Evaluation of embryonic ploidy
Yashchuk A.G., Gromenko D.D., Nasyrova S.F., Gromenko Iu.Iu.
Embryo aneuploidy is a leading cause of implantation failure and miscarriage during early pregnancy. Preimplantation genetic testing for aneuploidies (PGT-A) enables the assessment of embryo ploidy before transfer; however, it has several limitations. The integration of automated analysis algorithms into embryologists' workflows can significantly enhance embryo selection and mitigate human errors.
Objective: To evaluate the effectiveness of automated analysis algorithms in determining embryo ploidy across different age groups.
Materials and methods: This retrospective study was conducted from January to May 2022 at the Family Medical Center and included embryos from 51 patients who underwent in vitro fertilization (IVF) with PGT-A. The effectiveness of determining euploidy based on blastocyst images was compared with the results obtained through PGT-A. The study utilized the Embryo Ranking Intelligent Classification Algorithm (ERICA 1.0) software.
Results: A total of 117 blastocysts were obtained, of which 101 were subjected to PGT-A and automated analysis: 31 blastocysts from women under 35 years of age (mean age 30.7 years), 39 blastocysts from women aged 35–39 years (mean age 37.4 years), and 31 blastocysts from women over 40 years of age (mean age 42 years). According to the PGT-A results for 101 embryos, the euploidy rate was 51.5%. The accuracy, positive predictive value, negative predictive value, sensitivity, specificity, and area under the ROC curve were 0.74, 0.76, 0.73, 0.73, 0.76, and 0.78, respectively. The most significant results were observed in patients aged < 35 years.
Conclusion: Automated image analysis shows promise as an auxiliary tool for decision-making in embryo selection, particularly in patients over 35 years of age.
Authors' contributions: Yashchuk A.G., Nasyrova S.F. – conception and design of the study, editing of the manuscript; Gromenko Iu.Iu. – data collection; Gromenko D.D. –statistical analysis, drafting of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Acknowledgment: The authors express their gratitude to Dr. Alejandro Chavez-Badiola and his team for providing consent to use the ERICA 1.0 AI in this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the institution.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Yashchuk A.G., Gromenko D.D., Nasyrova S.F., Gromenko Iu.Iu. Evaluation of embryonic ploidy.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (9): 126-132 (in Russian)
https://dx.doi.org/10.18565/aig.2025.106
Keywords
Embryonic aneuploidy is the primary cause of implantation failure and miscarriage during the first trimester of pregnancy [1]. Matorras R. et al. (2024) analyzed 64,071 embryos and found that the incidence of aneuploid embryos is significantly higher in women over 35 compared to younger women (71.76% vs. 47.44%) [2]. Given that most patients who seek assisted reproductive technologies (ART) are women of advanced reproductive age, determining the ploidy status of embryos is critical for improving program outcomes [3].
Preimplantation genetic testing for aneuploidy (PGT-A) via trophectoderm biopsy enables embryologists to ascertain embryo ploidy before transfer and is currently regarded as the gold standard for detecting ploidy abnormalities in embryos. However, PGT-A has several limitations, including the necessity to cancel fresh transfers and implement freeze-all protocols, the potential for false results due to allele loss, loss of heterozygosity during genome amplification during testing, and the high prevalence of mosaicism in human embryos [4]. Additionally, this analysis incurs significant financial costs for patients, as it is not covered by mandatory or private medical insurance in the Russian Federation [5]. Consequently, scientific research is increasingly focusing on developing non-invasive methods for testing embryos for ploidy, aiming to create alternatives to PGT-A.
The standard Gardner embryo morphology assessment is correlated with the ploidy status of embryos; however, it cannot replace PGT-A due to considerable intra- and inter-laboratory variability in results and the potential for measurement errors arising from human factors [6–8]. Various minimally invasive successors to PGT-A have emerged over time, including techniques that analyze blastocyst fluid rather than embryo cells and non-invasive PGT-A that examines spent culture media. Unfortunately, these techniques remain experimental because of uncertainties regarding the origin of the DNA obtained for amplification [8]. Another research method, the study of embryo development kinetics using time-lapse imaging, has led to the identification of new markers for embryo selection, allowing for the documentation and evaluation of embryo morphology and development timelines, as well as the prediction of ploidy status through continuous real-time image tracking [9]. However, it is challenging to assess the information obtained solely through embryologists, as analyzing large amounts of data is required to identify prognostically significant morphodynamic parameters.
Advancements in artificial intelligence (AI) have the potential to bridge the considerable gap between the high demand for non-invasive embryo ploidy predictions and the inadequate accuracy of such assessments by shifting the research focus from a limited set of independent variables to the analysis of extensive databases [10, 11]. The most commonly employed AI algorithms in clinical practice are based on machine learning and its variants, emphasizing the use of neural networks and deep learning (DL), which help minimize the subjectivity of embryologists and enhance selection accuracy [12–14]. According to Bori L. et al. (2020), the synergy of AI and time-lapse imaging has improved research accuracy and yielded promising results [15].
This study aimed to evaluate the effectiveness of automated analysis algorithms in determining embryo ploidy across different age groups.
Materials and methods
This single-center retrospective study included 51 patients who underwent in vitro fertilization (IVF) with preimplantation genetic testing for aneuploidy (PGT-A) at Medical Center Family between January and May 2022.
The inclusion criteria were as follows: completed informed consent form for additional AI assessment, a geneticist's conclusion on the necessity of PGT-A, participation in a freeze-all program, and procurement of at least one blastocyst suitable for PGT-A.
Exclusion Criteria: Arrested development of all embryos; obtaining a morula or blastocyst of unsatisfactory quality for PGT-A on days 5–7 of development; performing a “fresh” transfer; refusal to participate in the study; ambiguous test results (indicating DNA degradation); and repeat embryo biopsy. Patients were categorized into three age groups: under 35, 35 to 39, and over 40, as this parameter significantly influences embryo ploidy [2].
Ovarian stimulation was performed considering the patient’s ovarian reserve. Transvaginal puncture of ovarian follicles occurred 34–36 h after the administration of the trigger—human chorionic gonadotropin (hCG). Oocyte maturity was assessed upon retrieval to select the suitable oocytes for fertilization. Sperm samples were collected and processed for IVF or intracytoplasmic sperm injection (ICSI). Fertilization was performed using either in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) upon oocyte retrieval. Further cultivation, assisted hatching, and standard Gardner embryo grading were performed in accordance with the clinical recommendations of the Russian Ministry of Health for "Female Infertility" and the methodological guidelines of the Russian Association of Human Reproduction (RAHR) [16, 17].
On days 5–7 of development, each blastocyst was photographed separately using an Olympus IX51 microscope at a magnification of 20X for subsequent uploading to the website erica.embryoranking.com [18]. The program available on this website analyzed the photographs using AI developed at the New Hope Fertility Center in Mexico, referred to as ERICA 1.0 (Embryo Ranking Intelligent Classification Algorithm), which consists of two modules [19]. The first module was designed to extract texture patterns from microphotographs and included a preprocessing stage to standardize the ratio of pixels to micrometers. Each image was convolved with 275 specially designed kernel filters to identify and crop the embryo. The images were then automatically segmented into four regions of interest: the background, pellucid zone, trophectoderm, and intracellular mass. Finally, a feature extractor quantitatively assessed the predictors of embryo viability, such as size, internal mass shape, total energy, and entropy measurements, generating a 94-dimensional feature vector for each micrograph. The second module ranks embryos based on the identification and evaluation of the blastocysts. To achieve this, ERICA uses image-based features combined with metadata for each embryo image. This module is a binary classification model created using a deep neural network built on the Python framework, trained on a dataset collected from New Hope centers, which included an additional 50 embryo images with precisely known PGT-A results and clinical outcomes from the Semya Medical Center. In addition to the embryo image and encoding of each cycle separately, the program incorporated information about the patient's age, timing of the procedures, selected method of fertilization, and name of the culture medium.
Subsequently, 5–10 trophoblast cells were biopsied from the blastocysts, and the samples were sent for PGT-A using next-generation sequencing (NGS). The test results were received at least 28 days after the material was sent to the laboratory.
Statistical analysis
Descriptive statistics for continuous variables of the subgroups are presented as the mean (M) ± standard error of the mean (m), given the normal distribution. One-way analysis of variance was used to assess intergroup differences.
The capabilities of automated euploidy determination were compared with those obtained from PGT-A testing. An ERICA score above 0.49 indicates embryo euploidy, whereas a score below 0.49 indicates aneuploidy. The results of PGT-A testing were converted into a binary system: the embryo's euploidy status, as well as low- and medium-level mosaicism, were assigned a value of 1 according to viability predictions, while aneuploid embryos and high-level mosaicism were assigned a value of 0 [20]. The effectiveness of AI was assessed by analyzing contingency tables and calculating the test accuracy, sensitivity, specificity, positive and negative predictive values, and area under the ROC curve (AUC) for all embryos collectively and for each subgroup separately. The website https://www.statskingdom.com/roc-calculator.html was utilized to calculate the latter parameter and construct the graph.
Results
The study included 51 patients: 14 in group I, 18 in group II, and 19 in group III. When comparing the clinical and anamnestic characteristics of the groups, they were comparable in terms of body mass index and duration of infertility (Table 1).
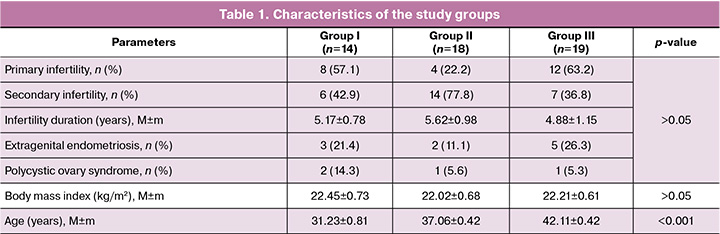
The indications for the study included recurrent miscarriage, maternal late reproductive age, repeated implantation failures, and the presence of chromosomal abnormalities in one of the parents or a previously born child. Additionally, in four cases, the reason for performing PGT-A was to select a Rh-negative euploid embryo due to the Rh conflict that arose in a previous pregnancy.
During ART procedures, 406 eggs were obtained, and 323 oocytes were successfully fertilized. On the 5th to 7th day, 117 blastocysts were obtained, 101 of which underwent PGT-A and automated analysis. To evaluate the effectiveness of the procedures, all embryos that underwent analysis were divided into three groups: those obtained from women younger than 35 years (mean age 30.7 years, 31 blastocysts), those from 35 to 39 years (mean age 37.4 years, 39 blastocysts), and those over 40 years (mean age 42 years, 31 blastocysts). According to the PGT-A results for 101 embryos, the frequency of euploidy was 51.5%.
On this test set, the accuracy of the automated analysis was 0.74, 95% CI [0.5996; 0.8856], positive predictive value – 0.76, 95% CI [0.591; 0.929], negative predictive value – 0.73, 95% CI [0.5565, 0.8945], specificity – 0.76, 95% CI [0.5821; 0.9281], sensitivity – 0.73, 95% CI [0.6108, 0.8508], and area under the ROC curve – 0.78, 95% CI [0.68, 0.87], SE=0.05, p<0.001 (Table 2, Figure).
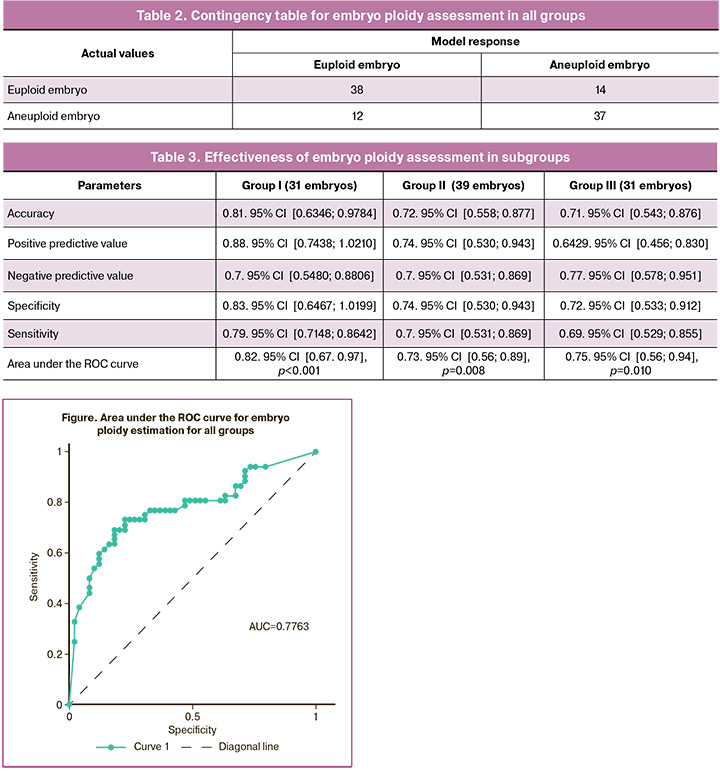
The accuracy of the automated ploidy assessment decreased with age (Table 3), reaching 0.81 for patients younger than 35 years and dropping to 0.71 for those older than 40 years. Overall, this indicator might have been even lower if not for the higher negative predictive value in group III, which exceeded that of the group II patients.
Discussion
The ERICA 1.0 program demonstrated a solid capacity to assess embryo ploidy across different age groups. This algorithm, similar to the standard Gardner classification, evaluates the morphological characteristics of blastocysts, including the trophectoderm, degree of blastocyst expansion, and internal cell mass. However, unlike assessments conducted by embryologists, it is free from subjectivity and can capture a broader range of details than human assessments.
According to a systematic review by Salih M. et al. (2023), when utilizing blind test datasets, the accuracy of embryologists' predictions regarding embryo morphological class averages 65.4% (range 47–75%), while the average prediction accuracy of currently available AI models is 75.5% (range 59–94%) [21]. A meta-analysis conducted by Xin X. et al. (2024) found that the cumulative sensitivity and specificity of various AI systems in predicting embryonic euploidy were 0.71 (95% CI: 0.59–0.81) and 0.75 (95% CI: 0.69–0.80), respectively. This data was derived from an analysis of 6,879 embryos, of which 3,110 were euploid and 3,769 were aneuploid [22]. Thus, our findings align with those of previous studies.
Regarding the prospects for AI integration in ART laboratories, Diakiw S.M. et al. (2022) suggested that AI models could potentially differentiate mosaic embryos based on their level of mosaicism, a capability that has been lacking in PGT-A [23]. Furthermore, the observed high effectiveness of ploidy assessment in patients under 35 years of age, who may not undergo PGT-A due to the absence of indications, could enhance clinical practice by helping to prevent the transfer of embryos with quantitative chromosome abnormalities caused by de novo mutations.
The combination of AI with traditional embryo selection methods, as demonstrated by Diakiw S.M. et al. (2022), presents clear advantages. Notably, this approach reduced the time to pregnancy by 12.2% compared to relying solely on routine morphological assessment, according to Gardner [24].
However, the retrospective nature of the study, small sample size, and potential selection bias towards patients with better prognoses in group I are limiting factors. These issues hinder our ability to confidently extrapolate the results to larger populations, necessitating prospective studies with a larger number of embryos per group.
In conclusion, the automated analysis of embryo ploidy could serve as a crucial tool for embryologists in selecting optimal embryos. Its application saves time on classification and does not require time-lapse imaging or an invasive biopsy. The ongoing enhancement of AI capabilities will result from increased clinical patient data volumes, expanded sample sizes, and algorithm optimization.
Conclusion
Our results demonstrated the high accuracy of embryo ploidy assessment using the ERICA program, with accuracy rates ranging from 0.81 for patients younger than 35 years to 0.71 for those older than 40 years. Additionally, there was a 77% probability that a low score would indicate embryo aneuploidy in women aged > 40 years. These findings suggest that AI algorithms have significant potential for predicting embryonic euploidy based on static images of embryos at 5–7 days of development. Although existing AI models cannot yet completely replace invasive methods for determining embryo ploidy, they represent a promising supplementary tool for embryo selection decisions, especially for patients over 35 years of age.
References
- Melo P., Dhillon-Smith R., Islam M.A., Devall A., Coomarasamy A. Genetic causes of sporadic and recurrent miscarriage. Fertil. Steril. 2023; 120(5): 940-4. https://dx.doi.org/10.1016/j.fertnstert.2023.08.952
- Matorras R., Pérez-Fernández S., Mercader A., Sierra S., Larreategui Z., Ferrando M. et al. Lessons learned from 64,071 embryos subjected to PGT for aneuploidies: results, recurrence pattern and indications analysis. Reprod. Biomed. Online. 2024; 49(5): 103979. https://dx.doi.org/10.1016/j.rbmo.2024.103979
- Российская Aссоциация Репродукции Человека. Регистр ВРТ. Отчет за 2022 год. Доступно по: https://www.rahr.ru/d_registr_otchet/RegistrVRT_2022.pdf [Russian Association of Human Reproduction. ART Register. 2022 Report. Available at: https://www.rahr.ru/d_registr_otchet/RegistrVRT_2022.pdf (in Russian)].
- Casper R.F. PGT-A: Houston, we have a problem. J. Assist. Reprod. Genet. 2023; 40(10): 2325-32. https://dx.doi.org/10.1007/s10815-023-02913-w
- Theobald R., SenGupta S., Harper J. The status of preimplantation genetic testing in the UK and USA. Hum. Reprod. 2020; 35(4): 986-98. https://dx.doi.org/10.1093/humrep/deaa034
- Roos Kulmann M.I., Lumertz Martello C., Bos-Mikich A., Frantz N. Pronuclear and blastocyst morphology are associated age-dependently with embryo ploidy in in vitro fertilization cycles. Hum. Fertil. (Camb). 2022; 25(2): 369-76. https://dx.doi.org/10.1080/14647273.2020.1808716
- Khosravi P., Kazemi E., Zhan Q., Malmsten J.E., Toschi M., Zisimopoulos P. et al. Deep learning enables robust assessment and selection of human blastocysts after in vitro fertilization. NPJ Digit. Med. 2019; 2: 21. https://dx.doi.org/10.1038/s41746-019-0096-y
- Cimadomo D., Rienzi L., Conforti A., Forman E., Canosa S., Innocenti F. et al. Opening the black box: why do euploid blastocysts fail to implant? A systematic review and meta-analysis. Hum. Reprod. Update. 2023; 29(5): 570-633. https://dx.doi.org/10.1093/humupd/dmad010
- Giménez C., Conversa L., Murria L., Meseguer M. Time-lapse imaging: morphokinetic analysis of in vitro fertilization outcomes. Fertil. Steril. 2023; 120(2): 218-27. https://dx.doi.org/10.1016/j.fertnstert.2023.06.015
- Riegler M.A., Stensen M.H., Witczak O., Andersen J.M., Hicks S.A., Hammer H.L. et al. Artificial intelligence in the fertility clinic: status, pitfalls and possibilities. Hum. Reprod. 2021; 36(9): 2429-42. https://dx.doi.org/10.1093/humrep/deab168
- Luong T.M., Le N.Q.K. Artificial intelligence in time-lapse system: advances, applications, and future perspectives in reproductive medicine. J. Assist. Reprod. Genet. 2024; 41(2): 239-52. https://dx.doi.org/10.1007/s10815-023-02973-y
- Diakiw S.M., Hall J.M.M., VerMilyea M.D., Amin J., Aizpurua J., Giardini L. et al. Development of an artificial intelligence model for predicting the likelihood of human embryo euploidy based on blastocyst images from multiple imaging systems during IVF. Hum. Reprod. 2022; 37(8): 1746-59. https://dx.doi.org/10.1093/humrep/deac131
- Paya E., Pulgarín C., Bori L., Colomer A., Naranjo V., Meseguer M. Deep learning system for classification of ploidy status using time-lapse videos. F. S. Sci. 2023; 4(3): 211-8. https://dx.doi.org/10.1016/j.xfss.2023.06.002
- Драпкина Ю.С., Макарова Н.П., Васильев Р.А., Амелин В.В., Франкевич В.Е., Калинина Е.А. Изучение аналитической обработки клинико-анамнестических и эмбриологических данных пациентов в программе вспомогательных репродуктивных технологий различными методами машинного обучения. Акушерство и гинекология. 2024; 3: 96-107. [Drapkina Yu.S., Makarova N.P., Vasilev R.A., Amelin V.V., Frankevich V.E., Kalinina E.A. Application of various machine learning techniques to the analysis of clinical, anamnestic, and embryological data of patients undergoing assisted reproductive technologies. Obstetrics and Gynecology. 2024; (3): 96-107 (in Russian)]. https://dx.doi.org/10.18565/aig.2023.281
- Bori L., Paya E., Alegre L., Viloria T.A., Remohi J.A., Naranjo V. et al. Novel and conventional embryo parameters as input data for artificial neural networks: an artificial intelligence model applied for prediction of the implantation potential. Fertil. Steril. 2020; 114(6): 1232-41. https://dx.doi.org/10.1016/j.fertnstert.2020.08.023
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Женское бесплодие. М.; 2024. [Ministry of Health of the Russian Federation. Clinical guidelines. Female infertility. Moscow; 2024. (in Russian)].
- Российская Ассоциация Репродукции Человека. Секция «Клиническая эмбриология». Оценка ооцитов и эмбрионов в лаборатории ВРТ. Методические рекомендации. М.; 2021. 17 с. [Russian Association of Human Reproduction. Section "Clinical embryology". Assessment of oocytes and embryos in the ART laboratory. Methodological recommendations. Moscow; 2021. 17 p. (in Russian)].
- ERICA. ERICA: artificial intelligence for embryo selection. Available at: https://embryoranking.com/
- Chavez-Badiola A., Flores-Saiffe-Farías A., Mendizabal-Ruiz G., Drakeley A.J., Cohen J. Embryo ranking intelligent classification algorithm (ERICA): artificial intelligence clinical assistant predicting embryo ploidy and implantation. Reprod. Biomed. Online. 2020; 41(4): 585-93. https://dx.doi.org/10.1016/j.rbmo.2020.07.003
- Capalbo A., Poli M., Rienzi L., Girardi L., Patassini C., Fabiani M. et al. Mosaic human preimplantation embryos and their developmental potential in a prospective, non-selection clinical trial. Am. J. Hum. Genet. 2021; 108(12): 2238-47. https://dx.doi.org/10.1016/j.ajhg.2021.11.002
- Salih M., Austin C., Warty R.R., Tiktin C., Rolnik D.L., Momeni M. et al. Embryo selection through artificial intelligence versus embryologists: a systematic review. Hum. Reprod. Open. 2023; 2023(3): hoad031. https://dx.doi.org/10.1093/hropen/hoad031
- Xin X., Wu S., Xu H., Ma Y., Bao N., Gao M. et al. Non-invasive prediction of human embryonic ploidy using artificial intelligence: a systematic review and meta-analysis. EClinicalMedicine. 2024; 77: 102897. https://dx.doi.org/10.1016/j.eclinm.2024.102897
- Diakiw S.M., Hall J.M.M., VerMilyea M.D., Amin J., Aizpurua J., Giardini L. et al. Development of an artificial intelligence model for predicting the likelihood of human embryo euploidy based on blastocyst images from multiple imaging systems during IVF. Hum. Reprod. 2022; 37(8): 1746-59. https://dx.doi.org/10.1093/humrep/deac131
- Diakiw S.M., Hall J.M.M., VerMilyea M., Lim A.Y.X., Quangkananurug W., Chanchamroen S. et al. An artificial intelligence model correlated with morphological and genetic features of blastocyst quality improves ranking of viable embryos. Reprod. Biomed. Online. 2022; 45(6): 1105-17. https://dx.doi.org/10.1016/j.rbmo.2022.07.018
Received 21.04.2025
Accepted 18.08.2025
About the Authors
Alfiya G. Yashchuk, Dr. Med. Sci., Professor, Head of the Department of Obstetrics and Gynaecology No. 2, Bashkir State Medical University, Ministry of Health of Russia, 450008, Russia, Republic of Bashkortostan, Ufa, Lenina str., 3, +7(917)343-17-15, https://orcid.org/0000-0003-2645-1662Daria D. Gromenko, PhD student at the Department of Obstetrics and Gynaecology No. 2, Bashkir State Medical University, Ministry of Health of Russia, 450008, Russia, Republic of Bashkortostan, Ufa, Lenina str., 3, +7(987)473-86-19, https://orcid.org/0000-0001-5638-1779
Svetlana F. Nasyrova, PhD, Associate Professor, Department of Obstetrics and Gynaecology No. 2, Bashkir State Medical University, Ministry of Health of Russia, 450008, Russia, Republic of Bashkortostan, Ufa, Lenina str., 3, +7(919)615-46-52, https://orcid.org/0000-0002-2313-7232
Iuliia Iu. Gromenko, PhD, Chief Physician, Medical Centre "Family", 450054, Russia, Republic of Bashkortostan, Ufa, Oktyabrya Ave., 73 build. 1, +7(917)348-69-86,
https://orcid.org/0000-0002-3373-0873
Corresponding author: Daria D. Gromenko, dasha.gromenko@mail.ru



