.32). The present study found that the likelihood of pregnancy resulting from the transfer of frozen-thawed embryos was 3.1 times higher after MI + α-LA supplementation in the preconception period of an IVF program than in the absence of such supplementation (95% CI: 1.2–8.3). The chance of pregnancy ending in childbirth was 3 times higher (95% CI: 1.05-8.53) with MI supplementation before IVF (p=0.04).
Conclusion: The combination of MI 600 mg + α-LA + folic acid 200 µg (Inofert Forte) may be a simple and effective solution when preparing patients of advanced reproductive age for IVF programs since it helps to reduce the total dose of gonadotropins, improve embryological parameters and increase the effectiveness of ART programs.
Authors' contributions: Saraeva N.V., Tugushev M.T., Montanino Oliva M. – developing the concept and design of the study; Saraeva N.V., Tugushev M.T. – collecting and processing the material; Tugushev M.T., Levin V.A. – statistical processing of the data; Tugushev M.T., Shurygina О.V., Devyatov I.M. – writing the text; Shurygina О.V., Levin V.A. – editing the article.
Conflicts of interest: The authors declare no possible conflicts of interest.
Funding: The study was conducted without sponsorship.
Ethical Approval: The study was approved by the Ethical Review Board of the Samara State Medical University, Ministry of Health of Russia.
Patient Consent for Publication: The patients signed informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Saraeva N.V., Tugushev M.T., Shurygina O.V., Montanino Oliva M., Levin V.A., Devyatov I.M.
Experience of myo-inositol use in patients of advanced reproductive age undergoing assisted reproduction.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (4): 104-111 (in Russian)
https://dx.doi.org/10.18565/aig.2025.99
Keywords
A review of the existing literature, as well as national and international registries of assisted reproductive technologies (ART), shows a change in the age profile of patients over the last 10–15 years [1, 2]. The number of patients of advanced reproductive age who seek infertility treatment using ART methods is increasing [3]. Evidence suggests that the rise in the proportion of women aged 35 years and over reduces the effectiveness of treatment and results in a higher incidence of early pregnancy loss after in vitro fertilization (IVF) programs [4, 5]. In addition, according to the literature, there is an increased risk of pregnancy complications in this group of patients due to a higher incidence of somatic pathology [6].
Current publications discuss the use of antioxidant therapy in patients of advanced reproductive age, in patients with decreased ovarian reserve, poor response to ovarian stimulation and poor embryo quality in ART programs. The use of plant polyphenols [4, 7, 8], melatonin [9], vitamins C and E [10], coenzyme Q10 [11] is discussed as an additional therapy. A number of foreign and Russian scientific publications are devoted to the role of myo-inositol (MI) in the treatment of women with poor ovarian response to stimulation and poor oocyte and embryo quality [12-14]. Inositol is a polyalcohol that has nine stereoisomers. Two of them have been shown to mediate the post-receptor effects of insulin: MI and D-chiro-inositol. Due to its antioxidant action (increase in the activity of superoxide dismutase, catalase and glutathione), MI improves cell morphology and growth, and affects the synthesis of lipids that make up cell membranes [15, 16]. MI is the most prevalent isomer of inositol in the human body. Inositol-dependent signals are of importance during the final stages of oocyte maturation when preparing for successful oocyte activation at the time of fertilization. An increase in the concentration of MI in follicular fluid during the pre-ovulatory and ovulatory periods is necessary for full follicular maturation. Its high concentration is one of the markers of good oocyte quality [17, 18]. During oocyte maturation, MI derivatives play a primary role in the formation of calcium-mediated signals from the gonadotropin-releasing hormone receptor, luteinizing and follicle-stimulating hormones. This, in turn, results in the indirect regulation of ovarian function, affecting oocyte quality, trophoblast invasion processes during blastocyst attachment and placental function.
MI bioavailability is particularly important since there have been the cases of resistance to this substance in patients diagnosed with metabolic syndrome and hypoestrogenic conditions associated with advanced reproductive age. This resistance has been linked to reduced microbial diversity within the intestines and lower genital tract. The gut microbiota, consisting of about 10 trillion bacteria, is the collection of microbes that colonize the human gut. The vaginal microbiota is usually dominated by Lactobacillus spp. By producing lactic acid, which lowers the pH, these bacteria protect the vagina from colonization by pathogenic bacteria. They also produce antimicrobial compounds and modulate the immunological and physical characteristics of the cervical and vaginal mucosa. Recently, there has been a suggestion that estrogen concentrations may influence the amount of Lactobacillus spp. in the vaginal microbiota. The vaginal microbiota in hypoestrogenic conditions is represented by increasing concentrations of anaerobic and aerobic bacteria. Microbiota has also been found to play a causal role in the development of metabolic diseases such as diabetes and obesity [16, 19, 20]. Alpha-lactalbumin (α-LA) has been shown to be effective in overcoming MI resistance. This protein within the milk of mammals improves the MI availability. α-LA is a factor that improves nutrient absorption in the intestines because the α-LA molecule is characterized by water solubility and thermostability [21]. Given that the quality and quantity of oocytes inevitably decrease with age, the rational stage of preparation for IVF treatment in patients of advanced reproductive age would be measures aimed at improving the quality of gametes, namely the administration of the combination of MI + α-LA, taking into account its high bioavailability.
The aim of the study was to evaluate the outcomes of ART programs in patients of advanced reproductive age after administration of the combination of MI 600 mg + α-LA + folic acid 200 µg in the preconception period.
Materials and Methods
The study included 167 patients who underwent infertility treatment using assisted reproduction on the premises of the Mother and Child Group of Companies in 2022–2023.
There were the following inclusion criteria: age of patients from 35 to 40 years, infertility, IVF treatment using own oocytes, absence of severe male factor infertility, embryo transfer in stimulated cycles (at the cleavage or blastocyst stage), cycles of frozen-thawed embryo transfer (at the blastocyst stage), absence of severe adenomyosis, absence of polycystic ovary syndrome (PCOS), endometrial thickness of 8 mm or more on days 11–14 of the menstrual cycle in the embryo transfer cycle.
There were the following exclusion criteria: age of patients younger than 35 years or 40 years and older, IVF treatment using donor oocytes, severe male factor infertility (oligozoospermia ≤1 million/ml), severe adenomyosis, PCOS, endometrial thickness less than 8 mm on days 11–14 of the menstrual cycle in the embryo transfer cycle.
The main group consisted of 68 patients who received MI 600 mg + α-LA + folic acid 200 mcg (Inofert Forte), one capsule twice daily, orally, three months before the IVF program. The control group consisted of 99 patients who were prescribed folic acid 400 mcg daily, orally, for three months. In both groups, controlled ovarian stimulation was performed according to a standard protocol with gonadotropin-releasing hormone antagonists (Ganirelix 0.25 mg). Human menopausal gonadotropin (Menopur), recombinant follicle-stimulating hormone (Primapur; Puregon) were used as gonadotropic hormones. The dose of gonadotropic preparations was selected individually taking into account the parameters of ovarian reserve, age and weight. Ovulation trigger was administered when ≥3 follicles ≥18 mm in diameter were present. Human chorionic gonadotropin 10,000 IU and recombinant human chorionic gonadotropin (Ovitrel 6500 IU) were used as ovulation triggers. Follicle puncture was performed 36 h after ovulation triggering. In both groups, embryos were cultured in universal G-TL medium (Virtolife, Sweden) using COOK mini-incubators. Embryo transfer was performed on the 3rd or 5th day of culturing, provided the endometrial thickness was 8 mm or more on the day of embryo transfer into the uterine cavity. Embryo quality was assessed on the 5th day of culturing, 116–118 h after fertilization. Embryo quality was evaluated using the alphanumeric system developed by Gardner D.K. and Schoolcraft W.B. (1999) [22]. From the day of follicle puncture, all patients were prescribed luteal phase support with micronized progesterone at a dosage of 400 mg/day (Iprozhin), vaginally. The transfer of frozen-thawed embryos was performed in a modified cycle: estradiol valerate was administered at a dose of 4–6 mg/day orally (Proginova) from the 5th day of the cycle. Micronized progesterone at a dosage of 600 mg/day (Iprozhin) was administered vaginally during the post-transfer period from the moment of embryo transfer into the uterine cavity and further until 12 weeks of gestation if pregnancy occurred [23].
Statistical analysis
Mathematical processing of the results was performed using the statistical package SPSS21, license number 20130626-3 (IBM Company; USA) and Microsoft Excel (Microsoft; USA). Normality of distribution was determined using the Kolmogorov-Smirnov test with Lilliefors correction. The distribution is considered non-normal if p-value is less than 0.05 (because the differences between distribution and normal distribution are statistically significant); the distribution is considered normal if p-value is greater than 0.05 (because the differences between the distribution in the study and the normal distribution are insignificant). Quantitative variables that had normal distribution were described using arithmetic mean (M), standard deviation (SD). In case of distribution other than normal, median (Me), interquartile range (Q1; Q3) were used to describe quantitative variables. Categorical data were presented using absolute values (n) and percentages (%). Two groups were compared on quantitative variables that had normal distribution using the Student’s t-test. In the case of quantitative variables that had a distribution different from normal, two groups were compared using the Mann–Whitney test. The comparison of two groups was performed using the Fisher’s exact test if the minimum expected number of observations was less than 10, or the Pearson’s chi-squared test if the minimum expected number of observations was greater than 10. Differences were considered statistically significant at p<0.05.
If the frequency of events in each group was not equal to 0 or 100%, an odds ratio (OR) with 95% confidence interval (CI) was calculated. The OR was defined as the ratio of the odds of an event occurring in the group exposed to a given risk factor to the odds of an event occurring in the group that is not exposed to that factor.
Results
The women of both groups were compared in age, 37.4 (1.87) and 37.0 (1.53) years, respectively (p=0.28); in body mass index 23.2 (2.27) and 22.8 (3.75) kg/m2, respectively (p=0.32); in order number of the present ART program, 2.0 (1.76) in the main group and 2.42 (2.02) in the control group (p=0.096); in mean duration of infertility, 6.18 (4.33) years in the main group and 6.66 (4.39) years in the control group (p=0.501).
The mean total dose of gonadotropins per treatment cycle of ovarian stimulation differed significantly between the study groups and was 2000 (1575; 2450) IU in the main group versus 2150 (1900; 2500) IU in the control group (p=0.037).
The main parameters and the embryological outcomes of the ART programs are presented in Table 1.

The frequency of normal fertilization in the main group and control group was 58/68 (85.3%) and 73/99 (73.7%), respectively (p=0.085). The frequency of embryos growing to the blastocyst stage in the main group was significantly higher than in the control group, 68.9% (40/58) and 49.3% (37/73), respectively (p<0.001); the mean number of blastocysts obtained in the groups was 2.82 (2.8) and 2.01 (1.9), respectively (p=0.026). The number of cycles without embryos (no fertilization, embryonic developmental arrest) did not differ significantly between groups: 9/68 cycles (13.2%) in the main group and 18/99 cycles (18.2%) in the control group (p=0.522).
The rate of patients with embryo transfer in a stimulated cycle was 52.9% (36/68 cycles) in the main group and 59.6% (59/99 cycles) in the control group (p=1.000). Due to the absence of other embryos in 4/68 (5.9%) patients of the main group, embryo transfer was performed at the cleavage stage in 14/99 (14.1%) patients of the control group (p=0.30). The rate of segmented cycles and cryopreservation of all embryos was 38.9% (23/59 cycles) in the main group and 27.1% (22/81 cycles) in the control group (p=0.139). There were the following indications for segmentation of the treatment cycle: endometrial thickness after follicle puncture was less than 7 mm in 31.0% of patients in the main group (18/58 cycles), and women in 8.6% of cases (5/58 cycles) complained of symptoms of acute respiratory disease prior to the day of embryo transfer; in the control group, endometrial thickness after follicle puncture was less than 7 mm in 20.1% of patients (17/81 cycles), and women in 4.9% of cases (4/81 cycles) complained of symptoms of acute respiratory disease prior to the day of embryo transfer, one case was due to a woman’s health condition (on the 3rd day after follicle puncture she suffered a musculoskeletal injury).
The rate of cycles with embryo transfer and cryopreservation of the remaining embryos of good and excellent quality was 38.9% (14/36 cycles) in the main group and 42.4% (25/59 cycles) in the control group (p=0.631). The mean number of embryos per transfer was 1.5 in the main group and 1.35 in the control group (p=0.09).
The results of embryo transfer into the uterine cavity in infertility treatment in the study groups are presented in Table 2.
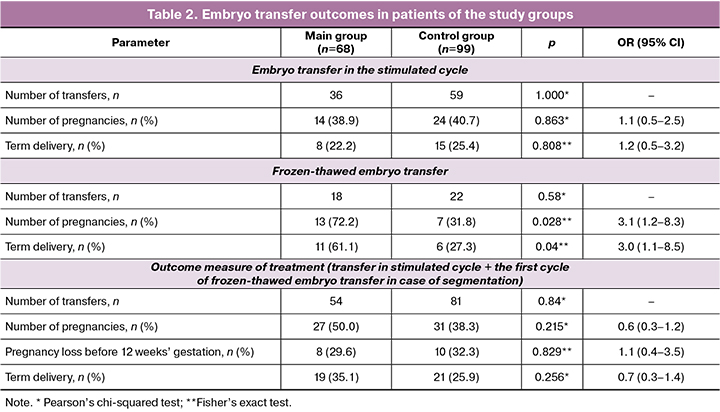
The clinical pregnancy rate for embryo transfer in a stimulated cycle was 38.9% in the main group (14 pregnancies in 36 cycles) and 40.7% in the control group (24 pregnancies in 59 cycles) (p=0.863).
In case of segmentation of the treatment cycle, only the first cycle with the transfer of frozen-thawed embryos was taken into account for further evaluation of treatment efficacy. The clinical pregnancy rate for frozen-thawed embryo transfer was 72.2% in the main group (13 pregnancies per 18 cycles of cryopreservation) and 31.8% in the control group (7 pregnancies per 22 cycles) (p=0.028). Based on the results, it was found that the likelihood of pregnancy resulting from the transfer of frozen-thawed embryos was 3.1 times higher if the patients received MI when preparing for an IVF program compared to those who did not receive it (95% CI: 1.2–8.3).
The outcome measure for achieving clinical pregnancy was 50.0% (27 pregnancies per 54 cycles) in the main group and 38.3% (31 pregnancies per 81 cycles) in the control group (p=0.32). The pregnancy ended in term delivery in 35.1% (19/54) of cases in the main group and 25.9% (21/81) of cases in the control group (p=0.36). When comparing the rate of births after transfer of frozen-thawed embryo into the uterine cavity depending on the presence/absence of MI prescription at the preconception stage, statistically significant differences were obtained (p=0.04). Thus, the likelihood of pregnancy ending in labor was 3 times higher (95% CI: 1.05-8.53) when MI was administered before IVF.
Discussion
It is evident from the current publications that MI plays a significant role in human reproduction, particularly in oogenesis, lipid synthesis, cell membrane structure and cell growth [15, 18]. There is a growing body of data on the effectiveness of using MI in patients diagnosed with PCOS, both for achieving spontaneous pregnancy and in stimulation cycles in ART programs [15, 16]. There is little information on the use and effects of MI in women of advanced reproductive age who do not have PCOS. The data on the benefits of MI in young women with decreased ovarian reserve after ovarian surgery have been presented in a few publications by Russian researchers (14). It is obvious that MI plays an important role as a secondary messenger in the process of oocyte maturation, and consequently, it can increase the clinical pregnancy rate. Therefore, the objective of this study was to evaluate the benefits of administering the biological supplement specifically in patients of advanced reproductive age without PCOS.
Some researchers have reported a decrease in the total dose of gonadotropins in ovarian stimulation programs, including the programs of the patients without PCOS when they are administered MI before ART [24, 25]. Laganà A. et al. (2018) in a systematic review of randomized controlled trials (RCTs) (8 RCTs, 812 patients), evaluated the total dose of gonadotropins and the duration of ovarian stimulation in women with and without PCOS in ART programs and concluded that MI was effective in both women with and without PCOS in case of reducing the total dose of gonadotropins, but the duration of ovarian stimulation was effectively reduced only in women with PCOS [26]. According to the results presented in a study by Simi G. et al. (2017), the administration of MI for 3 months prior to ovarian stimulation led to a significant improvement in hormonal responses, a decrease in the amount of follicle-stimulating hormone preparation, and improvement in the quality of oocytes due to a decrease in the number of degenerated and immature forms of oocytes [25]; these findings are consistent with the results of our study. Thus, the mean total dose of gonadotropins per treatment cycle in the main group was 2000 (1575; 2450) IU, while in the control group it was 2150 (1900; 2500) IU (p=0.037). This means that follicle growth requires a significantly lower dose of gonadotropins in the main group compared to the control group.
Nazari L. et al. (2020) demonstrated in their RCT that receiving MI one month before the start of ART treatment by the patients with ‘poor response’ in ovarian stimulation programs, with fertilization by intracytoplasmic sperm injection (ICSI) can improve embryological outcomes: it can improve fertilization rates and embryo quality, and increase the cumulative pregnancy rate [27]. The results of another double-blind placebo-controlled RCT showed that the number of obtained oocytes, the number of MII oocytes, the number of transferred embryos, the pregnancy rate per embryo transfer, and the rate of achieving clinical pregnancy did not show statistical significance in the group with MI prescription [28]; these results are inconsistent with our data. Our study showed that the number of mature oocytes collected for fertilization was significantly higher in the main group and was 5.18 (3.75) (95% CI: 4.27–6.08) versus 4.1 (2.56) (95% CI: 3.57–4.62) in the control group (p=0.04). The percentage of embryos growing to the blastocyst stage in the main group was 69.1% and it was also significantly higher than in the control group, namely 50.1% (p<0.001).
In a double-blind placebo-controlled RCT conducted by Seyedoshohadaei F. et al. (2022), the mean number of oocytes, MII oocytes and 2PN embryos was significantly higher in the MI group; moreover, the rate of clinical pregnancy and live birth was significantly higher in this group than in the control group (p=0.04). The researchers concluded that MI administration may increase the rate of clinical pregnancy and live birth by increasing the total number of oocytes and MII oocytes in infertile women undergoing ICSI [29]. In our study, the mean number of blastocysts obtained in groups was 2.82 (2.8) (95% CI: 2.15–3.5) versus 2.01 (1.9) (95% CI: 1.63–2.39), respectively (p=0.026). We detected significant differences in the effectiveness of the IVF program using such a criterion as the presence/absence of MI administration prior to IVF. The outcome measure for achieving clinical pregnancy was not significantly different between the groups and was 46.6% in the main group and 38.3% in the control group (p=0.32). Pregnancy ended in term delivery in 35.1% of cases in the main group and 25.9% of cases in the control group (p=0.36). The lack of differences between the two groups in clinical pregnancy rate, delivery rate and early pregnancy loss in our study may be due to the limitations of the study, namely small sample size. At the same time, the results of our study showed that the likelihood of pregnancy resulting from frozen-thawed embryo transfer was 3.1 times higher when the patients received MI at the preparatory stage for IVF program in comparison with the patients who did not receive it (95% CI: 1.2–8.3). The comparison of the frequency of births after the transfer of frozen-thawed embryo into the uterine cavity depending on the presence/absence of MI addition at the preconception stage also showed statistically significant differences. Thus, the likelihood of pregnancy ending in childbirth was 3 times higher (95% CI: 1.05-8.53) when MI was administered before IVF (p=0.04).
Conclusion
It should be noted that when preparing women of advanced reproductive age for IVF treatment, the administration of MI + α-LA may reduce the total dose of gonadotropins in the ovarian stimulation program, improve embryological outcomes (more mature oocytes and more embryos growing to the blastocyst stage) and increase the effectiveness of ART programs in this cohort of patients. The results of our study demonstrate that the combination of MI 600 mg + α-LA + folic acid 200 mcg (Inofert Forte) can be a simple and effective solution to prepare the patients of advanced reproductive age for ART treatment. However, it is important to note that the results of this study should be interpreted carefully because of its limitations. Further large multicenter randomized controlled trials are required to provide more evidence on this issue.
References
- Российская Ассоциация Репродукции Человека (РАРЧ). Регистр ВРТ. Отчет за 2022 год. Доступно по: https://www.rahr.ru/d_registr_otchet/RegistrVRT_2022.pdf [Russian Association for Human Reproduction (RAHR). ART register. 2022 report. Available at: https://www.rahr.ru/d_registr_otchet/RegistrVRT_2022.pdf (in Russian)].
- Smeenk J., Wyns C., De Geyter C., Kupka M., Bergh C., Cuevas Saiz I. et al.; European IVF Monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). ART in Europe, 2019: results generated from European registries by ESHRE. Hum. Reprod. 2023; 38(12): 2321-38. https://dx.doi.org/10.1093/humrep/dead197.
- Машаева Р.И., Марченко Л.А., Гус А.И., Костюков К.В. Овариальный возраст – ранний маркер преждевременной недостаточности яичников. Акушерство и гинекология. 2025; 3: 120-7. [Mashaeva R.I., Marchenko L.A., Gus A.I., Kostyukov K.V. Ovarian age – an early marker of premature ovarian insufficiency. Obstetrics and Gynecology. 2025; (3): 120-7 (in Russian)]. https://dx.doi.org/10.18565/aig.2024.271.
- Havrljenko J., Kopitovic V., Pjevic A.T., Milatovic S., Pavlica T., Andric N. et al. The prediction of IVF outcomes with autologous oocytes and the optimal MII oocyte/embryo number for live birth at advanced maternal age. Medicina (Kaunas). 2023; 59(10): 1799. https://dx.doi.org/10.3390/medicina59101799.
- Назаренко T.А., Пестова Т.И., Локшин В.Н., Джусубалиева Т.М., Серов В.Н., Баранов И.И., Беженарь В.Ф., Гависова А.А., Городнова Е.А., Долгушина Н.В., Калугина А.С., Квашнина Е.В., Коган И.Ю., Колода Ю.А., Корсак В.С., Краснопольская К.В., Молчанова И.В., Сабирова В.Л., Тапильская Н.И., Сухих Г.Т. Предикторы частоты наступления беременности при вспомогательных репродуктивных технологиях: результаты исследовательской программы «ИРИС» в популяции России и Казахстана. Акушерство и гинекология. 2025; 3: 144-58. [Nazarenko T.A., Pestova T.I., Lokshin V.N., Dzhusubalieva T.M., Serov V.N., Baranov I.I., Bezhenar V.F., Gavisova A.A., Gorodnova E.A., Dolgushina N.V., Kalugina A.S., Kvashnina E.V., Kogan I.Yu., Koloda Yu.A., Korsak V.S., Krasnopolskaya K.V., Molchanova I.V., Sabirova V.L., Tapilskaya N.I., Sukhikh G.T. Predictors of pregnancy rate in assisted reproductive technologies: results of the IRIS observational program in the population of Russia and Kazakhstan. Obstetrics and Gynecology. 2025; (3): 144-58 (in Russian)]. https://dx.doi.org/10.18565/aig.2025.82.
- Киракосян Е.В., Назаренко Т.А., Павлович С.В. Поиск причин формирования нарушений репродуктивной системы: обзор научных исследований. Акушерство и гинекология. 2021; 11: 18-25. [Kirakosyan E.V., Nazarenko T.A., Pavlovich S.V. Search for the causes of reproductive system disorders: a research review. Obstetrics and Gynecology. 2021; (11): 18-25 (in Russian)]. https://dx.doi.org/10.18565/aig.2021.11.18-25.
- Gou M., Li J., Yi L., Li H., Ye X., Wang H. et al. Reprogramming of ovarian aging epigenome by resveratrol. PNAS Nexus. 2022; 2(2): 310. https://dx.doi.org/10.1093/pnasnexus/pgac310.
- Yang S., Shao S., Huang B., Yang D., Zeng L., Gan Y. et al. Tea polyphenols alleviate tri-ortho-cresyl phosphate-induced autophagy of mouse ovarian granulosa cells. Environ. Toxicol. 2020; 35(4): 478-86. https://dx.doi.org/10.1002/tox.22883.
- Тamura H., Jozaki M., Tanabe M., Shirafuta Y., Mihara Y., Shinagawa M. et al. Importance of melatonin in assisted reproductive technology and ovarian aging. Int. J. Mol. Sci. 2020; 21(3): 1135. https://dx.doi.org/10.3390/ijms21031135.
- Abdollahifar M.A., Azad N., Sajadi E., Shams Mofarahe Z., Zare F., Moradi A. et al. Vitamin C restores ovarian follicular reservation in a mouse model of aging. Anat. Cell. Biol. 2019; 52(2): 196-203. https://dx.doi.org/10.5115/acb.2019.52.2.196.
- Blumenfeld Z. What is the best regimen for ovarian stimulation of poor responders in ART/IVF? Front. Endocrinol. (Lausanne). 2020; 11: 192. https://dx.doi.org/10.3389/fendo.2020.00192.
- Zheng X., Lin D., Zhang Y., Lin Y., Song J., Li S. et al. Inositol supplement improves clinical pregnancy rate in infertile women undergoing ovulation induction for ICSI or IVF-ET. Medicine (Baltimore). 2017; 96(49): e8842. https://dx.doi.org/10.1097/MD.0000000000008842.
- Caprio F., D'Eufemia M.D., Trotta C., Campitiello M.R., Ianniello R., Mele D. et al. Myo-inositol therapy for poor-responders during IVF: a prospective controlled observational trial. J. Ovarian Res. 2015; 8: 37. https://dx.doi.org/10.1186/s13048-015-0167-x.
- Вартанян Э.В., Цатурова К.А., Девятова Е.А., Михайлюкова А.С., Левин В.А., Сагамонова К.Ю., Громенко Д.С., Овсянникова Т.В., Эрлихман Н.М., Колосова Е.А., Сафронова Е.В., Фотина О.В., Красновская Е.В., Пожарищенская Т.Г., Аутлева С.Р., Гзгзян А.М., Нуриев И.Р., Воропаева Е.Е., Пестова Т.И., Здановский В.М., Ким Н.А., Котельников А.Н., Сафронов О.В., Назаренко Т.А., Ионова Р.М. Подготовка к лечению бесплодия методом экстракорпорального оплодотворения при сниженном овариальном резерве. Акушерство и гинекология. 2019; 8: 134-42. [Vartanyan E.V., Tsaturova K.A., Devyatova E.A., Mikhailyukova A.S., Levin V.A., Sagamonova K.Yu., Gromenko D.S., Ovsyannikova T.V., Erlikhman N.M., Kolosova E.A., Safronova E.V., Fotina O.V., Krasnovskaya E.V., Pozharischenskaya T.G., Autleva S.R., Gzgzyan A.M., Nuriev I.R., Voropaeva E.E., Pestova T.I., Zdanovsky V.M., Kim N.A., Kotelnikov A.N., Safronov O.V., Nazarenko T.A., Ionova R.M. Preparation for the in vitro fertilization treatment of infertility in diminished ovarian reserve. Obstetrics and Gynecology. 2019; (8): 134-42 (in Russian)]. https://dx.doi.org/10.18565/aig.2019.8.134-142.
- Merviel P., James P., Bouée S., Le Guillou M., Rince C., Nachtergaele C. et al. Impact of myo-inositol treatment in women with polycystic ovary syndrome in assisted reproductive technologies. Reprod. Health. 2021; 18(1): 13. https://dx.doi.org/10.1186/s12978-021-01073-3.
- Пустотина О.А. Инозитол и липоевая кислота в лечении инсулинорезистентности у женщин с синдромом поликистозных яичников. Акушерство и гинекология. 2020; 12: 209-16. [Pustotina O.A. Inositol and lipoic acid in the treatment of insulin resistance in women with polycystic ovary syndrome. Obstetrics and Gynecology. 2020; (12): 209-16 (in Russian)]. https://dx.doi.org/10.18565/aig.2020.12.209-216.
- Chiu T.T., Rogers M.S., Law E.L., Briton-Jones C.M., Cheung L.P., Haines C.J. Follicular fluid and serum concentrations of myo-inositol in patients undergoing IVF: relationship with oocyte quality. Hum. Reprod. 2002; 17(6): 1591-6. https://dx.doi.org/10.1093/humrep/17.6.1591.
- Квашнина Е.В., Гвоздикова Т.В., Дружинина А.Ю., Мастерова И.А., Мурунова С.В., Плотавская Т.Б., Тутаков М.А., Павлюченкова С.М., Шилова Н.В., Дикке Г.Б. Роль мио-инозитола в подготовке женщин к программам вспомогательных репродуктивных технологий. Акушерство и гинекология. 2020; 11: 139-46. [Kvashnina E.V., Gvozdikova T.V., Druzhinina A.Yu., Masterova I.A., Murunova S.V., Plotavskaya T.B., Tutakov M.A., Pavlyuchenkova S.M., Shilova N.V., Dikke G.B. The role of myo-inositol in preparing women for assisted reproductive technologies. Obstetrics and Gynecology. 2020; (11): 139-46 (in Russian)]. https://dx.doi.org/10.18565/aig.2020.11.139-146.
- Alessandri G., Mancabelli L., Fontana F., Lepore E., Forte G., Burratti M. et al. Disclosing α-lactalbumin impact on the intestinal and vaginal microbiota of women suffering from polycystic ovary syndrome. Microb. Biotechnol. 2024; 17(10): e14540. https://dx.doi.org/10.1111/1751-7915.14540.
- Yang X., Chang T., Yuan Q., Wei W., Wang P., Song X. et al. Changes in the composition of gut and vaginal microbiota in patients with postmenopausal osteoporosis. Front. Immunol. 2022; 13: 930244. https://dx.doi.org/10.3389/fimmu.2022.
- Kamenov Z., Gateva A., Dinicola S., Unfer V. Comparing the efficacy of myo-inositol plus α-lactalbumin vs. myo-inositol alone on reproductive and metabolic disturbances of polycystic ovary syndrome. Metabolites. 2023; 13: 717. https://dx.doi.org/10.3390/metabo13060717.
- Gardner D.K., Schoolcraft W.B. Culture and transfer of human blastocysts. Curr. Opin. Obstet. Gynecol. 1999; 11(3): 307-11. https://dx.doi.org/10.1097/00001703-199906000-00013.
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Женское бесплодие. 2024. [Ministry of Health of the Russian Federation. Clinical guidelines. Female infertility. 2024. (in Russian)].
- Lisi F., Carfagna P., Oliva M.M., Rago R., Lisi R., Poverini R. et al. Pretreatment with myo-inositol in non-polycystic ovary syndrome patients undergoing multiple follicular stimulation for IVF: a pilot study. Reprod. Biol. Endocrinol. 2012; 10: 52. https://dx.doi.org/10.1186/1477-7827-10-52.
- Simi G., Genazzani A.R., Obino M.E., Papini F., Pinelli S., Cela V. et al. Inositol and in vitro fertilization with embryo transfer. Int. J. Endocrinol. 2017; 2017: 5469409. https://dx.doi.org/10.1155/2017/5469409.
- Laganà A.S., Vitagliano A., Noventa M., Ambrosini G., D'Anna R. Myo-inositol supplementation reduces the amount of gonadotropins and length of ovarian stimulation in women undergoing IVF: a systematic review and meta-analysis of randomized controlled trials. Arch. Gynecol. Obstet. 2018; 298(4): 675-84. https://dx.doi.org/10.1007/s00404-018-4861-y.
- Nazari L., Salehpour S., Hosseini S., Saharkhiz N., Azizi E., Hashemi T. et al. Effect of myo-inositol supplementation on ICSI outcomes among poor ovarian responder patients: A randomized controlled trial. J. Gynecol. Obstet. Hum. Reprod. 2020; 49(5): 101698. https://dx.doi.org/10.1016/j.jogoh.2020.101698.
- Mohammadi S., Eini F., Bazarganipour F., Taghavi S.A., Kutenaee M.A. The effect of Myo-inositol on fertility rates in poor ovarian responder in women undergoing assisted reproductive technique: a randomized clinical trial. Reprod. Biol. Endocrinol. 2021; 19(1): 61. https://dx.doi.org/10.1186/s12958-021-00741-0.
- Seyedoshohadaei F., Abbasi S., Rezaie M., Allahvaisi A., Jafar Rezaie M., Soufizadeh N. et al. Myo-inositol effect on pregnancy outcomes in infertile women undergoing in vitro fertilization/intracytoplasmic sperm injection: A double-blind RCT. Int. J. Reprod. Biomed. 2022; 20(8): 643-50. https://dx.doi.org/10.18502/ijrm.v20i8.11753.
Received 09.04.2025
Accepted 21.04.2025
About the Authors
Natalya V. Saraeva, PhD, Associate Professor at the Department of Reproductive Medicine, Clinical Embryology and Genetics, Samara State Medical University,Ministry of Health of Russia, 443099, Russia, Samara, Chapaevskaya str., 89; Head of the Department, IDK Medical Company, Mother and Child Group of Companies,
443072, Russia, Samara, Volzhskoye Shosse, 70, +7(000)000-00-00, kuzichkina@gmail.com, https://orcid.org/0000-0003-0222-1803
Marat T. Tugushev, PhD, Head of the Department of Reproductive Medicine, Clinical Embryology and Genetics, Samara State Medical University, Ministry of Health of Russia, 443099, Russia, Samara, Chapaevskaya str., 89; Chief Physician, IDK Medical Company, Mother and Child Group of Companies, 443072, Russia, Samara, Volzhskoye Shosse, 70, https://orcid.org/0000-0002-3328-3217
Oksana V. Shurygina, Dr. Med. Sci., Professor, Department of Reproductive Medicine, Clinical Embryology and Genetics, Samara State Medical University, Ministry of Health
of Russia, 443099, Russia, Samara, Chapaevskaya str., 89; Head of the ART Laboratory, IDK Medical Company, Mother and Child Group of Companies,
443072, Russia, Samara, Volzhskoye Shosse, 70, https://orcid.org/0000-0002-1413-3328
Mario Montanino Oliva, Dr. Med. Sci., Professor, Scientific Consultant in Obstetrics and Gynecology, Clinica Santo Spirito, Rome, Italy,
https://orcid.org/0000-0001-8676-1465
Vitaly A. Levin, endocrinologist, Ornament Health AG, Lucerne, Switzerland, https://orcid.org/0009-0000-8758-0842
Ilya M. Devyatov, student of the Medical Institute, Patrice Lumumba Peoples’ Friendship University of Russia, 117198, Russia, Moscow, Miklukho-Maklaya str., 6,
https://orcid.org/0000-0002-6025-8148
Corresponding author: Natalya V. Saraeva, kuzichkina@gmail.com



