В настоящее время в большинстве развитых стран мира наблюдается тенденция к увеличению возраста, в котором женщина впервые рожает или планирует родить ребенка. Это явление все чаще наблюдается и в России [1, 2]. Однако с увеличением возраста женщины растет число сопутствующих соматических заболеваний и патологий репродуктивного тракта, что негативно влияет не только на вынашивание беременности, но и на саму возможность зачатия ребенка [3]. С возрастом женщины снижается как овариальный резерв, так и ухудшается качество яйцеклеток, особенно у женщин старше 36 лет [4]. Все эти факторы приводят к значительному снижению вероятности зачатия ребенка не только самостоятельно, но и с использованием вспомогательных репродуктивных технологий (ВРТ).
В данной работе представлено клиническое наблюдение успешного применения ВРТ с получением одноплодной беременности и рождением здорового ребенка женщиной 42 лет с использованием собственных яйцеклеток и спермы мужа 54 лет, после 13 неудачных попыток ЭКО.
Клиническое наблюдение
В июне 2016 г. пациентка Б., 39 лет, впервые обратилась в Клинику репродукции человека «АльтраВита» по причине отсутствия беременности в течение двух лет при ведении регулярной половой жизни с целью зачатия ребенка. Предварительный клинический диагноз: вторичное бесплодие, обусловленное трубно-перитонеальным фактором и эндометриозом. Из анамнеза известно о наличии множественной миомы матки, спаечного процесса в малом тазу, аденомиоза, сниженного овариального резерва. В браке с 37 лет (брак 1-й). В анамнезе две беременности (вне брака), обе без родоразрешения (в возрасте 27 лет внематочная трубная беременность слева, с последующей лапаротомией и тубэктомией слева; в 29 лет – фармакологический аборт на сроке 6–7 недель, без особенностей).
В 38 лет в результате лапароскопического исследования диагностирован наружный генитальный эндометриоз III степени, эндометриоидная киста левого яичника, аденомиоз и спаечный процесс (III–IV степени) в малом тазу. В результате оперативного лечения были проведены удаление эндометриоидных кист яичника, разделение спаек, коагуляция очагов эндометриоза. При проведении гистероскопии было осуществлено раздельное диагностическое выскабливание.
Кариотип нормальный женский (46, ХХ). По результатам тестирования была выявлена генетическая предрасположенность к тромбофилии (по гену PAI-1).
Эндокринный статус: на момент обращения (39 лет) уровень АМГ в сыворотке крови составил 0,60 нг/мл, с прогрессивным снижением до 0,55 нг/мл – в 40 лет и 0,41 нг/мл – к 41 году. Патологий со стороны щитовидной железы не выявлено. Индекс массы тела – 27,0 кг/м2. С целью снижения избыточной массы тела с 38 лет принимает «Глюкофаж» по 500 мг/сутки.
Возраст мужа Б. на момент обращения в клинику составлял 52 года. Детей нет. Вредные привычки и профессиональные вредности отсутствуют. Перенесенные заболевания и хронические соматические патологии отсутствуют. Анализы на половые инфекции отрицательные, мазок из уретры в норме. Эндокринный статус: ТТГ, ЛГ, ФСГ, эстрадиол, тестостерон общий, глобулин, связывающий половые гормоны – в пределах нормы. Отмечалась гиперпролактинемия (680,8 мМЕ/л). Согласно данным предварительной оценки спермы – астенотератозооспермия. Концентрация сперматозоидов в эякуляте – 71 млн/мл, 29% прогрессивно-подвижных форм и 1% морфологически-нормальных (по Крюгеру) сперматозоидов, MAR-тест – отрицательный, 6% сперматозоидов с фрагментацией ДНК (TUNEL). По результатам ультразвукового исследования (УЗИ) выявлены признаки двустороннего гидроцеле. Кариотип нормальный мужской (46, ХУ).
Дифференциальный диагноз
Вторичное бесплодие сочетанное, обусловленное: трубно-перитонеальным фактором (N97.1), эндометриозом (N80), связанное с мужскими факторами (N97.4), хроническими воспалительными заболеваниями матки (N71.1), хроническим цервицитом (N72), полипом тела матки (N84.0), множественной миомой матки (D25).
Лечение
Данные программ ЭКО, не приведших к наступлению беременности (всего 13), и результаты соответствующих эмбриологических этапов представлены в таблице. Перед проведением первой и последующих программ ЭКО, до 14-й включительно, супружеской паре была назначена соответствующая прегравидарная подготовка.
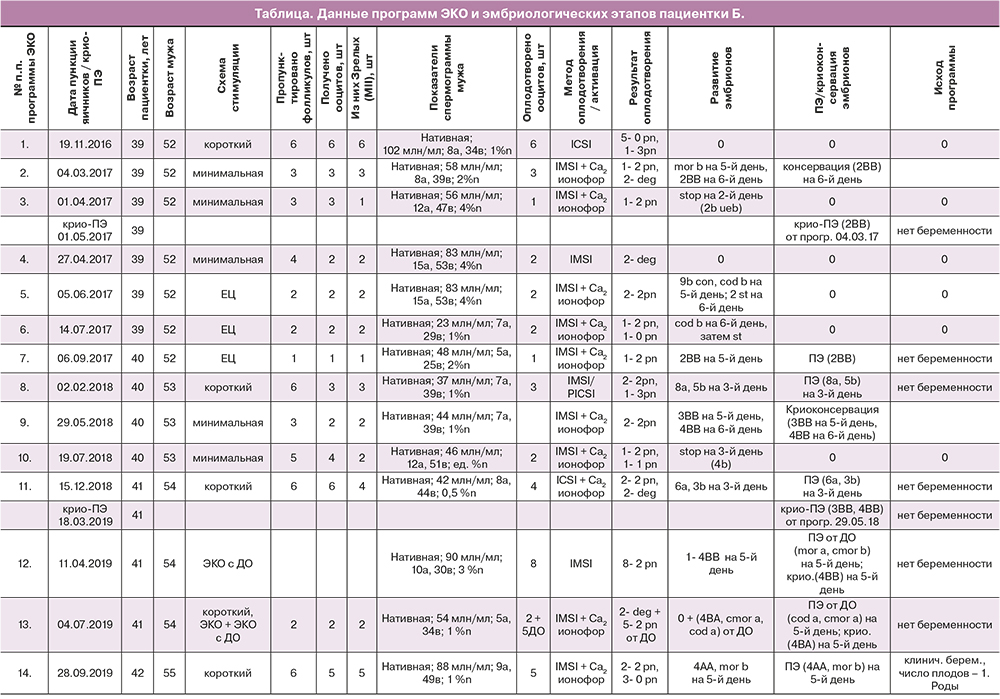
Данные 14-й (успешной) программы ЭКО, завершившейся рождением ребенка
По результатам осмотра пациентки Б. в сентябре 2019 г. начали стимуляцию суперовуляции в коротком протоколе. В течение 4 дней с 10-го дня менструального цикла пациентка получала рекомбинантный ФСГ по 150 МЕ/сутки подкожно (суммарная доза гонадотропина составила 600 МЕ). При достижении размера фолликулов 14 мм был назначен антагонист гонадотропин рилизинг гормона (ГнРГ) 0,25 мг подкожно. На 13-й день менструального цикла при УЗИ в правом яичнике визуализировались 2 антральных фолликула размером 11,0 мм, в левом – 5 фолликулов: 3 – размером 20 мм, 1–19 мм и 1–13,5 мм. На вечер 13-го дня менструального цикла был назначен двойной триггер овуляции: агонист ГнРГ (0,2 мг) подкожно и гонадотропин хорионический (9000 МЕ) внутримышечно. В день введения триггера овуляции содержание гормонов в плазме крови пациентки Б. составило: ЛГ – 1,2 мМЕ/мл, эстрадиол – 3937,0 пмоль/л, прогестерон – 2,62 нмоль/л. Пункция фолликулов была проведена через 35 ч после введения триггера овуляции, т. е. наутро 15-го дня менструального цикла.
Трансвагинальная пункция яичников
Аспирацию фолликулов выполняли под внутривенным наркозом. При помощи иглы K-OPSD-1735 B-L (Cook) было пропунктировано 6 фолликулов, получено 5 ооцит-кумулюсных комплексов. Через 3 ч после проведения пункции, пациентка Б. в удовлетворительном состоянии была выписана из палаты дневного стационара клиники.
Подготовка спермы
Для оплодотворения использовался свежий образец спермы мужа пациентки Б. Подвижные сперматозоиды отбирали путем осаждения в двухступенчатом градиенте плотности в среде ALLGrad (LifeGlobal, Belgium), после чего дважды отмыли и осадили в среде Sperm Wash (VitroMed, Germany). Для оплодотворения использовали свободно флотирующие сперматозоиды.
Оплодотворение
Через 3 ч после трансвагинальной пункции 5 ооцит-кумулюсных комплексов были денудированы посредством мягкого пипетирования в растворе гиалуронидазы (Hylase Vitromed, Germany). Спустя 2 часа после денудации (5 ч после пункции) 5 зрелых ооцитов MII были оплодотворены методом IMSI. Иммобилизацию сперматозоидов проводили в растворе PVP (LifeGlobal, Belgium). Процедура IMSI выполнялась на инвертированном микроскопе Leitz Fluovert FU (Wild Leitz GmbH, Germany), оборудованном комплектом микроманипуляторов Narishige (Narishige, Tokyo, Japan). Спустя 1 ч после процедуры IMSI оплодотворенные яйцеклетки подвергли дополнительной химической активации к развитию Са-ионофором A23187 (20 µg\ml) в течение 2 мин.
Культивирование эмбрионов
Культивирование осуществляли в СО2-инкубаторе при 36,8°C в увлажненной атмосфере с 6,0% СО2 и 20% O2. С 0-го по 4-й день развития эмбрионы культивировали на фидерном слое, состоящем из эпителиальных клеток эндоцервикса человека в 4-луночном планшете (Nunclon Delta Treated 4-Well IVF Dish, Thermo Scientific, Denmark) в 200 мкл каплях среды Onestep Vitromed (Germany) с добавлением 5 мг/мл белка (LifeGlobal Protein Supplement, Belgium) под слоем парафинового масла (Light Paraffin Oil, Vitromed, Germany). Эмбрионы перемещали в новую каплю на клеточный монослой со свежей средой через каждые 36 часов. Вечером 4-го дня развития эмбрионы рассаживали в индивидуальные 25 мкл капли среды Onestep Vitromed (Germany) с добавлением 5 мг/мл белка (LifeGlobal Protein Supplement, Belgium), покрытые слоем парафинового масла (Light Paraffin Oil, Vitromed, Germany). Оценку развития эмбрионов проводили на 1, 3 и 5-й дни развития.
Оценка качества эмбрионов
Через 17 ч после процедуры IMSI, производилась оценка оплодотворения. В цитоплазме двух из 5 зигот визуализировалось по два пронуклеуса, а в перивителлиновом пространстве наблюдались выделившиеся вторые полярные тельца. В 3 из 5 клеток – пронуклеусов и вторых полярных телец не наблюдалось. На 3-й день развития два эмбриона с нормальным оплодотворением были оценены как 7а и 9а. На 5-е сутки развития эмбрионы были оценены как морула, качества b и бластоциста, качества 4AA (по Gardner D.K., 2000) [5].
Перенос эмбрионов (ПЭ)
Перенос двух эмбрионов в полость матки был осуществлен на 5-й день после оплодотворения (21-й день менструального цикла). На момент переноса, эмбрионы оценивались как морула, качества b и бластоциста, качества 4AA. ПЭ осуществляли с использованием катетера Labotect Embryo Transfer Catheter Set (Germany) под абдоминальным контролем УЗИ. Эмбрионы в минимальном объеме (≈ 10 мкл) культуральной среды Onestep Vitromed (Germany) с добавлением 7,5 мг/мл белка (LifeGlobal Protein Supplement, Belgium) были инжектированы в полость матки. Толщина эндометрия на момент ПЭ составила 10 мм. ПЭ прошел без особенностей.
Подготовка эндометрия и оценка наступления беременности
После ПЭ в течение 15 дней были назначены: микронизированный прогестерон 600 мг/сутки, вагинально; фолиевая кислота 800 мкг/день, per os. На 14, 16 и 20-й день после ПЭ был назначен анализ крови на содержание β-хорионического гонадотропина. На 23-й и 33-й день после ПЭ – УЗИ органов малого таза, была диагностирована клиническая беременность (5–6 недель). В полости матки визуализировалось одно плодное яйцо, размером 16 мм. На 33-й день после ПЭ в полости матки визуализировалось 1 плодное яйцо, размером 39×26,7 мм, с 1 живым эмбрионом. Беременность протекала без особенностей. На 39-й неделе гестации пациентка самостоятельно родила здоровую девочку весом 3190 г.
Учитывая анамнез пациентки (сниженный овариальный резерв) и опыт неудачных попыток ЭКО (повторяющиеся неудачи в оплодотворении и развитии эмбрионов), коллегиально было принято решение об экстенсивном накоплении эмбрионов пригодных для ПЭ или криоконсервации. Для этого в естественном цикле (ЕЦ) или в ЕЦ с минимальной стимуляцией получали ооциты пациентки с последующим их оплодотворением. В зависимости от развития эмбрионов проводился либо «свежий» ПЭ, либо криоконсервация и перенос размороженных эмбрионов. Для повышения шансов на получение беременности может быть реализовано две стратегии, касательно стадии развития и числа переносимых эмбрионов: перенос нескольких эмбрионов на стадии дробления; перенос 1–2 бластоцист в «свежем» или криоцикле. В случае с пациенткой Б., в программах с использованием собственных яйцеклеток, были применены обе стратегии. В программах № 2, 7, 9 была реализована стратегия ПЭ на стадии бластоцисты в цикле стимуляции (№7) или криоцикле (№ 2, 9). ПЭ 3-го дня развития на стадии дробления был предпринят в программе № 8 и 11. Однако в программах с 1-й по 13-ю, при использовании как собственных яйцеклеток, так и ооцитов донора (ДО), получить беременность не удалось. В 14-й (успешной) программе, на 5-й день развития был осуществлен перенос 2 эмбрионов, бластоцисты 4АА и морулы качества b (таблица).
Надо отметить, что в связи со сложностью получения эмбрионов хорошего качества, пригодных для ПЭ, коллегиально, было принято решение об отказе от преимплантационного генетического тестирования. После наступления беременности, на 9-й неделе гестации был проведен неинвазивный пренатальный ДНК тест, по результатам которого было получено заключение: вероятность специфических аномалий хромосом плода – низкая, пол плода – женский.
В результате, выбранная стратегия ведения бесплодной пары, оказалась успешной и привела к рождению здорового ребенка.
Обсуждение
Основной целью ВРТ является рождение здорового ребенка. Однако полученный результат будет зависеть от множества различных независимых факторов [6]. При выборе стратегии терапии врач-репродуктолог вынужден учитывать множество индивидуальных особенностей пациентки, маркеры ее овариального резерва, историю болезни и неудачных попыток ЭКО (при наличии), а также прогнозировать ответ на овариальную стимуляцию [7].
Количество ооцитов, получаемых после стимуляции суперовуляции, признано одним из самых значимых факторов, влияющих на эффективность ВРТ. Большинство специалистов считают оптимальным получение 10–15 ооцитов [8]. Однако значительная часть пациенток, проходящих лечение бесплодия методами ЭКО, имеет либо бедный (<4 ооцитов), либо субоптимальный (4–9 ооцитов) ответ яичников на стимуляцию [7, 8]. Как следствие, число эмбрионов, пригодных для переноса или криоконсервации, может значительно сократиться, поставив под угрозу успех всей программы [7]. Также, кумулятивные финансовые затраты на проведение успешного цикла ЭКО, выше у пациенток со сниженным или субоптимальным ответом, чем у женщин с нормальным овариальным ответом. Совокупно эти факторы приводят к эмоциональному, физическому и финансовому истощению для пары, в особенности, когда требуется многократное проведение циклов ЭКО.
У большинства женщин со сниженным овариальным резервом, вне зависимости от их возраста, велика вероятность отмены ПЭ в связи с отсутствием эмбрионов надлежащего качества для переноса [9]. Поэтому при ведении таких «сложных» пациенток целесообразно использование индивидуальных стратегий для увеличения результативности циклов ЭКО [7]. При этом стратегии, направленные на достижение беременности, могут затрагивать не только сферу непосредственно стимуляции суперовуляции, но также касаться и остальных этапов ВРТ и здоровья супружеской пары в целом. Диагностические мероприятия, направленные на выявление хронических заболеваний репродуктивного тракта и экстрагенитальной сферы, а также комплекс мер по их лечению; корректировка гормонального статуса как мужчины, так и женщины; терапия по увеличению мужской фертильности; способ оплодотворения и дополнительная активация к развитию эмбриона; система культивирования эмбрионов, направленная на минимизацию влияния стресс-факторов на развитие эмбриона – совокупность данных мероприятий позволяет значительно увеличить вероятность получения беременности и рождения ребенка.
Снижение фертильности женщины при старении обусловлено, как прогрессивным уменьшением числа первичных фолликулов в яичниках, так и усугубляющимися дисфункциональными состояниями ооплазмы и увеличением числа хромосомных аномалий в ооцитах [10]. Предимплантационное генетическое тестирование с использованием сравнительной геномной гибридизации и высокопроизводительного секвенирования NGS показывают, что показатели эуплоидии эмбрионов у женщин моложе 35 лет заметно выше, чем у более возрастных пациенток [11]. Эуплоидность эмбрионов является одним из ведущих факторов, объясняющих различия в показателях успешности ЭКО у молодых и возрастных женщин [12].
Вероятность получения у пациенток после ЭКО, по крайней мере, одной эуплоидной бластоцисты для ПЭ, возрастает с увеличением общего числа полученных бластоцист, вне зависимости от возраста женщины [11]. Поэтому в случае с подобными «сложными» парами, стратегия получения беременности методами ЭКО, может заключаться в экстенсивном наборе «из цикла в цикл» ооцитов, их оплодотворении и получении максимально возможного количества эмбрионов для переноса. На получение необходимого количества эмбрионов, пригодных для переноса, может влиять не только количество и качество ооцитов, но и фертильные свойства спермы, так как от способности сперматозоидов к снятию у ооцитов блока MII и последующей активации к развитию будет зависеть доля зигот с нормальным оплодотворением (наличие 2 пронуклеусов) и развитие эмбрионов до предимплантационных стадий. С целью повышения оплодотворяемости, особенно в случаях с повторными неудачами при оплодотворении, после процедуры ICSI/ IMSI, ряд авторов рекомендует проведение вспомогательной активации яйцеклеток Са-ионофором А 23187 [13]. В 10 из 14 программ ЭКО пациентки Б. была использована процедура активации. В последней удачной программе, закончившейся беременностью и родами, также были использованы IMSI и активация Са-ионофором.
На эффективность эмбриологического этапа, а именно, на получение эмбрионов хорошего качества пригодных для ПЭ или криоконсервации, большое влияние оказывает, непосредственно, система культивирования in vitro. В ряде работ было показано, что использование системы сокультивирования эмбрионов с соматическими клетками позволяет улучшить качество самих эмбрионов и приводит к увеличению доли беременностей [14–16]. Было отмечено, что при сокультивировании эмбрионов на фидерном слое клеток (клетки Vero) происходит более ранее экспандирование бластоцист, синтез эмбрионом хорионического гонадотропина и хетчинг [14–16]. Крупнейшие исследования в области ЭКО человека показали положительное влияние сокультивирования на клинические исходы программ ВРТ при использовании аутологичных клеток матки [15, 16], клеток эндометрия [14] и клеток Vero. Положительное влияние сокультивирования становится еще более заметным в случае проведения программ у бесплодных пар с повторяющимися неудачными попытками ЭКО. Также была обнаружена существенная разница по показателям жизнеспособности после криоконсервации и оттаивания между бластоцистами, полученными при сокультивировании и бластоцистами, полученными в обычном протоколе культивирования [17, 18].
В исследованиях, касающихся сокультивирования эмбрионов, было продемонстрировано, что при переносе таких бластоцист, соотношение полов детей при рождении не изменено (1 014 мальчиков/945 девочек: 51,7%), а вес при рождении – не увеличен [19, 20]. Неоднократно сообщалось, что при переносе эмбрионов человека 5–6-го дня развития, культивировавшихся in vitro, наблюдается увеличение частоты формирования монозиготных двоен. Однако, впоследствии, было выявлено, что возникновение данного феномена, как правило, связано с само́й средой для культивирования, которая не обладает достаточными антиоксидантными свойствами, способными предотвратить апоптоз [21, 22]. При использовании системы сокультивирования, число монозиготных двоен не увеличивается. Клетки фидерного слоя выделяют в среду культивирования серосодержащие антиоксиданты, такие как гипотаурин, являющийся естественным акцептором свободных радикалов в среде, где развиваются эмбрионы [23, 24].
При сокультивировании эмбрионов на фидерном слое значительно повышается доля образования бластоцист с использованием донорских ооцитов (70,5% против 56,4%, р<0,0001) [25]. При использовании фидерного слоя из клеток Vero у пациентов с повторными неудачными имплантациями в анамнезе удалось получить клиническую беременность в 39,3% случаев в программах с переносом одной селективной бластоцисты [26]. В метаанализе [27] было показано наличие статистически значимого положительного влияния сокультивирования эмбрионов на частоту имплантации, клинической и прогрессирующей беременности.
Заключение
Индивидуальный подход в выборе методических решений (со стороны эмбриолога) и терапии (со стороны врача-репродуктолога и андролога) для конкретной бесплодной пары, позволяет получать беременность и роды у возрастных пар с многократными повторяющимися неудачными попытками ЭКО.
Согласие пациентов
От пациентки Б. и ее мужа получено письменное добровольное информированное согласие на публикацию персональной медицинской информации в обезличенной форме в журнале «Акушерство и гинекология».



