On the possibility of mosaic embryos transfer in the programmes of assisted reproductive technologies
Sidorchuk M.A., Ekimov A.N., Karetnikova N.A., Zaretskaya N.V., Sadelov I.O., Stupko O.K.
Chromosomal mosaicism is a common finding in human embryos.
Objective: To determine the possibility of mosaic embryos transfer in the absence of euploid embryos and the importance of medical genetic counseling.
Materials and methods: 15 women with no prospects for obtaining euploid embryos were examined in the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia during the period of 2022–2024. Preimplantation genetic testing (PGT) of all embryos was performed by high-performance next-generation sequencing on the Illumina platform (AneuPGT). Confirmatory prenatal diagnostics in 5 (33.3%) cases was performed in amniotic fluid by chromosomal microarray analysis and in one case a FISH method was additionally used.
Results: The following chromosomes were involved in mosaicism: 2, 5, 7, 8, 9, 10, 11, 14, 15, 16, 19, 20, 22, Y. Among the 16 (two mosaic embryos were transferred in one case) analyzed embryos, 10 (62.5%) had numerical mosaicism and in 6 (37.5%) embryos segmental mosaicism was found. Successful implantation occurred in 10 cases (with 5 cases of segmental mosaicism and 5 cases of numerical mosaicism), in one case a 9-week non-developing pregnancy took place, in 4 cases pregnancy did not occur. Afterwards 10 clinically healthy children were born.
Conclusion: Selection and transfer of mosaic embryos is possible and indicated for spouses with a burdened medical history and reduced reproductive potential according to medical genetic counseling, which allows the couple to give birth to a healthy child in the absence of euploid embryos.
Authors’ contributions: Karetnikova N.A. – study concept and design, manuscript editing, genetic counseling; Zaretskaya N.V. – general supervision, genetic counseling; Sidorchuk M.A. – data collection and processing, statistical processing; Ekimov A.N. – conduction of PGT using NGS-method; Sadelov I.O., Stupko O.K. – conduction of prenatal genetic and cytogenetic testing.
Conflicts of interest: The authors declare no conflicts of interest.
Funding: The study had no sponsorship.
Ethical Approval: The study was approved by the local ethics committee of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Sidorchuk M.A., Ekimov A.N., Karetnikova N.A., Zaretskaya N.V., Sadelov I.O., Stupko O.K. On the possibility of mosaic embryos transfer in the programmes of assisted reproductive technologies.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (9): 109-116 (in Russian)
https://dx.doi.org/10.18565/aig.2025.153
Keywords
Nowadays, around 17.5% of the adult population in the world, approximately 1 in 6, suffer from infertility [1]. The number of infertile couples all over the world is increasing, as evidenced by the growth in registered assisted reproductive technology (ART) cycles from approximately 140,000 in 1991 to more than 3.2 million in 2018 [2].
The use of ART methods with preimplantation genetic testing of embryos for chromosomal abnormalities (PGT-A) made it possible to determine the chromosomal status of the analyzed sample. However, some problems occurred, among which is the embryo transfer with segmental aneuploidies and mosaicism [3].
Embryo mosaicism is defined as the presence of two or more cell populations with different genotypes. It was first identified using fluorescent in situ hybridization [4–6]. According to the data, its frequency in PGT-A ranges from 2 to 40% [7, 8].
The reasons for mosaic embryos may be:
- individual embryo characteristics;
- biopsy techniques;
- technical characteristics of laboratory methods (artifact);
- patient-associated factors (karyotype characteristics).
Embryo mosaicism can be differentiated by: degree – percentage of aneuploidies; number of chromosomes involved: simple – one chromosome, or complex – two or more chromosomes; prevalence – segmental or numerical; type of cells affected – inner cell mass, trophectoderm, or both [8–11].
PGDIS was the first organization to announce criteria for the selection of mosaic embryos. According to the current 2021 guidelines, consideration should be given to:
- level of mosaicism: low mosaicism embryos prevail over high mosaicism embryos (cutoff threshold not defined);
- type of mosaicism: segmental mosaicism dominates over total mosaicism of the whole chromosome;
- embryo morphology;
- need for invasive prenatal diagnostics [12].
The aim of the study is to determine the possibility of mosaic embryos transfer in the absence of euploid embryos and the importance of medical genetic counseling. The cases comprise the transfer of mosaic embryos in ART programs.
Materials and methods
During the period of 2022–2024, ART cycles with PGT-A were performed in 2965 women at the V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. Chromosomal mosaicism was detected in 20% of embryos. This article represents cases of 15 women patients aged 28–48 (with mean age of 41.2 years) in whom euploid embryos for transplantation were not identified. A total of 7,500 embryos were analyzed by PGT-A; mosaicism was detected in 16 (0.21%) blastocysts (one woman had 2 mosaic embryos).
PGT-A of all embryos was conducted using the ReproLine reagent kits for detection of chromosomal abnormalities in single cells on the F-Genetics system, as well as using a reagent kit for detection of chromosomal abnormalities in single cells by high-throughput NGS sequencing on the Illumina platform (AneuPGT).
Mosaic embryo transfer was performed in 15 women who signed informed consent for embryo implantation (in 2 cases – two mosaic embryos were available, in 13 cases there was only one mosaic blastocyst). All patients were informed about the possible adverse effects of the mosaic embryo transfer: the absence of pregnancy, the increased risk of non-viable pregnancy and fetal malformations, the risk of placental insufficiency and premature birth. All women with a favorable transplantation outcome are recommended to undergo invasive prenatal testing using transabdominal amniocentesis at 17–20 weeks. Three patients had contraindications for intrauterine intervention and were recommended a dynamic monitoring of pregnancy progression.
Chromosomal microarray analysis of amniotic fluid cells using CytoScan Optima Suite by Thermofisher (USA) was performed in 5 women and 6 fetuses (transfer of 2 mosaic embryos in one case), diagnostics with FISH-mehod of chromosome 8 in one fetus was carried out using an Axio Imager A2 microscope, Zeiss (Germany) on Isis software, MetaSystems (Germany).
Results
The most prevailing reasons for conducting IVF with PGT-A included: advanced reproductive age of women (over 35 years) and a complicated gynecological history in 13 (86.7%) cases, the advanced reproductive age of the woman in 1 (6.7%) case and male factor infertility in 1 (6.7%) case as well. The rate of primary infertility cases correlated with secondary infertility and was observed in 8 (53.3%) and 7 (46.7%) cases, respectively. The average duration of infertility was 5.8 years. Reduced ovarian reserve was noted in 13 (86.7%) women. The level of anti-mullerian hormone (AMH) comprised 0.45–3.37 ng/ml with the average AMH level of 1.52 ng/ml. The number of in vitro fertilization (IVF) cycles ranged from 1 to 8 (with 3.4 on average). Donor sperm was used in 4 (26.7%) cases, donor oocytes in 3 (20%) cases, and surrogacy in 1 (6.7%) case.
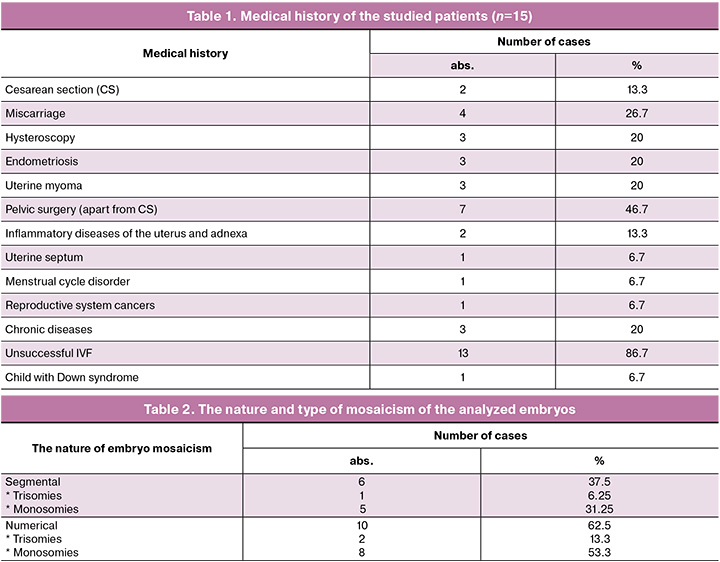
All patients had a complicated obstetric history, either isolated or in combination with gynecological and other diseases (Tables 1 and 3).
The main condition for mosaic embryos transfer was the lack of prospects for obtaining euploid embryos in the studied women patients.
Pregnancies confirmed by biochemical and ultrasound diagnostic methods occurred in 10/15 (66.7%) cases of mosaic embryos transplantation for chromosomes 2, 5, 8, 10, 11, 14, 15, 16, 19, 20, 22. Healthy children were born in 9/10 (90%) successful pregnancies. According to the ultrasound data no abnormalities in the fetal phenotype were detected in any of the observations. No abnormalities in the growth and weight indicators of the newborns were found. In 1/10 (10%) cases, pregnancy progresses and proceeds without complications. In 1/15 (6.7%) women, a miscarriage occurred at 9 weeks of gestation due to the mosaicism of the embryo on the Y chromosome. Pregnancy did not happen in 4 (26.7%) cases – with mosaicism of the embryo on chromosomes 7, 9 and in 2 cases – on chromosome 22 (Table 3).
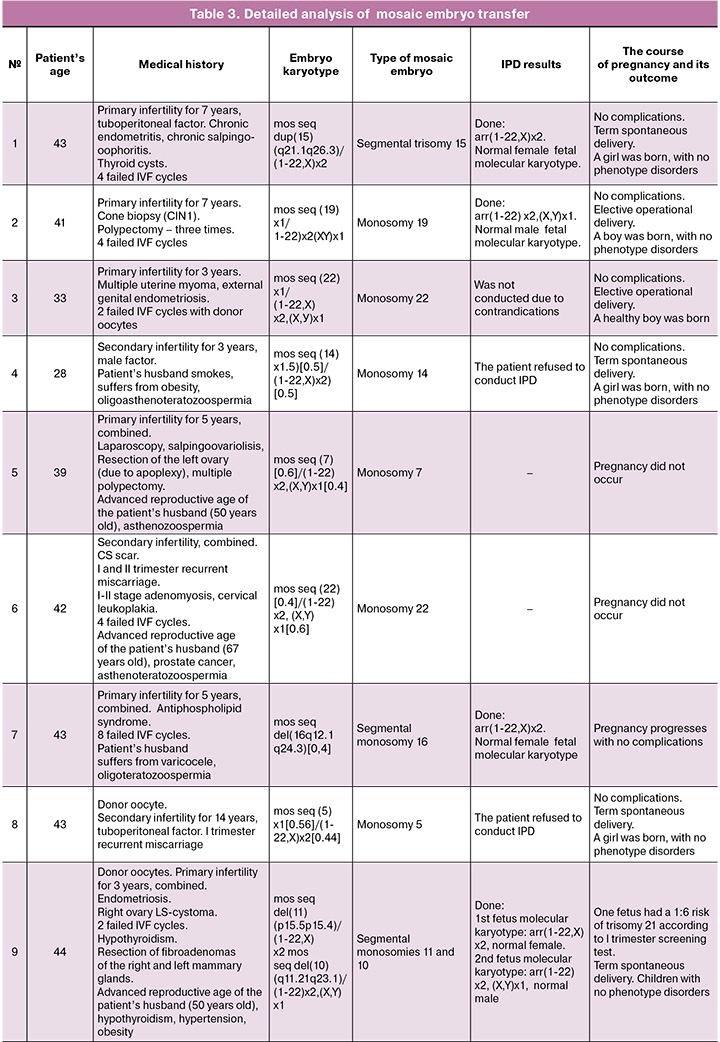
Numerical mosaicism was identified in 10/16 (62.5%) of the analyzed embryos (in one case two mosaic embryos were transferred) and segmental mosaicism – in 6/16 (37.5%) cases. Numerical mosaicism dominated over segmental, as did monosomies over trisomies. At the same time, numerical monosomies were detected in 8 (50%) embryos, numerical trisomies in 2 (12.5%) cases, segmental monosomies in 5 (31.25%) embryos, and segmental trisomy was observed in 1 (6.25%) case. The following chromosomes contained mosaicism: 2, 5, 7, 8, 9, 10, 11, 14, 15, 16, 19, 20, 22, Y. Similarity was noted in 3 (18.75%) embryos on chromosome 22. The number, nature and type of mosaicism of embryos are presented in Table 2.
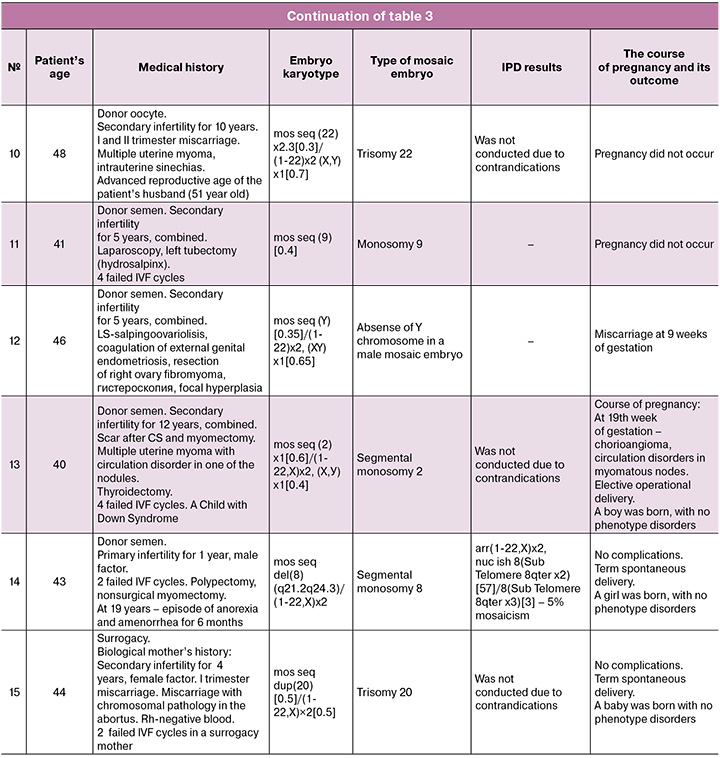
Due to their unique nature, we provide a detailed description of each case in Table 3.
According to the above mentioned data (Table 3), all patients had various combinations of the age with gynecological and other pathology. 5 women became pregnant when transferring embryos with segmental mosaicism on chromosomes 2, 8, 10, 11, 15, 16 (in one case two embryos with segmental monosomies were transferred) (Table 3, cases 1, 7, 9, 13, 14). Among them, invasive prenatal diagnostics was conducted in 4 women (Table 3, observations 1, 7, 9, 14); and in one case amniocentesis was not performed due to the presence of multiple uterine fibroids with circulatory disorders in one of the myomatous nodes (Table 3, case 13).
Transfer of embryos with numerical mosaicism led to pregnancy only in 5/10 (50%) women. These were embryos with trisomy of chromosome 20 (Table 3, case 15) and monosomies of chromosomes 5, 14, 19, 22 (Table 3, cases 2, 3, 4, 8). Amniocentesis was carried out in one case (Table 3, case 2) and was not performed in 2 cases due to contraindications (Table 3, cases 3 and 15), in 2 cases patients refused to undergo the procedure (Table 3, cases 4 and 8).
In 5/10 (50%) normally progressing pregnancies with invasive prenatal testing, normal molecular karyotypes of the fetuses were identified. 3/10 (30%) pregnant women had contraindications for intrauterine intervention during pregnancy, and 2/10 (20%) refused to undergo invasive diagnostics.
When transferring a male embryo with the absence of chromosome Y in mosaic form (Table 3, case 12), the pregnancy was arrested at 9 weeks. Transferring of embryos with trisomy on chromosome 22 (Table 3, case 10) and with monosomies on chromosomes 7, 9, 22 (Table 3, cases 5, 6, 11) did not induce pregnancy. As seen from Table 3, all these women had severe gynecological pathology.
Discussion
This article is the first national experience based on a small number of cases with mosaic embryos transfer into the uterine cavity in married couples with various reproductive disorders and the inability to obtain euploid embryos. In some publications there are data on the onset of pregnancy and the outcome of childbirth after mosaic embryos transfer [13]. Although, there is no detailed medical history of spouses in any of these studies.
It is known that the risk of aneuploidies in the offspring increases with the advancing age of women [14, 15]. There is no data on the relationship between these indicators in mosaicism. In the present study (Table 3), mosaic embryos were identified in women aged 28 and 33 (cases 4 and 3). We noted that in one of the spouses or in a married couple there was thyroid dysfunction, isolated or in combination with other hormonal disorders: thyroid cysts (Table 3, case 1); hypothyroidism, fibroadenomas of both mammary glands, cystoma of the right ovary in the woman and hypothyroidism with obesity in the husband (Table 3, case 9); diffuse toxic goiter and thyrotoxicosis in combination with thyroidectomy and myomectomy (Table 3, case 13); polypectomy, myomectomy and episodes of amenorrhea (Table 3, case 14); the presence of antiphospholipid syndrome (Table 3, case 7); hypothyroidism in the husband and in the woman with the use of donor oocytes (Table 3, case 9). Segmental type of embryo mosaicism was identified in all 5 cases. Pregnancies in these women proceeded without complications. Karyotypes of fetuses according to invasive prenatal diagnostics were normal (in case 13 IPD was not performed due to circulatory disorder in one of the myomatous nodes). The newborns are clinically healthy. Thus, it is possible to assume that there is a connection between thyroid dysfunction and an increased risk of chromosomal abnormalities in the trophoblast. At the same time, the cellular mass remains intact.
Failure to conceive and early pregnancy loss differed between embryos carrying segmental and numerical chromosomal abnormalities, which is consistent with foreign data [16]. According to the results of this study, pregnancy occurred and resulted in the birth of a clinically healthy child in all (100%) cases of transfer of an embryo with segmental mosaicism, whereas in the case of an embryo transfer with numerical mosaicism postive outcomes occurred only in 50% of cases, which confirms the high potential of transfer of embryos with segmental mosaicism.
The number of mosaic monosomies in embryos was higher than mosaic trisomies: 13 (81.25%) and 3 (18.75%), respectively. There is currently insufficient data to explain this phenomenon, and a larger patient population is needed for further study.
According to the world community, after assessing the ploidy of embryos, it is recommended to take into account the level of mosaicism and the type of chromosomes [12]. The present study demonstrates more positive data regarding the transfer of embryos with a high level of numerical mosaicism regardless of the chromosome. Pregnancy occurred upon implantation of embryos with a high level of mosaicism in 4 (26.7%) cases: 50% for chromosomes 14 and 20, monosomy and trisomy, respectively (Table 3, cases 4 and 15); 56% – monosomy for chromosome 5 (Table 3, case 8); 60% – monosomy for chromosome 2 (Table 3, case 13). Only in 1 case (26.7%) (Table 3, case 5) with mosaicism on chromosome 7, an embryo transfer was noted without effect, which is probably due to the woman’s obstetric and gynecological history and multiple surgical interventions on the pelvic organs.
It has been established that the transfer of embryos with mosaic aneuploidies in chromosomes 21, 18, 13, which suggest the development of chromosomal syndromes with viability, is not recommended. Such cases are not presented in this article. A non-developing pregnancy with a male fetus in the absence of a Y chromosome in a mosaic form is most likely associated with mortality and incompatibility with live birth.
After mosaic embryos transfer and the onset of pregnancy, invasive prenatal testing is indicated. The best period to conduct the analysis in amniotic fluid is 17–20 weeks of gestation, due to the absence of placental mosaicism and the lowest risk of complications [17, 18]. In the present study, invasive prenatal testing with chromosomal microarray analysis was performed in 5 (50%) out of 10 normal pregnancies (Table 3, cases 1, 2, 7, 9, 14) with normal molecular karyotypes of the fetuses obtained. In one case FISH diagnostics was carried out (Table 3, case 14). We expect that in pregnancy without fetal abnormalities, the management tactics will not change based on the level of mosaicism. Therefore, chromosomal microarray analysis is sufficient and there is no practical need for FISH testing. However, this article does not provide enough data to confirm this.
Conclusion
Being the only available option in ART programs, transfer of mosaic embryos is possible and recommended for couples with a burdened medical history and reduced reproductive potential.
All patients undergoing IVF with PGT-A should be recommended to have genetic testing at the preimplantation stage and, if pregnancy occurs, to undergo the test at the prenatal stage. They should be counselled on the potential risks, benefits, and limitations of diagnostic methods, the potential clinical outcomes of mosaic embryo transfer, and the importance of dynamic monitoring of such pregnancies.
Selection and transfer of mosaic embryos should be carried out according to recommendations. The results of our own research show that with the onset of a normal pregnancy without ultrasound markers of chromosomal pathology in the fetus in the first trimester, and even without invasive prenatal diagnostics, pregnancy outcomes are expected to be favorable, especially when transferring embryos with segmental mosaicism of any level. This indicates a high potential for transplantation of mosaic blastocysts. However, transfer of such embryos, regardless of the level of mosaicism and chromosome (except for aneuploidies 21, 18, 13), should be approached individually in each specific case.
When pregnancy occurs, dynamic monitoring is required. In the absence of contraindications invasive prenatal testing with chromosomal microarray analysis is indicated.
It is necessary to collect information on clinical cases of mosaic blastocyst transfer with further studies of the factors influencing the formation of embryo mosaicism and the success of their transfer. In each case, a detailed examination of the couple's medical history, the course and outcome of the pregnancy is required before this approach can be evaluated for routine integration into PGT-A programs in women undergoing IVF.
References
- World Health Organization. News release: 1 in 6 people globally affected by infertility: WHO. 4 April 2023. Available at: https://www.who.int/news/item/04-04-2023-1-in-6-people-globally-affected-by-infertility
- Adamson G.D., Zegers-Hochschild F., Dyer S. Global fertility care with assisted reproductive technology. Fertil. Steril. 2023; 120(3 Pt 1): 473-82. https://dx.doi.org/10.1016/j.fertnstert.2023.01.013
- Morales C. Current applications and controversies in preimplantation genetic testing for aneuploidies (PGT-A) in in vitro fertilization. Reprod. Sci. 2024; 31(1): 66-80. https://dx.doi.org/10.1007/s43032-023-01301-0
- Delhanty J.D.A., Griffin D.K., Handyside A.H., Harper J., Atkinson G.H., Pieters M.H. et al. Detection of aneuploidy and chromosomal mosaicism in human embryos during preimplantation sex determination by fluorescent in situ hybridisation, (FISH). Hum. Mol. Genet. 1993; 2(8): 1183-5. https://dx.doi.org/10.1093/hmg/2.8.1183
- Evsikov S., Verlinsky Y. Mosaicism in the inner cell mass of human blastocysts. Hum. Reprod. 1998; 13(11): 3151-5. https://dx.doi.org/10.1093/humrep/13.11.3151
- Munné S., Lee A., Rosenwaks Z., Grifo J., Cohen J. Fertilization and early embryology: Diagnosis of major chromosome aneuploidies in human preimplantation embryos. Hum. Reprod. 1993; 8(12): 2185-91. https://dx.doi.org/10.1093/oxfordjournals.humrep.a138001
- Capalbo A., Rienzi L. Mosaicism between trophectoderm and inner cell mass. Fertil. Steril. 2017; 107(5): 1098-106. https://dx.doi.org/10.1016/j.fertnstert.2017.03.023
- Muñoz E., Bronet F., Lledo B., Palacios-Verdú G., Martinez-Rocca L., Altmäe S. et al. To transfer or not to transfer: the dilemma of mosaic embryos – a narrative review. Reprod. Biomed. Online. 2024; 48(3): 103664. https://dx.doi.org/10.1016/j.rbmo.2023.103664
- Lee C.I., Cheng E.H., Lee M.S., Lin P.Y., Chen Y.C., Chen C.H. et al. Healthy live births from transfer of low-mosaicism embryos after preimplantation genetic testing for aneuploidy. J. Assist. Reprod. Genet. 2020; 37(9): 2305-13. https://dx.doi.org/10.1007/s10815-020-01876-6
- Munné S., Blazek J., Large M., Martinez-Ortiz P.A., Nisson H., Liu E. et al. Detailed investigation into the cytogenetic constitution and pregnancy outcome of replacing mosaic blastocysts detected with the use of high-resolution next-generation sequencing. Fertil. Steril. 2017; 108(1): 62-71.e8. https://dx.doi.org/10.1016/j.fertnstert.2017.05.002
- Spinella F., Fiorentino F., Biricik A., Bono S., Ruberti A., Cotroneo E. et al. Extent of chromosomal mosaicism influences the clinical outcome of in vitro fertilization treatments. Fertil. Steril. 2018; 109(1): 77-83. https://dx.doi.org/10.1016/j.fertnstert.2017.09.025
- Leigh D., Cram D.S., Rechitsky S., Handyside A., Wells D., Munne S. et al. PGDIS position statement on the transfer of mosaic embryos 2021. Reprod. Biomed. Online. 2022; 45(1): 19-25. https://dx.doi.org/0.1016/j.rbmo.2022.03.013
- Abhari S., Kawwass J.F. Pregnancy and neonatal outcomes after transfer of mosaic embryos: a review. J. Clin. Med. 2021; 10(7): 1369. https://dx.doi.org/10.3390/jcm10071369
- Lee H.L., McCulloh D.H., Hodes-Wertz B., Adler A., McCaffrey C., Grifo J.A. In vitro fertilization with preimplantation genetic screening improves implantation and live birth in women age 40 through 43. J. Assist. Reprod. Genet. 2015; 32(3): 435-44. https://dx.doi.org/10.1007/s10815-014-0417-7
- Schoolcraft W.B., Katz-Jaffe M.G. Comprehensive chromosome screening of trophectoderm with vitrification facilitates elective single-embryo transfer for infertile women with advanced maternal age. Fertil. Steril. 2013; 100(3): 615-9. https://dx.doi.org/10.1016/j.fertnstert.2013.07.1972
- Zhang Y.X., Chen J.J., Nabu S., Yeung Q.S.Y., Li Y., Tan J.H. et al. The pregnancy outcome of mosaic embryo transfer: a prospective multicenter study and meta-analysis. Genes (Basel). 2020; 11(9): 973. https://dx.doi.org/10.3390/genes11090973
- Kalousek D.K., Vekemans M. Confined placental mosaicism. J. Med. Genet. 1996; 33(7): 529-33. https://dx.doi.org/10.1136/jmg.33.7.529
- Kolibianakis E., Osmanagaoglu K., De Catte L., Camus M., Bonduelle M., Liebaers I. et al. Prenatal genetic testing by amniocentesis appears to result in a lower risk of fetal loss than chorionic villus sampling in singleton pregnancies achieved by intracytoplasmic sperm injection. Fertil. Steril. 2003; 79(2): 374-8. https://dx.doi.org/10.1016/S0015-0282(02)04578-8
Received 11.06.2025
Accepted 01.09.2025
About the Authors
Mariia A. Sidorchuk, Geneticist at the Department of Clinical Genetics, Institute of Reproductive Genetics, Academician V.I. Kulakov National Medical Research Centerfor Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, 117997, Russia, Moscow, Ac. Oparin str., 4, +7(977)4470373,
m_sidorchuk@oparina4.ru, https://orcid.org/0009-0008-1373-7905
Alexey N. Ekimov, PhD, Head of the Laboratory of Preimplantation Genetic Testing and Genetic Diagnostics, Institute of Reproductive Genetics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, 117997, Russia, Moscow, Ac. Oparin str., 4, +7(926)980-58-60, a_ekimov@oparina4.ru, https://orcid.org/0000-0001-5029-0462
Natalia A. Karetnikova, Dr. Med. Sci., Obstetrician-Gynecologist, Geneticist, Leading Researcher at the Department of Clinical Genetics, Institute of Reproductive Genetics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation,
117997, Russia, Moscow, Ac. Oparin str., 4, +7(495)438-24-10, n_karetnikova@oparina4.ru, https://orcid.org/0000-0002-5060-8239
Nadezhda V. Zaretskaya, PhD, Obstetrician-Gynecologist, Geneticist at the Department of Clinical Genetics, Institute of Reproductive Genetics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, 117997, Russia, Moscow, Ac. Oparin str., 4, +7(495)438-24-11, n_zaretskaya@oparina4.ru, https://orcid.org/0000-0001-6754-3833
Igor O. Sadelov, Geneticist of Laboratory of Genomic Data Analysis, Institute of Reproductive Genetics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, 117997, Russia, Moscow, Ac. Oparin str., 4, +7(495)438-24-10,
i_sadelov@oparina4.ru, https://orcid.org/0000-0002-5144-6307
Olga K. Stupko, Cytogeneticist at the Laboratory of Molecular Genetic Methods, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, 117997, Russia, Moscow, Ac. Oparin str., 4, +7(916)621-76-15, o_stupko@oparina4.ru,
https://orcid.org/0009-0000-7205-1964
Сorresponding author: Mariia A. Sidorchuk, m_sidorchuk@oparina4.ru



