Optimization of ART programs in women of late reproductive age by co-cultivation of oocytes with donor extracellular vesicles from follicular fluid
Akhmedova Z.F., Sysoeva A.P., Gavrilov M.Yu., Zingerenko B.V., Shevtsova Yu.A., Silachev D.N., Ekimov A.N., Makarova N.P., Kulakova E.V., Kalinina E.A.
Background: The effectiveness of infertility treatment using assisted reproductive technologies (ART) decreases with the age of the woman due to the aging of the germ cells. It has been previously shown that co-cultivation of embryos with autologous extracellular vesicles from follicular fluid (EVFF) improves the embryological stage of treatment. However, in women of late reproductive age, biological fluids senesce and lose their effectiveness. Therefore, a hypothesis about the use of donor EVFF to improve the results of ART has been proposed.
Objective: To evaluate the effectiveness of donor EVFF in co-cultivation of embryos of women over 40 years.
Materials and methods: The study was conducted among 79 married infertile couples. Inclusion criteria: the woman's age > 40 years, normal karyotype both in a woman and a man. The patients were divided into two groups: the main group (n=40) with co-cultivation of oocytes with donor EVFF and the comparison group (n=39) with standard cultivation. Stimulation of ovarian function was performed according to the protocol with gonadotropin-releasing hormone antagonists. Transvaginal puncture was done under anesthesia. In group 1, donor EVFF were added to fertilized oocytes. The next day, they were transferred to fresh culture medium. The main evaluation parameters included: fertilization rate, blastocyst formation, and clinical pregnancy.
Results: In the group with donor EVFF, the fertilization rate was higher (84.8% vs. 70%, p=0.008), the proportion of embryo transfer cancellations was lower (22.5% vs. 46.1%, p=0.021). The frequency of pregnancy and genetically normal embryos did not statistically differ.
Conclusion: Donor EVFF improve embryological parameters but do not affect clinical outcomes in women of late reproductive age.
Authors’ contributions: Akhmedova Z.F. – study concept, data collection, data processing, text composition; Sysoeva A.P. – conduction of embryo stage of infertility treatment, critical re-evaluation of obtained results; Gavrilov M.Yu. – conduction of embryo stage of infertility treatment, extracellular vesicles identification; Zingerenko B.V. – conduction of embryo stage of infertility treatment, data processing; Shevtsova Yu.A. – extracellular vesicles identification using ultracentrifugation; Silachev D.N. – critical re-evaluation of the manuscript and the obtained results; Ekimov A.N. – preimplantation genetic testing conduction; Makarova N.P. – study concept, data processing, text composition; Kulakova E.V. – critical re-evaluation of obtained results from the point of obstetrics and gynecology; Kalinina E.A. – manuscript final approval.
Conflicts of interest: The authors declare no conflicts of interest in connection with the publication of this article.
Funding: The study was carried out within the initiative scientific project “Study of the influence of extracellular vesicles from reproductive organs and tissues biological fluids on gametes, the process of fertilization and early human embryogenesis and implantation” (2025–2027, supervisor — N.P. Makarova), Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia.
Ethical Approval: The study was approved by the bioethics committee of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia (Protocol #10 of October 20, 2022).
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Akhmedova Z.F., Sysoeva A.P., Gavrilov M.Yu., Zingerenko B.V., Shevtsova Yu.A., Silachev D.N., Ekimov A.N., Makarova N.P., Kulakova E.V., Kalinina E.A.. Optimization of ART programs in women of late reproductive age by co-cultivation of oocytes with donor extracellular vesicles from follicular fluid.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (8): 133-142 (in Russian)
https://dx.doi.org/10.18565/aig.2025.104
Keywords
Successful pregnancy in infertility treatment programs with the use of assisted reproductive technologies (ART) is the result of interaction of many factors, including adequate folliculogenesis and correct early embryo development. In women of late reproductive age (over 40 years), the effectiveness of infertility treatment remains extremely low due to the natural aging of female germ cells and, consequently, bad quality of embryos obtained from such oocytes [1, 2]. According to global statistics, the frequency of clinical pregnancy with their own oocytes in patients over 40 years of age does not exceed 12–13% [1]. New approaches to ART are required to improve pregnancy outcomes in this cohort of patients.
Our previous studies showed that the use of autologous extracellular vesicles (EV) of follicular fluid (FF) can improve the effectiveness of infertility treatment programs through ART methods [3–5]. At the embryological stage of ART, the use of EVFF increases sperm motility, makes it possible to select male gametes and to improve fertilization. However, it was also shown, including in animal models, that in late reproductive age, the biological fluids of the reproductive organs and tissues undergo aging and do not provide valuable results in ART programs. The composition of the EVFF in patients over 40 years of age differs significantly from that in younger women. Lipidome, concentration of important small non-coding RNAs, proteome – all these indicators deteriorate within the body general aging and the weakening of female reproductive function [6, 7]. That is why we put forward a hypothesis about the possibility of improving reproductive outcomes when using donor EVFF at the embryological stage of the infertility treatment program.
In connection with the abovesaid, the objective of this study was to evaluate the clinical and embryological effectiveness of a young donor EVFF in co-cultivation with oocytes of women of late reproductive age in the treatment of infertility using ART methods.
Materials and methods
Patients’ characteristics
Selection of patients was carried out among patients, who applied for infertility treatment and ART program during the period of 2023 to 2024 at the Prof. B.V. Leonov Department of Assistive Technologies in Infertility Treatment (Head – Dr. Med. Sci., Professor E.A. Kalinina), Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia (Director – Academician of the RAS, Prof. G.T. Sukhikh).
The inclusion criteria for the study were the following parameters: normal karyotype of both spouses; woman's age 40 years and over; mild pathozoospermia in the spouse. The exclusion criteria comprised: absence of cumulus-oocyte complexes during transvaginal puncture of follicles, abnormal fertilization (3 or more pronuclei), use of donor oocytes in the ART cycle; surrogacy; uterine factor infertility, obesity. All married couples were examined in accordance with Russian regulatory legal acts: Order of the Ministry of Health of Russia No. 803n of July 31, 2020 "On the procedure for using assisted reproductive technologies, contraindications and restrictions on their use"; clinical guidelines "Female Infertility" (2022, 2024), clinical guidelines "Male Infertility" (2022). No contraindications to the use of ART were identified in the studied couples. Before entering the ART program, the couples signed an informed voluntary consent. This study was supported at the Bioethics Commission of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia (Protocol No. 10 of October 20, 2022).
According to the inclusion/exclusion criteria, 79 married couples were chosen for the study. All women were randomly divided into two groups: main group 1 (n=40) – co-cultivation of oocytes with donor EVFF; comparison group 2 (n=39) – standard cultivation. Due to current clinical guidelines, in most cases, the selected women underwent biopsy of the obtained blastocysts, followed by preimplantation genetic testing for aneuploidies (PGT-A). If the couple refused to do PGT-A, the embryo was transferred into the uterine cavity.
Infertility treatment with ART methods
Stimulation of ovarian function was performed based on the protocol with gonadotropin-releasing hormone antagonists. The ovulation trigger was administered if the ovaries contained follicles with a diameter of more than 17 mm. Human chorionic gonadotropin (hCG) at a dosage of 10,000 IU or a gonadotropin-releasing hormone agonist of 0.2 mg were used as an ovulation trigger.
Transvaginal puncture of follicles was conducted under general anesthesia with ultrasound control using atraumatic needles (Vitrolife, Sweden). The whole embryological stage was Carried out on GLOBAL culture media (CooperSurgical, Denmark) according to the manufacturer's instructions. If PGT-A was not performed, then one or two embryos were transferred into the uterine cavity on the 5th day after fertilization. In case of trophectoderm cell biopsy, the blastocyst was cryopreserved and stored in liquid nitrogen. Cryopreservation was performed on B-VIT media according to the manufacturer's instructions (Proteinsynthesis, Russia). Post-transfer support during native transfer was done in accordance with a standard protocol using micronized progesterone 600 mg/day. In case of transfer of a thawed genetically tested embryo we conducted the support in the menstrual cycle with standard cyclic hormonal therapy according to the “Female Infertility” clinical guidelines. Clinical pregnancy was diagnosed on the 21st day after embryo transfer by performing a pelvic ultrasound based on the presence of a fertilized egg in the uterine cavity.
Isolation of donor EVFF and co-cultivation procedure
Laboratory study was conducted by the specialists of the Cell Technologies Laboratory (Head of Laboratory – Dr. Bio. Sci. D.N. Silachev), Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia.
A healthy donor woman (24 y.o.) with her own children was selected at the Kulakov Center. Donor stimulation was carried out according to a standard protocol with GnRH antagonists (total gonadotropin at a dose of 1890 IU). As ovulation trigger we used hCG at a dose of 10 000 IU. 15 ml of follicular fluid with no blood and granulosa cells was collected at the day of transvaginal puncture. EVFF was isolated using sequential ultracentrifugation with high-speed centrifuge Avanti JXN-30 (Beckman Coulter Life Sciences, USA), as previously described [4]. The obtained FF was being centrifuged at 4°С for 10 minutes at 400 g to remove cell debris. Then supernatant FF was transferred to a centrifuging glass to process it with PBS solution (Gibco, USA) to 40 ml with the following centrifuging for the period of 30 minutes at 10 000 g (4°С, acceleration 4, deceleration 4). Supernatant was moved to new sterile probes, then it was being centrifuged for 1,5 hours at 108 000 g (4°С, acceleration 4, deceleration 4). The sediment was resuspended at PBS (Gibco, USA), the end volume was processed to 40 ml and again it was being centrifuged for 1,5 hours at 108 000 g (4°С, acceleration 4, deceleration 4). The EVFF sediment was diluted with GLOBAL culture medium (CooperSurgical, Denmark) at the end volume of 100 mcl, which was aliquoted at 5 mcl parts and kept at -80°С till its use.
The obtained MII oocytes of the late reproductive age women from Group 1 (n=40) were co-cultivated for 24 hours at the day of transvaginal puncture with donor EVFF after the fertilization using ICSI method. To add the EVFF one should follow the scheme. 2 mcl of medium with donor EVFF was added into the cup of a 4-cup tablet (NUNC, USA) with 0,3 ml culture medium (Global, CooperSurgical, Denmark) and oocytes after ICSI, and left for 24 hours. On the 1st day of co-cultivation zygote stage embryos (2PN2PB) were transferred into the classical culture medium, where they continued to develop till blastocyte stage. Morphological assessment of patients’ embryos at the cleavage stage was not carried out. On the 5th day of co-cultivation embryo assessment and quality classification was conducted for further analysis.
Embryo assessment and method of their quality classification
Embryo quality assessment at the embryological stage of infertility treatment program using ART methods was done in accordance with Recommendations of the Russian Association for Human Reproduction (2021) [8]. On the 5th day of cultivation the morphological analysis of the expansion of blastocyte, trophectoderm cells and inner cell mass was conducted. Sallem A. et al. classification [9] was used to analyze and classify the embryos (Table 1).
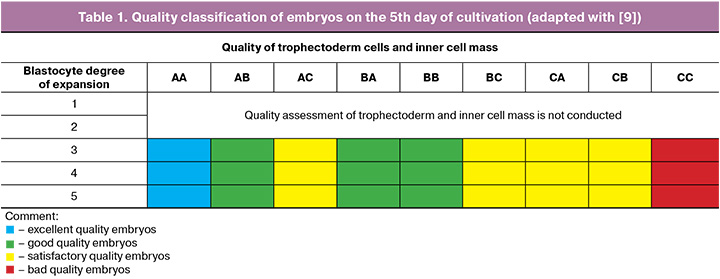
Trophectoderm cell biopsy and PGT-A analysis
On the 5th and 6th days of cultivation no lower than 3ВВ quality blastocytes underwent trophectoderm cell biopsy according to the standards applied within the laboratory [10]. The obtained trophectoderm cells were transferred into the 0,5 ml probes, containing lysing buffer solution, and handled to the laboratory for PGT testing by high-production next generation sequencing (NGS). PGT-A was performed in the Laboratory of Preimplantation Genetic Screening and Genetic Diagnostics of the Institute of Reproductive Genetics (Director – Dr. Bio. Sci. D.Yu. Trofimov), using certified test-systems (DNA-Technology, Russia).
Data analysis
The primary endpoints of the study were the fertilization rate (number of zygotes/number of mature MII oocytes, in %) and the frequency of formation of excellent and good quality blastocysts depending on the type of culture (number of blastocysts/number of zygotes, in %). The endpoints of the study also were the frequency of genetically normal embryos (number of genetically normal embryos/number of blastocysts analyzed, in %) in terms of PGT-A and the frequency of clinical pregnancy per embryo transfer (number of pregnant women/number of transfers, in %) in case of native embryos transfer into the uterine cavity.
Statistical analysis
For statistical processing of the results, the standard Microsoft Excel soft and the IBM SPSS Statistics v. 27.0.1.0 statistical software (USA) were used. The concordance of the analyzed parameters to the normal distribution law was checked according to the Shapiro–Wilk test. Absolute N numbers and percentages of the total number of patients were used to describe categorical binary data (%). To describe quantitative data with a normal distribution, the mean parameter value and the standard deviation were used (M (SD)). When the distribution of the parameters differed from the normal, nonparametric statistics methods were used with the indication of the median, upper and lower quartiles – Me [Q25; Q75]. When comparing quantitative parameters, we used the nonparametric Mann–Whitney U-test, and the Pearson chi-square test – for qualitative features. The threshold significance level p-value was 0.05.
Results
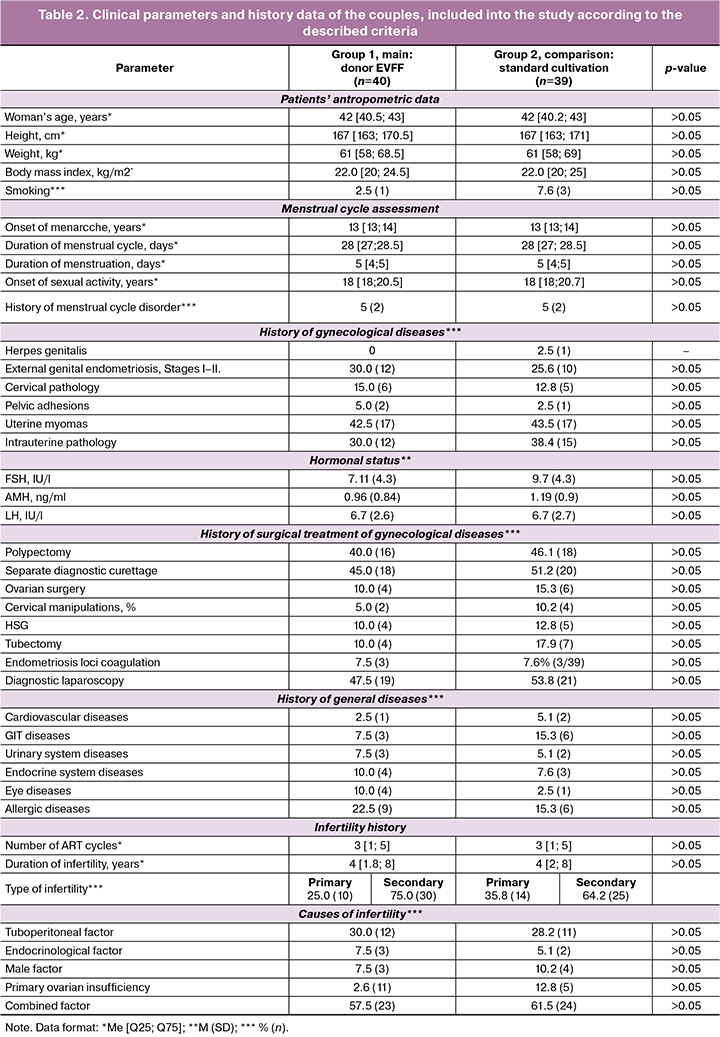
At the first stage of the study, we performed the analysis of clinical data and medical history of the selected married couples. Table 2 presents the results of descriptive statistics. The average age of women in the groups was 42 years, with the average height – 167 cm, and average weight – 61 kg. When assessing the menstrual function of patients, no differences were found either: the average age of menarche was 13 years, the duration of the menstrual cycle was 28 days, the duration of bleeding was 5 days. Gynecological diseases in both groups were as follows. History of cervical pathology in group 1 – 15.0%, in group 2 – 12.8%; adhesions in the small pelvis in group 1– 5.0%, in group 2 – 2.5%. It is notable that stage I and II external genital endometriosis was present in 30.0% of patients in group 1 and in 25.6% in group 2; uterine fibroids were diagnosed in 42.5% in group 1 and in 43.5% in group 2. All studied patients had a history of surgical treatment for gynecological diseases. History of separate diagnostic curettage was present in 45.0% of patients in group 1 and in 51.2% in group 2; diagnostic laparoscopy in 47.5% patients in group 1 and in 53.8% in group 2. According to the their physical state, history of allergy was most often recorded in 22.5% of women in group 1 and in 15.3% in group 2. It means that patients of late reproductive age included into the study may be considered a special group in terms of gynecological status for the treatment of infertility using ART methods and for subsequent pregnancy. Leading obstetricians and gynecologists, both in Russia and worldwide, have drawn attention to this fact in their publications [1]. The duration of infertility in women of late reproductive age was 4 years in both groups, but in general the range turned out to be 8 years or more. The studied couples in both groups had a history of 3 or more ART treatment for infertility. Also it is remarkable that the most common cause of infertility was a combined factor, which was found in 57.5% of couples in group 1, and in 61.5% in group 2 (Table 2).
The hormonal status at the time of entry into the ART program of women in the groups did not differ significantly. All had preserved ovarian reserve and had the opportunity to perform ovarian stimulation. The features of the ovarian stimulation and spermatogenesis indices are shown in Table 3. The total dose of gonadotropins in the groups was similar – 1851.7 (486.5) IU in group 1 and 1931.8 (581.5) IU in group 2. On average, the duration of stimulation in all women lasted for 9 days. On the day of transvaginal puncture, an average of three cumulus-oocyte complexes were obtained in both groups (Table 3). Ejaculate parameters on the day of fertilization included mild pathozoospermia, which, nevertheless, required the use of ICSI fertilization methods. In group 1, the sperm concentration was 47.5 million/ml, their progressive motility was 55%, whereas in group 2 these parameters were 56 million/ml and 54.5%, respectively. The proportion of morphologically normal sperm in both groups did not differ and accounted 2%, i.e. we observed a two-fold decrease compared to the standard values.
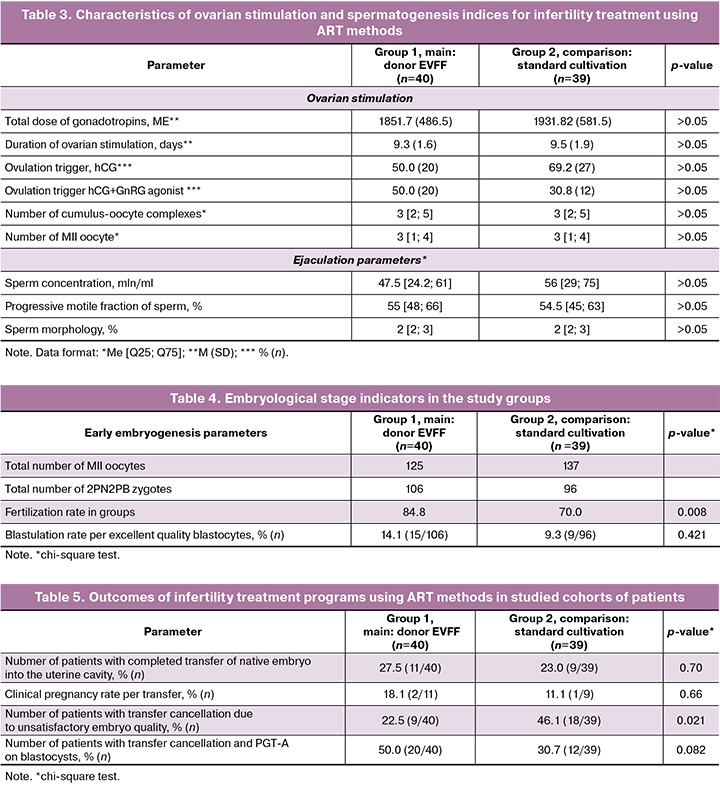
According to the study design, the oocytes of women in group 1 were co-cultured with donor EVFF. The results are presented in Table 4. As shown by the data, in the group with co-cultured oocytes in women of late reproductive age, the fertilization rate, which is 84.8%, turned out to be higher than in group 2 without co-culture – 70.0% (p=0.008, chi-square test). The percentage of blastulation between groups did not differ significantly: 14.1% in group 1 and 9.3% in group 2. Thus, we can affirm that co-cultivation of young donor ovarian fluid with oocytes of women of late reproductive age over 40 years old immediately after the ICSI procedure allows to increase the chances for normal fertilization, therefore improving the embryological stage of the infertility treatment program using ART methods..
Then the outcomes of the infertility treatment programs in the study groups were analyzed. The results are shown in Table 5. As the table shows, when the embryo was transferred into the uterine cavity, the pregnancy rate in groups 1 and 2 did not differ and was 18.1% in group 1 and 11.1% in group 2 (p=0.66). It should be noted that in group 1 with co-cultivation of oocytes with EVFF of a young donor, two live full-term children were born from two clinical pregnancies. Nevertheless, the proportion of patients with transfer cancellation due to unsatisfactory quality of embryos in group 1 is significantly lower (22.5%), compared to group 2 without co-cultivation (46.1%) (p=0.021, chi-square test). Thus, we can conclude that the higher fertilization rate identified at the previous stage of the study caused the development of a larger number of blastocysts and their transfer into the uterine cavity or the implementation of PGT-A.
The frequency of aneuploidy in the biopsed blastocysts was then evaluated. In group 1 with co-cultivation of oocytes with donor EVFF, a total of 25 blastocysts (including poor quality blastocysts) underwent PGT-A, the frequency of euploid embryos was 8.0% (2/25). However, in group 2 without co-cultivation, the frequency of euploid embryos accounted for 10.5% (2/19) (p=0.77). According to the presented data, the frequency of euploid embryos with PGT-A in women aged over 40 years did not change and remained extremely low.
Thus, the results of the study show that co-cultivation of oocytes from women of late reproductive age with EVFF of a young donor improves embryological parameters, and increases the percentage of fertilization, but at the same time the clinical outcomes in this cohort of patients remain unchanged.
Discussion
In the recent decades, ART has developed significantly, offering new approaches to infertility treatment. One promising direction in this field is the study of extracellular vesicles contained in FF. These vesicles carry important biomolecules and play a key role in optimization the embryological stage of ART programs. The folicular fluid surrounding mature oocytes is a rich source of extracellular vesicles. They participate in the processes of oocyte maturation, fertilization and embryo development, providing the necessary support for preimplantation.
Modern technologies of extraction and application of EVs have now opened up new high-potential methods of patient-oriented infertility treatment. The prospects include development of additives to culture media, research on identification of specific markers of embryo quality by isolated EVs, improvement of protocols of patient management based on analysis of EV composition in reproductive organs and tissues.
In this study, an attempt was made to improve the conditions for culturing embryos of women of late reproductive age with a young donor EVFF. No similar studies on humans have been found in the published studies. The results of this study showed that with the help of EVFF of a young donor, the fertilization rate increases from 70.0% to 84.8% (p=0.008). These results are not surprising since EVFF contain important molecules for cytoplasmic maturation of female gametes, including small noncoding RNAs [11–13]. Animal models have shown an increased degree of absorption by granulosa cells of EVs obtained from small ovarian follicles, compared with EVs from preovulatory follicles. In addition, these EVs have been observed to induce changes in metabolic and developmental gene expression, as well as in microRNA profiles and global patterns of cellular DNA methylation and hydroxymethylation. These results indicate a significant involvement of follicular EVs in oocyte maturation and early embryo development in vitro [14]. This study indicated that the addition of EVFF during in vitro maturation resulted in an increase in fertilization rates and EV from small ovarian follicles increased the embryo cleavage rate compared to controls.
We also observed an increase in the formation of blastocysts suitable for transfer into the uterine cavity and/or cryopreservation. This is promising factor when adding donor EVFF in women of late reproductive age. It allows to reduce the frequency of transfer cancellation due to unsatisfactory parameters of early embryogenesis. The frequency of cycle cancellation decreases almost by 2 times, from 46.1% to 22.5%, however, the outcomes of infertility treatment programs using ART methods in women over 40 years of age remain unchanged. The frequency of formation of genetically normal blastocysts, even in the group with co-cultivation with the young donor's EVFF, does not exceed 10%. It means a possible reduction in the number of subsequent stimulation cycles to search for an euploid embryo, but the chance still remains low.
This study demonstrated the possibility of donating EVFF, its safety and efficacy. It should be noted that in recent decades EVs have been recognized as “very important particles” in aging and age-related diseases. The measurement, quantification and characterics of plasma EVs have formed the basis for their potential use in clinics. They also may be useful as a biomarker for disease diagnosis and progression control of age-related diseases and physiological aging [15].
The present study showed that the use of EVFF represents a promising direction for the improvement of embryological stage of infertility treatment programs using ART methods. Their potential to improve embryo quality and increase the chances for successful pregnancy opens up new horizons for reproductive medicine. Further research in this area could lead to significant breakthroughs in infertility treatment and improved ART outcomes.
Conclusion
The results presented in the study indicate that co-cultivation of oocytes from women of late reproductive age with donor EVs isolated from ovarian FF is a promising approach to the enhancement of embryological stage of infertility treatment program with ART methods. Co-cultivation makes it possible to improve both the fertilization rate and the formation rate of excellent and good quality blastocysts. The obtained data reveals new possibilities for donating the secretome of biological fluids from reproductive organs and tissues to increase the effectiveness of infertility treatment, including oogenesis and spermatogenesis disorders.
References
- Долгушина Н.В., Адамян Л.В., Шешко Е.Л. Поздний репродуктивный возраст женщины: риски нарушения репродуктивной функции (обзор литературы). Проблемы репродукции. 2023; 29(4): 99-106. [Dolgushina N.V., Adamyan L.V., Sheshko E.L. Late reproductive age of a woman: risks of reproductive dysfunction (literature review). Russian Journal of Human Reproduction. 2023; 29(4): 99-106 (in Russian)] https://dx.doi.org/10.17116/repro20232904199
- Зингеренко Б.В., Бурменская О.В., Сысоева А.П., Шевцова Ю.А., Силачев Д.Н., Макарова Н.П., Калинина Е.А. Изменение транскрипции генов в клетках кумулюса возрастных женщин под действием внеклеточных везикул фолликулярной жидкости молодых доноров. Бюллетень экспериментальной биологии и медицины. 2024; 178(12): 767-73. [Zingerenko B.V., Burmenskaya O.V., Sysoeva A.P., Shevtsova Yu.A., Silachev D.N., Makarova N.P., Kalinina E.A. Gene transcription changes in advanced maternal aged women cumulus cells under influence of young donors follicular fluid extracellular vesicles. Bulletin of experimental biology and medicine. 2024; 178(12): 767-73 (in Russian)] https://dx.doi.org/10.47056/0365-9615-2024-178-12-767-773
- Патент №2801339 C1 Российская Федерация, МПК G01N 33/50, A61B 17/435. Способ использования внеклеточных везикул фолликулярной жидкости для увеличения подвижности сперматозоидов человека в программах экстракорпорального оплодотворения: №2022131353: заявл. 01.12.2022: опубл. 07.08.2023. А.П. Сысоева, Н.П. Макарова, Д.Н. Силачев [и др.]; заявитель Федеральное государственное бюджетное учреждение "Национальный медицинский исследовательский центр акушерства, гинекологии и перинатологии имени академика В.И. Кулакова" Министерства здравоохранения Российской Федерации. [Patent No. 2801339 C1 Russian Federation, IPC G01N 33/50, A61B 17/435. Method of using extracellular vesicles of follicular fluid to increase the motility of human sperm in in vitro fertilization programs: No. 2022131353: appl. 01.12.2022: publ. 07.08.2023. A.P. Sysoeva, N.P. Makarova, D.N. Silachev [et al.]; applicant Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology of the Ministry of Health of the Russian Federation. (in Russian)].
- Sysoeva A., Akhmedova Z., Nepsha O., Makarova N., Silachev D., Shevtsova Y. et al. Characteristics of the follicular fluid extracellular vesicle molecular profile in women in different age groups in ART programs. Life (Basel). 2024; 14(5): 541. https://dx.doi.org/10.3390/life14050541
- Sysoeva A.P., Nepsha O.S., Makarova N.P., Silachev D.N., Lobanova N.N., Timofeeva A.V. et al. Influence of extracellular vesicles from the follicular fluid of young women and women of advanced maternal age with different miRNA profiles on sperm functional properties. Bull. Exp. Biol. Med. 2022; 173(4): 560-8. https://dx.doi.org/10.1007/s10517-022-05589-x
- Nepsha O.S., Burmenskaya O.V., Akhmedova Z.F., Romanov E.A., Sysoeva A.P., Goryunov K.V. et al. Changes in the transcription of proliferation- and apoptosis-related genes in embryos in women of different ages under the influence of extracellular vesicles from donor follicular fluid in vitro. Bull. Exp. Biol. Med. 2024; 176(5): 658-65. https://dx.doi.org/10.1007/s10517-024-06087-y
- da Silveira J.C., Andrade G.M., Del Collado M., Sampaio R.V., Sangalli J.R., Silva L.A. et al. Supplementation with small-extracellular vesicles from ovarian follicular fluid during in vitro production modulates bovine embryo development. PLOS One. 2017; 12(6): e0179451. https://dx.doi.org/10.1371/journal.pone.0179451
- Российская Aссоциация Репродукции Человека. Оценка ооцитов и эмбрионов в лаборатории ВРТ. Методические рекомендации. М.; 2021. 17 с. [Russian Association of Human Reproduction. Assessment of oocytes and embryos in the ART laboratory. Methodological guidelines. Moscow; 2021. 17 p. (in Russian)].
- Sallem A., Santulli P., Barraud-Lange V., Foll N.L., Ferreux L., Maignien C. et al. Extended culture of poor-quality supernumerary embryos improves ART outcomes. J. Assist. Reprod. Genet. 2018; 35(2): 311-9. https://dx.doi.org/10.1007/s10815-017-1063-7
- Биопсия эмбриона на стадии бластоцисты: учебно-методическое пособие для практических занятий по специальности «эмбриология». М.: ФГБУ «НМИЦ АГП им. В.И. Кулакова» Минздрава России; 2024. 51 с. [Embryo biopsy at the blastocyst stage: a teaching manual for practical exercises in the specialty "embryology". Moscow: V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology; 2024. 51 p. (in Russian)].
- Довгань А.А., Ахмедова З.Ф., Сысоева А.П., Зингеренко Б.В., Романов Е.А., Силачев Д.Н., Макарова Н.П., Калинина Е.А. Внеклеточные везикулы фолликулярной жидкости: клинические аспекты и молекулярная биология. Акушерство и гинекология. 2023; 6: 38-43. [Dovgan A.A., Akhmedova Z.F., Sysoeva A.P., Zingerenko B.V., Romanov E.A., Silachev D.N., Makarova N.P., Kalinina E.A. Extracellular vesicles in follicular fluid: clinical aspects and molecular biology. Obstetrics and Gynecology. 2023; (6): 38-43 (in Russian)]. https://dx.doi.org/10.18565/aig.2022.320
- Pan Y., Pan C., Zhang C. Unraveling the complexity of follicular fluid: insights into its composition, function, and clinical implications. J. Ovarian Res. 2024; 17(1): 237. https://dx.doi.org/10.1186/s13048-024-01551-9
- Nejabati H.R., Roshangar L., Nouri M. Follicular fluid extracellular vesicle miRNAs and ovarian aging. Clin. Chim. Acta. 2023; 538: 29-35. https://dx.doi.org/10.1016/j.cca.2022.11.003
- Gabryś J., Pietras N., Kowal-Mierzwa W., Karnas E., Andronowska A., Nowak A. et al. Investigating the impact of extracellular vesicle addition during IVM on the fertilization rate of equine oocytes following ICSI. Reprod. Biol. 2024; 24(4): 100967. https://dx.doi.org/10.1016/j.repbio.2024.100967
- Mas-Bargues C., Alique M. Extracellular vesicles as «very important particles» (VIPs) in aging. Int. J. Mol. Sci. 2023; 24(4): 4250. https://dx.doi.org/10.3390/ijms24044250
Received 17.04.2025
Accepted 11.08.2025
About the Authors
Zumriiat F. Akhmedova, PhD student at the Prof. B.V. Leonov Department for Assisted Technologies in Infertility Treatment, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, zyuka-1997@mail.ru,https://orcid.org/0000-0002-4483-8820
Anastasia P. Sysoeva, PhD, Clinical Embryologist at the Prof. B.V. Leonov Department for Assisted Technologies in Infertility Treatment, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, a_sysoeva@oparina4.ru, https://orcid.org/0000-0002-6502-4498
Maxim Yu. Gavrilov, Junior Researcher at the Prof. B.V. Leonov Department of Assistive Technologies in Infertility Treatment, Academician V.I. Kulakov National
Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, maxgavr67@gmail.com, https://orcid.org/0000-0001-6189-0287
Boris V. Zingerenko, Junior Researcher at the Prof. B.V. Leonov Department of Assistive Technologies in Infertility Treatment, Academician V.I. Kulakov National
Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, b_zingerenko@oparina4.ru, https://orcid.org/0000-0002-8784-5502
Yulia A. Shevtsova, Junior Researcher at the Cell Technologies Laboratory, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, u_shevtsova@oparina4.ru
Denis N. Silachev, Dr. Bio. Sci., Head of the Cell Technologies Laboratory, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, d_silachev@oparina4.ru, https://orcid.org/0000-0003-0581-9755
Alexey N. Ekimov, PhD, Head of the Laboratory of Preimplantation Genetic Testing and Genetic Diagnostics, Academician V.I. Kulakov National Medical Research Center
for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, a_ekimov@oparina4.ru,
https://orcid.org/0000-0001-5029-0462
Natalya P. Makarova, Dr. Bio. Sci., Leading Researcher at the Prof. B.V. Leonov Department of Assistive Technologies in Infertility Treatment, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4,
np_makarova@oparina4.ru, https://orcid.org/0000-0003-1396-7272
Elena V. Kulakova, Dr. Med. Sci., Senior Researcher at the Prof. B.V. Leonov Department of Assistive Technologies in Infertility Treatment, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, e_kulakova@oparina4.ru, https://orcid.org/0000-0002-4433-4163
Elena A. Kalinina, Dr. Med. Sci., Professor, Head of the Prof. B.V. Leonov Department of Assistive Technologies in Infertility Treatment, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, e_kalinina@oparina4.ru, https://orcid.org/0000-0002-8922-2878



