Use of extracellular vesicles from multipotent mesenchymal stromal cells in preparation for in vitro fertilization programs in patients with diminished ovarian reserve
Martirosyan Ya.O., Biryukova A.M., Silachev D.N., Popov K.V., Nazarenko T.A., Sukhikh G.T.
Objective: To evaluate the clinical efficacy of intraovarian injection of extracellular vesicles isolated from placental multipotent mesenchymal stromal cells in patients with diminished ovarian reserve undergoing in vitro fertilization (IVF).
Materials and methods: This prospective randomized controlled trial (open-label design) included 100 women with a diminished ovarian reserve. Patients randomized to the study group (n=50) received intraovarian injections of extracellular vesicles before stimulation, whereas those in the control group (n=50) underwent standard stimulation. The primary outcome was the clinical pregnancy rate, and secondary outcomes included the number of retrieved and mature oocytes, number of 2PN zygotes, blastocysts, blastulation rate, and live births.
Results: The study group showed a statistically significant increase in the number of mature oocytes (4.1 (1.3) vs. 1.5 (1.0), p<0.001), blastocysts (1.4 (0.6) vs. 0.8 (0.4), p<0.001), and blastulation rate (58.2% vs. 31.1%, p=0.008). The clinical pregnancy rate was 24% in the study group compared to 6% in the control group (RR=4.0; 95% CI 1.2–13.3), and the live birth rate was 18% in the study group and 2% in the control group (RR=9.0; 95% CI 1.2–68.4).
Conclusion: Intraovarian administration of extracellular vesicles from mesenchymal stromal cells in patients with diminished ovarian reserve enhances ovarian response, embryo quality, clinical pregnancy rates, and live birth rates in IVF. This technique shows high potential but requires confirmation through larger studies.
Authors' contributions: Sukhikh G.T. – conception and design of the study; Silachev D.N., Popov K.V. – review of relevant literature; Nazarenko T.A. – Analysis and interpretation of data; Martirosyan Ya.O., Biryukova A.M. – drafting of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: R&D 121040600410-7 Nazarenko T.A. Solving the problem of infertility in modern conditions by developing a clinical and diagnostic model of infertile marriage and using innovative technologies in assisted reproduction programs.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Martirosyan Ya.O., Biryukova A.M., Silachev D.N., Popov K.V., Nazarenko T.A., Sukhikh G.T.
Use of extracellular vesicles from multipotent mesenchymal stromal cells in preparation for
in vitro fertilization programs in patients with diminished ovarian reserve.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (9): 117-125 (in Russian)
https://dx.doi.org/10.18565/aig.2025.228
Keywords
The effectiveness of assisted reproductive technology (ART) programs, including in vitro fertilization (IVF), is largely determined by the functional state of the ovaries. Patients with diminished ovarian reserve exhibit a decrease in the antral follicle counts, reduced anti-Müllerian hormone (AMH) levels, elevated follicle-stimulating hormone (FSH) levels, and a reduced ovarian response to gonadotropic stimulation [1–3]. The most severe form of diminished ovarian reserve is premature ovarian insufficiency (POI), characterized by amenorrhea and persistent hypergonadotropic insufficiency in women aged < 40 years [4, 5]. The literature indicates that 9–24% of women entering IVF programs display signs of a “poor” response to stimulation [6]. This group of patients is traditionally deemed prognostically unfavorable for the attainment of mature oocytes, blastocyst formation, and pregnancy [7]. Therefore, developing methods to activate the functional potential of the ovaries without using donor material is of paramount importance [8].
A promising area of regenerative medicine within reproductive science is the use of mesenchymal stromal cells (MSCs), which can migrate into tissues, undergo partial differentiation, and secrete biologically active molecules in a paracrine manner [9]. Recently, however, researchers have focused not on the cells themselves but on their secretory products: a heterogeneous population of extracellular vesicles (EVs), which includes exosomes and microvesicles [10, 11].
Despite the umbrella term "extracellular vesicles", exosomes and microvesicles are distinct subpopulations that differ in biogenesis, size, and molecular composition. Exosomes (30–150 nm) are formed in intracellular multivesicular bodies and contain a rich array of nucleic acids (microRNA and mRNA), cytokines, and signaling proteins. Microvesicles (100–1000 nm), on the other hand, bud directly from the plasma membrane and often carry membrane receptors and adhesion molecules from the parent cell on their surface [12]. These differences likely lead to varied mechanisms of interaction with target cells and a potentially synergistic therapeutic effect. Studies using animal models have shown that the introduction of MSC-derived exosomes reduces granulosa cell apoptosis, activates proliferation, and restores angiogenesis and steroid secretion in the ovarian tissue [13, 14]. In preclinical conditions, exosome therapy has demonstrated effects comparable to those of MSC transplantation, with several advantages: reduced immunogenic risk, absence of a nucleus and DNA, potential for standardization, and ease of long-term storage and transportation [8, 10]. Furthermore, exosomes serve as ideal carriers for microRNAs and regulatory proteins that act on the PI3K/AKT and Hippo signaling pathways, which are crucial for regulating oogenesis [13, 15].
Despite substantial preclinical data, clinical studies on the use of MSC-derived extracellular vesicles in IVF programs are extremely limited. Some pilot studies have suggested the potential effectiveness of this approach in cases of POI and resistance to standard stimulation [16]. However, systematic data on the impact of MSC-derived exosomes on IVF outcomes, such as the frequency of mature oocyte retrieval and pregnancy, remain insufficient.
Thus, this study aimed to address the gap in the research evidence by evaluating the therapeutic efficacy of intraovarian administration of EVs obtained from placental MSCs in women with a diminished ovarian reserve and a "poor" response to IVF programs. We hypothesized that intraovarian administration of EV-MSCs would significantly improve ovarian response and pregnancy rates compared with standard care in these patients.
Materials and methods
Study design
This prospective randomized controlled trial (1:1) was conducted at the Department of Assisted Reproductive Technologies of V.I. Kulakov NMRC for OG&P from January 2022 to March 2024. This study employed an open (unblinded) design. We assessed the effect of intraovarian administration of EVs derived from placental MSCs on ovarian response, embryogenesis parameters, and pregnancy rates in women with a diminished ovarian reserve undergoing IVF/ICSI. The primary outcome was the clinical pregnancy rate, defined as the presence of a gestational sac on ultrasound. The secondary outcomes included quantitative indicators of ovarian response and embryogenesis, such as the number of retrieved oocytes, number of mature (MII) oocytes, number of 2PN zygotes, number of blastocysts retrieved, blastulation rate, and live birth rate.
The null hypothesis stated that there was no difference in the effectiveness of standard ART protocols compared to protocols involving intraovarian EV administration. The alternative hypothesis proposed that the use of EVs is superior to the standard ART protocol in terms of the number of mature oocytes (MII), blastocyst formation rate, and clinical pregnancy rate.
The study was reviewed and approved by the Research Ethics Committee of V.I. Kulakov NMRC for OG&P. All patients provided informed consent to participate in the study and publish anonymized data in accordance with the Declaration of Helsinki (2013).
The inclusion criteria were as follows: age 18–40 years; diminished ovarian reserve (AMH<1.1 ng/ml and/or antral follicle count (AFC) ≤ 7 according to transvaginal ultrasound on days 2–4 of the cycle); history of ≥3 unsuccessful IVF/ICIS attempts; body mass index 18–25 kg/m²; normal karyotype; no contraindications to ART according to the order of the Ministry of Health of the Russian Federation No. 803n dated July 31, 2020 [17]; and signed informed consent.
The exclusion criteria were as follows: severe external endometriosis (grades III–IV); uterine fibroids with cavity deformation; chronic endometritis (histologically confirmed); tumor and tumor-like ovarian masses; history of systemic diseases, immunodeficiencies, and oncopathology; acute or chronic infections (HIV, viral hepatitis B and C, syphilis); use of donor gametes; absolute male factor infertility (cryptozoospermia, aspermia); and mental illness.
Sample size
The sample size was determined based on the expected difference in the clinical pregnancy rate (the primary outcome). According to the literature, in women with diminished ovarian reserve, the clinical pregnancy rate with standard ART protocols is approximately 8–10%. It is assumed that the use of intraovarian administration of EVs will increase the success rate to 25%. To detect such a difference at a significance level of a=0,05 and a study power of 80%, it was necessary to include at least 46 patients in each group. Considering possible losses and dropouts, the number of patients was increased to 50 per group, which amounted to 100 women.
Randomization and group assignment
The study included 100 women who met the inclusion criteria. Patients were randomly allocated to two equal groups using simple randomization with the random number function of Microsoft Excel. The study group (n=50) received intraovarian injections of extracellular vesicles before stimulation, whereas the control group (n=50) underwent standard stimulation. The study did not include placebo injections in the control group, resulting in an open-label design. At baseline, the groups were comparable in terms of age, duration of infertility, body mass index, and baseline hormonal parameters (FSH, AMH, LH, and estradiol levels); the baseline characteristics are presented in the Results section.
Obtaining EVs
EVs were obtained from the conditioned medium of multipotent MSCs isolated from postpartum placenta. The donors of the biomaterial were healthy women aged 18–35 years, with a healthy pregnancy and negative results of examination for TORCH infections, HIV, viral hepatitis B and C, and syphilis (in accordance with clinical recommendations and SanPiN [18].
Multipotent MSCs were cultured in DMEM/F12 medium enriched with 10% fetal bovine serum, followed by passaging on the 4th–5th day. Immunophenotyping was performed using flow cytometry with antibodies against CD73, CD90, and CD105 (positive markers) and CD45, CD34, CD11b, CD19, and HLA-DR (negative markers). According to the International Society for Cellular Therapy (ISCT) criteria, the expression of CD73/CD90/CD105 was ≥98%, and the expression of negative markers was ≤ 1% [9].
EVs were isolated by sequential differential centrifugation (up to 108,000 × g for 2 h) using an Avanti JXN-30 ultracentrifuge). The resulting EV pellet was resuspended in 200 μl of sterile saline and frozen at -80°C. The size, concentration, and morphology of the vesicles were assessed using nanoparticle trajectory analysis (NanoSight NTA LM10) and electron microscopy. The EV characteristics met the MISEV 2018 criteria [19].
To confirm the integrity of the vesicles after cryopreservation at -80°C, additional analysis was performed. After defrosting the samples, NTA confirmed that the particle size profile and concentration (3.5±0.8×1010 particles/ml) did not change significantly compared to the native preparation (absence of aggregation and significant loss of particle number), indicating the stability of the preparation under the conditions of the freezing protocol used. Western blot analysis confirmed the presence of canonical exosome markers (CD63, CD81, and TSG101) and the absence of apoptotic bodies (cytochrome C) in the thawed preparation. The EV preparation was a heterogeneous population consisting mainly of exosomes (60–70%, 50–150 nm in size, formed by the fusion of multivesicular bodies with the plasma membrane) and, to a lesser extent, microvesicles (20–30%, 100–500 nm in size, pinched off directly from the plasma membrane). Given the differences in the mechanisms of biogenesis and, as a consequence, the potentially different composition of elements (microRNA, proteins, lipids) and mechanisms of interaction with target cells, the composition of the preparation was considered synergistic, providing complex paracrine and regulatory effects on the follicular pool.
EV-MMSC administration technique
On days 4–5 of the menstrual cycle, ultrasound-guided transvaginal ovarian puncture was performed in patients in the study group. A total of 200 μl of EV suspension containing approximately 60 million particles was injected into each ovary. The injection was performed once, 2–3 days before the initiation of gonadotropin stimulation.
Ovarian stimulation protocol
All participants (both groups) underwent controlled ovarian stimulation according to the standard protocol with a gonadotropin-releasing hormone antagonist: gonadotropins (menotropins or recombinant FSH) at a starting dose of 225–300 IU; from the 6–7th day of stimulation, a gonadotropin-releasing hormone antagonist (cetrotide 0.25 mg/day) was added; ovulation trigger – recombinant human chorionic gonadotropin (hCG) 250 mcg subcutaneously. Follicle puncture was performed 36 h after the trigger, followed by fertilization of the obtained oocytes. The fertilization method (IVF or ICSI) was selected by an embryologist, considering the quality of the partner's sperm and medical history.
Embryological stage and embryo transfer
Embryos were cultured in vitro until the blastocyst stage (day 5). For transfer, 1–2 high-quality embryos were selected; if the conditions for transfer were not met, the embryos were vitrified for transfer in subsequent cycles. The embryological parameters recorded for all patients included the number of oocytes obtained, the number of mature (MII) oocytes, the number of 2PN zygotes, the number of blastocysts, and the blastulation rate. Outcome assessment
The fertilization rate was calculated as the ratio of the number of 2PN zygotes to the number of mature oocytes (%), and the blastulation rate was calculated as the ratio of the number of blastocysts to the number of fertilized zygotes (%). Clinical pregnancy was defined as the presence of a gestational sac (chorion) on ultrasound examination approximately 3 weeks after a positive β-hCG test. Live birth was defined as successful delivery after embryo transfer within a given cycle.
Statistical analysis
All randomized patients (n=100) were included in the final analysis according to the group to which they were randomized, regardless of whether they completed the full treatment cycle. Thus, the main analysis was conducted based on the intention-to-treat principle. The absence of embryo transfer or cycle cancellation was considered an adverse outcome (no pregnancy). Additionally, a per-protocol analysis was performed, including only patients who completed stimulation and embryo transfer, to assess the stability of the results. Statistical analysis was performed using Statistica 12.0 (StatSoft Inc., USA). Continuous variables with a normal distribution were expressed as means (M) and standard deviations (SD) and presented as M (SD). For non-normally distributed data, the median with interquartile range was reported as [Me (Q1–Q3)]. The normality of the distribution was tested using the Shapiro–Wilk test, and the homogeneity of variances among groups was assessed using the Levene test. Parametric methods were applied only when the appropriate conditions were met: for normal distribution and equality of variances, groups were compared using the two-tailed Student's t-test; when these conditions were not met, the nonparametric Mann–Whitney U-test was used. The χ² test or Fisher's exact test (when the expected frequency of one or more cells was less than five) was used to compare categorical variables. Differences were considered statistically significant at a significance level of p<0.05.
Results
Analysis of population and baseline characteristics
In a preliminary analysis of 2500 IVF/ICSI cycles performed at the Center in 2022–2024, 900 patients (36%) had a “poor” ovarian response (≤ 3 oocytes after standard stimulation). From this cohort, 100 women who met the inclusion criteria were selected and randomized into the study and control groups. The flowchart (Figure) shows the inclusion, randomization, and analysis of patients.

The mean age of patients in the study group was 38 years (Me=38; Q1–Q3: 34.5–41), and in the control group, it was 35 years (32–39); the difference was not significant (p=0.30). The median duration of infertility was 6 years (3–8) in the study group and 5 years (2–7) in the control group (p=0.30). The number of previous ART attempts in the anamnesis did not differ: 5 (2–7) versus 6 (2–9) (p=0.20). Primary infertility was observed in 35/50 (70%) patients in the study group and 36/50 (72%) in the control group (p=0.84). All baseline differences were statistically insignificant, and the groups were comparable in terms of demographic and anamnestic parameters (Table 1).
Baseline ovarian reserve parameters were similarly reduced in both groups. The median AMH level was 1.0 ng/ml (0.7–1.6) in the study group and 1.1 ng/ml (0.8–1.7) in the control group (p>0.05). The median AFC on days 2–4 of the cycle did not differ between the groups: 5 (4–8) versus 6 (4–9) (p>0.05). The FSH level was also similar: 7.9 mIU/ml (6.1–10.6) in the study group and 7.2 mIU/ml (5.2–8.2) in the control group (p>0.05). Thus, both groups demonstrated a pronounced deficiency in ovarian reserve according to hormonal and ultrasound criteria, which met the inclusion criteria (Table 2).
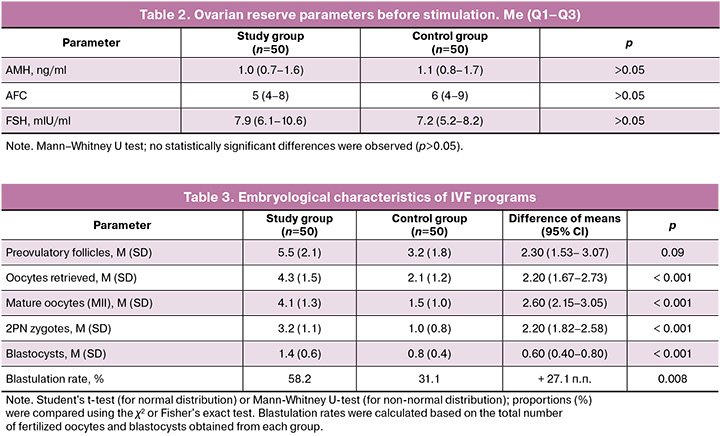
Thus, despite comparable age and medical history, patients in the study and control groups had more pronounced signs of ovarian reserve deficiency according to hormonal and ultrasonographic criteria.
Embryological parameters and follicular response
All patients in both groups received an identical controlled ovarian stimulation regimen. The mean total dose of gonadotropins was approximately 268 IU and did not differ significantly between the groups. The median duration of stimulation was 9 days (Q1–Q3: 8–11) in all patients. Against the background of EV therapy, a more pronounced follicular response was noted in the study group: the number of preovulatory follicles tended to increase (5.5 (2.1) versus 3.2 (1.8), p=0.09), and the number of oocytes obtained was significantly higher than that in the control group (4.3 (1.5) versus 2.1 (1.2), p<0.001). The number of mature oocytes (MII) in the study group was 4.1 (1.3), which was statistically significantly higher than that in the control group (1.5 (1.0), p<0.001). The number of 2PN zygotes was also higher in the EV group (3.2 (1.1) vs. 1.0 (0.8), p<0.001). The number of blastocysts obtained per cycle in the study group was 1.4 (0.6) versus 0.8 (0.4) in the control group (p<0.001). Thus, the use of MSCs exosomes led to a statistically significant improvement in the indicators of the embryological stage of IVF compared to standard therapy (Table 3). Additionally, the blastulation rate (the proportion of blastocysts obtained from the number of fertilized oocytes) in the study group was 58.2%, which was almost twice as high as that in the control group (31.1%, p=0.008). This indicates better embryo quality in patients treated with EV-MSCs.
In addition, the blastulation rate (per fertilization) in the study group was 58.2%, which was statistically higher than that of the control group (31.1%; p=0.008).
Reproductive outcomes
The pregnancy rate was higher in the study group. Clinical pregnancy (visualization of the chorion by ultrasound examination) was achieved in 12/50 (24%) patients in the study group and 3/50 (6 %) patients in the control group (p=0.012). This corresponds to a relative risk (RR) of pregnancy of 4.0 (95% confidence interval (CI), 1.2–13.3) when using EV-MSCs compared to the control. The live birth rate was 9/50 (18%) in the study group versus 1/50 (2%) in the control group (p=0.008), which means an approximately 9-fold increase in the chance of a live birth (RR=9.0; 95% CI 1.2–68.4) in patients who received EVs. The frequency of biochemical pregnancy (transient increase in β-hCG) was also higher in the study group (14/50 [28 %]) than in the control group (3/50 (6 %)) (p=0.003). Thus, the use of EVs was associated with a statistically significant increase in both the probability of pregnancy and the probability of successful delivery compared with standard care (Table 4).

Thus, despite the lower AFC and higher FSH in the study group, the use of EV-MSCs allowed for statistically significant improvement not only in the embryological stage indicators but also in the frequency of clinical pregnancy and live birth within the IVF program.
Discussion
The study results demonstrated a statistically significant clinical effect of EV-MSCs in patients with reduced ovarian reserve. Despite the initial unfavorable parameters in the study group (low AFC and increased FSH levels), these patients exhibited greater treatment efficacy than the control group. The number of mature oocytes, zygotes, and blastocysts, as well as the pregnancy and live birth rates, were significantly higher in the EV-MSCs group. In the intervention group, the probability of clinical pregnancy increased approximately four-fold, and the probability of live birth increased nine-fold compared to the absence of EV therapy, although the wide confidence intervals reflect the limited sample size. These findings indicate a high potential for exosome MSCs as an adjuvant therapy in IVF programs for patients with poor response.
Our results are consistent with those of preclinical studies. It has been shown that a heterogeneous population of EVs secreted by MSCs (including both exosomes and microvesicles) is capable of modulating regenerative processes in the ovaries by delivering functional molecules – microRNA, growth factors, signaling proteins [10, 13]. Despite the commonality of the final effects, different subpopulations of vesicles can contribute to the observed therapeutic outcome, owing to differences in the biogenesis mechanism and, consequently, molecular composition. Exosomes formed via the endosomal pathway are especially rich in nucleic acids (e.g., microRNA) and enzymes, whereas microvesicles pinched off from the plasma membrane often carry an increased number of membrane receptors and adhesion molecules on their surface [12]. In animal model experiments, intraovarian administration of exosomes and microvesicles resulted in the activation of the PI3K/AKT signaling pathway, increased proliferation of granulosa cells, and decreased expression of pro-apoptotic markers (Bax and Caspase-3) [14, 20]. Presumably, the effect observed in our study is associated with the complex paracrine effect of the heterogeneous EV-MSCs preparation, which helps restore the follicle microenvironment, normalize angiogenesis, and increase sensitivity to gonadotropins. According to data, MSCs exosomes increase the expression of FSH receptors (FSHR) and enhance local estradiol production by ovarian granulosa cells [21]. This is consistent with the increase in the number of mature MII oocytes and zygotes found in the main group, despite the initial AFC deficiency in the group.
One of the key effects of exosome use was a statistically significant increase in the frequency of blastocyst formation (blastulation), which may indicate an improvement in oocyte quality. Introduction of MSCs exosomes increased the expression of mitochondrial function genes and reduced the level of oxidative stress in oocytes [22]. These mechanisms may underlie improvements in embryological efficiency. In our study, the blastulation frequency in the main group was ~58%, which was almost twice that of the control group (31%). This means that in patients who received EVs, fertilized oocytes more frequently developed to the blastocyst stage.
It is interesting to note that a pronounced effect was achieved after a single administration of the complex EV preparation before the start of stimulation. This indicates a high biological activity of the MSCs secretome and the validity of its use as an independent therapeutic agent. The synergistic effect of different types of vesicles can provide a more powerful and multifactorial therapeutic response than the use of isolated fractions. In contrast to the direct transplantation of MSCs, the use of EVs is associated with lower ethical and immunological risks, the absence of oncogenic potential, and greater standardization of the drug [10, 23]. The present study is one of the first prospective controlled trials to demonstrate the improvement of both embryological and final reproductive outcomes with the use of MSCs exosomes in women with a reduced ovarian reserve.
The limitations of our study are as follows.
- Lack of blinding (masked) design. The study was open-label, which could theoretically influence the interpretation of subjective parameters. However, the main outcomes (quantitative parameters and pregnancy) were objective, which mitigated this drawback.
- Limited sample size. The sample size (50+50 patients) was insufficient to ensure high statistical power, especially for rare events (live births). The small number of observations led to wide confidence intervals for the effect estimate (for example, the 95% CI for the relative risk of live birth was very wide); therefore, the obtained results should be interpreted with caution. An expansion of the sample is required in future studies to improve the accuracy of the estimates and the power of the statistical tests.
- Lack of study of molecular markers. In this study, we did not evaluate changes in ovarian tissue (such as the expression of AMHR2, FOXL2, CYP19A1 receptors, or other local factors) after EVs administration. In addition, the study did not aim to separate individual vesicle subpopulations (exosomes and microvesicles) into the observed clinical effects. Such an analysis would allow us to better understand the mechanisms of exosome action in the ovaries and confirm their regenerative effects at the molecular level.
Future research should include multicenter randomized trials with a larger number of patients and extended follow-up. This is essential to evaluate not only the immediate but also the long-term reproductive outcomes of therapy, including embryo quality, implantation rates, and neonatal outcomes. Expanding the scale and duration of follow-up will enable a more accurate assessment of the therapeutic effect of exosomes and help narrow the confidence intervals, thereby confirming or refuting our findings with a higher degree of evidence.
Conclusion
Intraovarian administration of EVs isolated from placental MSCs in patients with reduced ovarian reserve during IVF cycles is associated with a statistically significant increase in the number of mature oocytes, zygotes, and blastocysts, as well as an increase in the frequency of clinical pregnancy and live birth. In other words, despite the initially more pronounced deficiency in ovarian reserve, women who received EV-MSC therapy demonstrated higher treatment efficacy than the control group. Given their favorable safety profile, biocompatibility, and potential for standardization, EV-MSCs can be considered a promising avenue for personalized therapy for "poor" ovarian response in ART. However, due to the limited sample size and lack of blinding, these findings require confirmation. Further randomized trials with larger sample sizes and long-term follow-ups are necessary to fully assess the clinical efficacy and clarify the molecular mechanisms of exosomes in reproductive medicine.
References
- Марченко Л.А., Машаева Р.И. Клинико-лабораторная оценка овариального резерва с позиции репродуктолога. Акушерство и гинекология. 2018; 8: 22-5. [Marchenko L.A., Mashaeva R.I. Clinical and laboratory assessment of ovarian reserve from a reproductologist’s point of view. Obstetrics and Gynecology. 2018; (8): 22-5. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.8.22-25
- Барановская Е.И. Антимюллеров гормон в оценке овариального резерва: возможности и ограничения. Российский вестник акушера-гинеколога. 2022; 22(2): 65-70. [Baranouskaya E.I. Anti-Müllerian hormone in the assessment of ovarian reserve: possibilities and limitations. Russian Bulletin of Obstetrician-Gynecologist. 2022; 22(2): 65-70. (in Russian)]. https://dx.doi.org/10.17116/rosakush20222202165
- Денисова В.М., Ярмолинская М.И., Закураева К.А. Преждевременная недостаточность яичников: генетические причины и тактика ведения пациенток (обзор литературы). Журнал акушерства и женских болезней. 2021; 70(3): 75-91. [Denisova V.M., Yarmolinskaya M.I., Zakurayeva K.A. Genetic causes and treatment options. A literature review. Journal of Obstetrics and Women’s Diseases. 2021; 70(3): 75-91 (in Russian)]. https://dx.doi.org/10.17816/JOWD59987
- Салимова М.Д., Данусевич И.Н., Наделяева Я.Г., Лазарева Л.М., Аталян А.В., Новикова Е.А., Шолохов Л.Ф., Рашидова М.А., Сутурина Л.В. Клинико-лабораторные показатели сниженного овариального резерва у женщин репродуктивного возраста: кросс-секционное исследование. Экология человека. 2022; 29(8): 587-97. [Salimova M.D., Danusevich I.N., Nadelyaeva Y.G., Lazareva L.M., Atalyan A.V., Novikova E.A., Sholokhov L.F., Rashidova M.A., Suturina L.V. Clinical manifestations of decreased ovarian reserve in premenopausal women: a cross-sectional study. Human Ecology. 2022; 29(8): 587-97 (in Russian)]. https://dx.doi.org/10.17816/humeco106718
- Nelson L.M. Clinical practice. Primary ovarian insufficiency. N. Engl. J. Med. 2009; 360(6): 606-4. https://dx.doi.org/10.1056/NEJMcp0808697
- Polyzos N.P., Devroey P. A systematic review of randomized trials for the treatment of poor ovarian responders: is there any light at the end of the tunnel? Fertil. Steril. 2011; 96(5): 1058-61. https://dx.doi.org/10.1016/j.fertnstert.2011.09.048
- Ferraretti A.P., La Marca A., Fauser B.C., Tarlatzis B., Nargund G., Gianaroli L. ESHRE consensus on the definition of 'poor response' to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum. Reprod. 2011; 26(7): 1616-24. https://dx.doi.org/10.1093/humrep/der092
- Vizoso F.J., Eiro N., Cid S., Schneider J., Perez-Fernandez R. Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int. J. Mol. Sci. 2017; 18(9): 1852. https://dx.doi.org/10.3390/ijms18091852
- Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D. et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International society for cellular therapy position statement. Cytotherapy. 2006; 8(4): 315-7. https://dx.doi.org/10.1080/14653240600855905
- Lai R.C., Arslan F., Lee M.M., Sze N.S., Choo A., Chen T.S. et al. Exosome secreted by MSC reduces myocardial ischemia / reperfusion injury. Stem Cell Res. 2010; 4(3): 214-22. https://dx.doi.org/10.1016/j.scr.2009.12.003
- Toh W.S., Lai R.C., Hui J.H.P., Lim S.K. MSC exosome as a cell-free MSC therapy for cartilage regeneration: implications for osteoarthritis treatment. Semin. Cell Dev. Biol. 2017; 67: 56-64. https://dx.doi.org/10.1016/j.semcdb.2016.11.008
- Raposo G., Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 2013; 200(4): 373-83. https://dx.doi.org/10.1083/jcb.201211138
- Li Z., Liu Y., Tian Y., Li Q., Shi W., Zhang J. et al. Human umbilical cord mesenchymal stem cell derived exosomes improve ovarian function in natural aging by inhibiting apoptosis. Int. J. Mol. Med. 2023; 52(4): 94. https://dx.doi.org/10.3892/ijmm.2023.5297
- Zhang S., Zhu D., Li Z., Huang K., Hu S., Lutz H. et al. A stem cell-derived ovarian regenerative patch restores ovarian function and rescues fertility in rats with primary ovarian insufficiency. Theranostics. 2021; 11(18): 8894-908. https://dx.doi.org/10.7150/thno.61690
- Yahyavi Y., Kheradi N., Karimi A., Ebrahimi-Kalan A., Ramezani F., Yousefi S. et al. Novel advances in cell-free therapy for premature ovarian failure (POF): A comprehensive review. Adv. Pharm. Bull. 2024; 14(3): 543-57. https://dx.doi.org/10.34172/apb.2024.059
- Zhang J., Tian X., Li Y., Fang C., Yang F., Dong L. et al. Stem cell-derived exosomes: a comprehensive review of biomedical applications, challenges, and future directions. Int. J. Nanomedicine. 2025; 20: 10857-905. https://dx.doi.org/10.2147/IJN.S527137
- Приказ Минздрава России от 31.07.2020 № 803н «Об утверждении порядка использования вспомогательных репродуктивных технологий». Доступно по: https://normativ.kontur.ru/document?moduleId=1&documentId=373901 [Order of the Ministry of Health of the Russian Federation dated 07/31/2020 No. 803n "On approval of the procedure for the use of assisted reproductive technologies". Available at: https://normativ.kontur.ru/document?moduleId=1&documentId=373901 (in Russian)].
- Постановление Главного государственного санитарного врача РФ от 28 января 2021 г. № 4. СанПиН 3.3686-21. Профилактика инфекционных болезней. Доступно по: https://base.garant.ru/400342149/#block_1000 [Resolution of the Chief State Sanitary Physician of the Russian Federation dated January 28, 2021 No. 4. SanPiN 3.3686-21. Prevention of infectious diseases. Available at: https://base.garant.ru/400342149/#block_1000 (in Russian)].
- Théry C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R. et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 2018; 7 (1): 1535750. https://dx.doi.org/10.1080/20013078.2018.1535750
- Elsherbiny N.M., Abdel-Maksoud M.S., Prabahar K., Mohammedsaleh Z.M., Badr O.A.M., Dessouky A.A. et al. MSCs-derived EVs protect against chemotherapy-induced ovarian toxicity: role of PI3K/AKT/mTOR axis. J. Ovarian Res. 2024; 17(1): 222. https://dx.doi.org/10.1186/s13048-024-01545-7
- He J., Ao C., Li M., Deng T., Zheng S., Zhang K. et al. Clusterin-carrying extracellular vesicles derived from human umbilical cord mesenchymal stem cells restore the ovarian function of premature ovarian failure mice through activating the PI3K/AKT pathway. Stem Cell Res. Ther. 2024; 15(1): 300. https://dx.doi.org/10.1186/s13287-024-03926-7
- Zhou Y., Huang J., Zeng L., Yang Q., Bai F., Mai Q. et al. Human mesenchymal stem cells derived exosomes improve ovarian function in chemotherapy-induced premature ovarian insufficiency mice by inhibiting ferroptosis through Nrf2/GPX4 pathway. J. Ovarian Res. 2024; 17(1): 80. https://dx.doi.org/10.1186/s13048-024-01403-6.
- Phinney D.G., Pittenger M.F. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells. 2017; 35(4): 851-8. https://dx.doi.org/10.1002/stem.2575
Received 21.08.2025
Accepted 03.09.2025
About the Authors
Yana O. Martirosyan, PhD, Researcher at the F. Paulsen Research and Educational Center for ART with the Clinical Department, V.I. Kulakov NMRC for OG&P,Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, +7(925) 124-99-99, marti-yana@yandex.ru, https://orcid.org/0000-0002-9304-4410
Almina M. Biryukova, PhD, Clinical Supervisor at the F. Paulsen Research and Educational Center for ART with the Clinical Department, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, alma21@list.ru
Denis N. Silachev, Dr. Bio. Sci., Head of the Laboratory of Cell Technologies, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow,
Ac. Oparin str., 4, +7(905)792-13-01, d_silachev@oparina4.ru, https://orcid.org/0000-0003-0581-9755
Konstantin V. Popov, PhD (Bio), Head of the Center for Personalized High-Tech Drugs, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, +7(916)580-68-05, k_popov@oparina4.ru, https://orcid.org/0000-0002-3436-3235
Tatiana A. Nazarenko, Dr. Med. Sci., Professor, Head of the Institute of Reproductive Medicine, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
117997, Russia, Moscow, Ac. Oparin str., 4, t_nazarenko@oparina4.ru, https://orcid.org/0000-0002-5823-1667
Gennady T. Sukhikh, Academician of the RAS, Dr. Med. Sci., Professor, Director, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow,
Ac. Oparin str., 4, g_sukhikh@oparina4.ru, https://orcid.org/0000-0002-7712-1260



