Outcomes of assisted reproductive technologies in patients with persistence оf antiphospholipid antibodies
Objective. To identify the connection between persistence of antiphospholipid antibodies (APLAs) and pregnancy rate in infertile women undergoing assisted reproductive technologies (ART).Kraevaya Е.Е., Dolgushina N.V., Menzhinskaya I.V., Shpilyuk М.А., Beznoshchenko О.S., Krechetova L.V.
Materials and methods. The prospective case-control study included 97 couples who presented for infertility treatment with ART programs. The stratification of the patients was the following: group 1 included patients who achieved pregnancy, group 2 included patients without pregnancy. Antibodies of the IgM and IgC classes to cardiolipin, β2-glycoprotein-1 (β2-GP-1), annexin V, phosphatidylserine (PS) and phosphatidylethanolamine (PE) were determined using enzyme-linked
immuno-sorbent assay (ELISA). Plasma lupus anticoagulant was detected by Russel Viper Venom Time test. APLAs were determined before ovarian stimulation and 8-12 weeks after it. Hemostasis was assessed using thrombodynamics (TD).
Results. In patients of group 2, there was the significantly higher level of criterial (β2-GP-1) and non-criterial IgM antibodies (PS, PE, annexin V). The ALPA level higher than the reference level was revealed twice with 8-12 weeks interval in 18 patients: in 2 patients of group 1 (6.7%) and in 16 patients of group 2 (23.9%) (р=0.04). It was accompanied by non-criterial antibodies persistence (PS, PE and annexin V) with IgM to IgG conversion. The significant correlation between level of antibodies and TD parameters was detected. It was suggestive of association between the APLA level and state of blood coagulation.
Conclusion. Persistence of APLAs, especially of the so-called non-criterial antibodies, decreased the chances of pregnancy by 4.34 times (95% CI=1.04; 20.22). The APLA level had a strong correlation with TD parameters showing hypercoagulation and subsequently developed hypocoagulation which in its turn can be one of pathogenetic mechanisms of implantation failures in ART programs.
Keywords
Pregnancy is known to occur due to the combination of two factors: presence of euploid embryo of good quality as well as receptive endometrium and high implantation potential of the embryo [1]. Inherited and acquired thrombophilia can result in recurrent pregnancy loss and other pregnancy complications, such as preeclampsia, intrauterine growth restriction (IUGR) and stillbirth [2, 3]. In thrombophilia the mechanisms that may be responsible for pregnancy complications are thrombosis of the placental vessels, endotheliopathy and inflammation which may cause blood circulation disturbances and impaired placental function [4]. Similar disorders may occur during early implantation of the embryo and placentation in the assisted reproductive technologies (ART) programs. The influence of thrombophilia on ART outcomes was confirmed by several studies [5-7]; however, some researchers failed to demonstrate this association [8]. The effect of persistence of antiphospholipid antibodies (APLAs) on ART outcomes has not been proved either. A number of scientists do not consider that APLAs are responsible for the decrease in the pregnancy rate in the ART programs [9]. Other studies demonstrate the results proving the connection between the persistence of APLAs and pregnancy rate [10–12] with special attention paid to the so called non-criterial APLAs. The decrease in pregnancy rate associated with persistence of APLAs in the ART programs may be caused by endotheliopathy and microvascular thrombosis in the place of trophoblast invasion, the mechanism being similar to pathogenesis of the great obstetrical syndromes, and also by the direct impact of APLAs on trophoblast cells. Thus, APLAs can displace protective layer of annexin V from the surface of trophoblast that may result in the loss of its structural integrity. APLAs can also initiate apoptosis in trophoblast cells, impair proliferation, invasiveness, adhesion molecule expression and secretion of human chorionic gonadotropin (hCG), cause proinflammatory reaction, namely the release of proinflammatory cytokines and complement activation, that may lead to slower trophoblast development rate [4, 6, 13]. Therefore, evaluation of hemostasis system in patients with persistence of APLAs continues to be relevant. The debate over the role of thrombophilia in ART has been fired up by the new data on the influence of thrombophilia on the increased pregnancy rate in patients with previous recurrent ART failures [13, 14].
The aim of the study was to identify the connection between pregnancy rate in infertile women undergoing ART and persistence of APLAs.
Materials and Methods
The prospective case-control study included 97 couples who presented for infertility treatment with ART programs between 2016 and 2018. ART was not contraindicated for the patients and informed consent was obtained from all participants of the study. The couples were stratified into groups with 1:2 ratio depending on the pregnancy achievement with this program of ovarian stimulation and embryo transfer. The couples selected for the case-control study met the criteria of the specialist in obstetrics and gynecology and embryologist performing the procedures. There were two groups of patients: group 1 included 30 patients with pregnancy, group 2 included 67 patients who did not achieve pregnancy.
Inclusion criteria were as follows: normal karyotype in both partners, women’s age from 18 to 40, body mass index (BMI) from 18 to 29.9 kg/m2. Exclusion criteria were contraindications for ART, pathospermia in partner, the use of donor gametes or surrogate mothers, decrease of the ovarian reserve, poor ovarian response to the ovarian stimulation, the absence of blastocysts for the transfer to the uterine cavity in the protocol of the ovarian stimulation, and also ART complications in the studied cycle.
Before starting the IVF program all the patients were examined in accordance with the order of the Russian Ministry of Health №107n dated 30.08.2012 «On approval of the use of assisted reproductive technologies, contraindications and limitations to their use» [15]. Ovarian stimulation was performed with recombinant follicle stimulating hormone (rFSH) or follicle stimulating hormone (FSH) combined with luteinizing hormone (LH) and controlled with gonadotropin-releasing hormone (GnRH) antagonists. The trigger of ovulation at a dose of 8000-10000 IU was injected if the leading follicle was greater than or equal to 18 mm. Human chorionic gonadotropin was used for ovulation triggering. Transvaginal oocyte retrieval (TOR) was performed 36 hours after injecting the trigger of ovulation.
Oocytes were fertilized by in vitro fertilization (IVF) or if indicated by intracytoplasmic sperm injection (ICSI). Morphological assessment of the embryos was carried out on the 5th day after TOR according to classification of Gardner et al. [16].
Embryo was transferred to the uterine cavity on the 5th day after TOR during the native cycle. One good or excellent quality blastocyst was transferred to the uterine cavity. The steps following embryo transfer were made in accordance with the standard protocol.
Pregnancy was defined by the increase in the level of serum β-hCG and visualization of the gestational sac in the uterine cavity under ultrasound guidance in 21 days after embryo transfer.
Antibodies of the IgM and IgG classes for cardiolipin, β2-glycoprotein-1 (β2-GP-1), annexin V, phosphatidylserine (PS) and phosphatidylethanolamine (PE) were identified using enzyme-linked immunoassay kits «ORGENTEC Diagnostika GmbH» and «IBL International GmbH» (Germany) twice: before ovarian stimulation and 8-12 weeks after it.
Plasma lupus anticoagulant was detected using automated blood coagulation analyzer SYSMEX CA-1500 (Japan) with «Siemens Healthcare Diagnostics Products GmbH» reagents, Germany. Antibodies were determined before ovarian stimulation and 8–12 weeks after it.
The evaluation of hemostasis system was carried out using thrombodynamics (TD) test three times: before ovarian stimulation, on the day of TOR and on the day of embryo transfer. TD was assessed using diagnostic laboratory system «Thrombodynamics T-2 Registrator», HemaCore LLC, Russia. The assessed parameters of TD test included the initial velocity of the blood clot formation (Vi, μm/min), velocity of blood clot growth (V, μm/ min), fibrin clot size in 30 sec (Cs, μm), spontaneous clotting formation in the space of the plasma not associated with the main clot growth (T).
Statistical analysis
Statistical processing of the data was performed using Statistica 10 software package (USA). Statistical analysis was conducted with χ2-test for the comparison of categorical variables, Student’s t-test and Mann-Whitney U-test for the comparison of medians. The relative association for binary outcomes was quantified using an odds ratio (OR); to estimate OR the logistic regression approach was implemented. The degree of association between two variables was measured with non-parametric test, Spearman rank correlation. The results were considered statistically significant at a significance level of р˂0.05.
The study was approved by the Local Ethics Committee of the National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow, Russia.
Results
The analysis of the clinical, laboratory and embryological parameters of the patients demonstrated that the couples who achieved pregnancy were younger and more often were administered rFSH for the ovarian stimulation. The rest parameters did not differ significantly in the groups (Table 1). Multifactorial analysis of the assessment of APLA impact on the IVF effectiveness was performed using these factors.
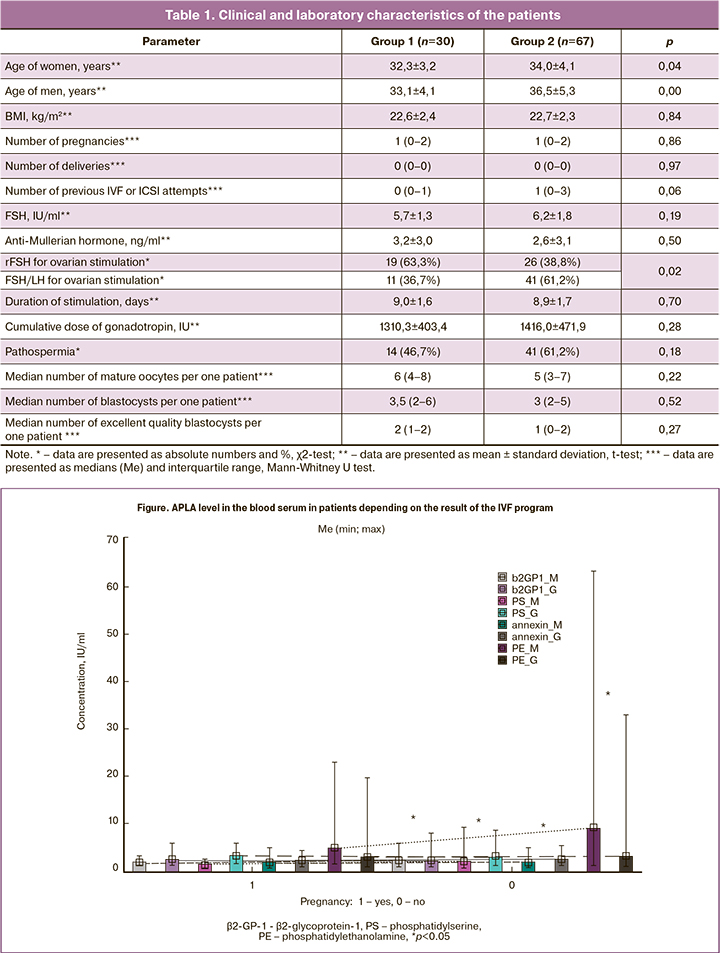
The study of APLA level revealed that a significant increase in the level of criterial anti-β2-GP-1 antibodies and non-criterial anti-PS, anti-PE and antiannexin V antibodies was noted more frequently in patients of group 2 (Figure). In our research we admit that the presence of criterial and non-criterial APLAs is evidence of thrombophilia taking into consideration that criterial antibodies in obstetric antiphospholipid syndrome are often within the normal range.
The ALPA level higher than the reference level was revealed twice with 8–12 weeks interval in 18 patients, namely in 2 patients of group 1 (6.7%) and in 16 patients of group 2 (23.9%) (р=0.04). It was accompanied by non-criterial antibodies persistence (PS, PE and annexin V) with IgM to IgG conversion.
The evaluation of hemostasis system with TD test depending on pregnancy did not reveal any significant difference. However, the significant correlation between level of antibodies and TD parameters was detected (Table 2).
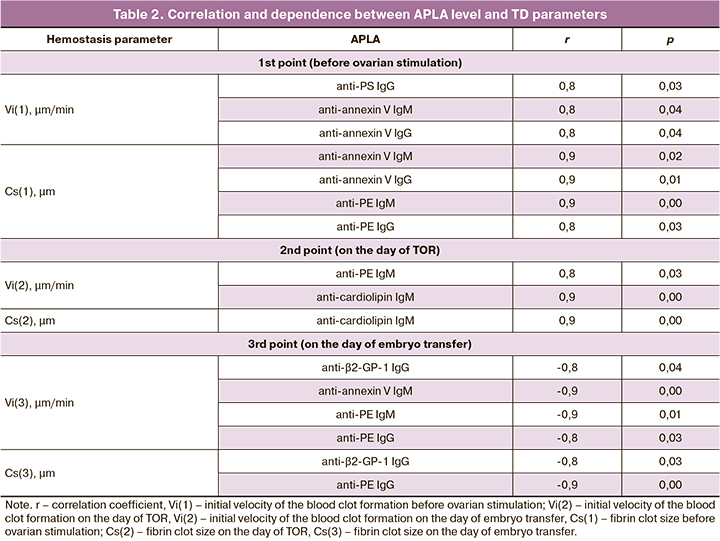
It was noted that positive correlation between the APLA level and initial velocity of the blood clot formation (Vi) and fibrin clot size (Cs) was revealed before ovarian stimulation and on the day of TOR. It means that the higher APLA level caused the higher velocity of clot formation and clot size which was evidence of association between APLA level and the development of hypercoagulation. On the day of embryo transfer, not positive but negative correlations of APLA level and initial velocity of the blood clot formation (Vi) and fibrin clot size (Cs) were revealed that were suggestive of the association between APLA level and the development of hypocoagulation by the day of embryo transfer. Correlations were mainly noted in non-criterial antibodies (anti-annexin V, anti-PE, anti-PS).
On assessing the fact of spontaneous clotting in the plasma not associated with the main clot growth (T), it was revealed that the level of anti-annexin V IgM antibodies was almost significantly higher in the patients with clotting on the day of embryo transfer (р=0.07).
Correlation analysis of TD parameters and the dose of gonadotropins when injected exogenously for the ovarian stimulation showed a statistically significant positive correlation between the dose of gonadotropin and Cs(3) parameter (r=0.7368, p=0.05).
Discussion
The pathogenesis of infertility and pregnancy complications in antiphospholipid syndrome is based on the effect of APLAs on the endothelial cells with the development of endothelial dysfunction and increased risk for clotting. APLAs also affect trophoblast cells that may cause the development of proinflammatory reaction, neutrophil infiltration, production of proinflammatory cytokines and complement system activation with all these resulting in a slower rate of trophoblast growth [17].
There was a number of studies focused on the research of the interconnection between antiphospholipid syndrome, presence of APLAs and ART outcomes. If the role of antiphospholipid syndrome and APLAs in miscarriages is obvious and confirmed, their function in the development of infertility and ART failures remains unclear. Our study identified this association and showed that the persistence of the so-called non-criterial antibodies reduced the chances of achieving pregnancy by 4.34 times.
The obtained results are not consistent with the data of Chighizola et al. (2014), who analyzed 29 studies investigating the association between the presence of APLAs and ART outcomes. The dependence of ART outcomes on increased APLA titers was detected in 44.8% of studies, however, the authors of the review noted the extremely high heterogeneity of patients’ groups, different diagnostic criteria for APLA titer assessment, the study of different APLA types and classes. This led to the conclusion that currently there is no evidence of association between increased APLA titers and infertility, as well as ART outcomes [8].
The results of our research are consistent with the findings of Di Rosa et al. (2019) who conducted a study with 520 patients and revealed the association of APLAs and ART outcomes [3]. Increased titers of lupus anticoagulant (53.49%), anti-cardiolipin (44.19%) and anti-β2-GP-1 (25.58%) antibodies were identified more frequently. Systemic autoimmune disease was diagnosed in 6.73% of patients, antiphospholipid syndrome was revealed in 3.27% of cases. According to the authors, classical clinical manifestations of antiphospholipid syndrome were absent in some patients (4.6%) with increased titer of APLA, however, these patients had more frequently adverse outcomes of pregnancy including unsuccessful implantations in ART programs in their histories. Therefore, it was assumed that in the presence of the laboratory criteria of antiphospholipid syndrome, the range of clinical manifestations can be wider than the approved classical clinical manifestations. The authors of the study recommend to evaluate APLAs in all patients undergoing ART programs for the earliest detection of women at a higher risk for miscarriages and pregnancy morbidity [3].
Similar results were obtained by Di Nisio et al. in their systematic review [12]. They conducted a metaanalysis of 29 cohort and case control studies including 5270 patients and revealed that persistence of APLAs increased the risk for ART failures by 3.3 times (OR=3.33; 95% CI=1.77–6.26). APLAs which contributed to implantation failures were non-criterial.
According to the data of the national studies (Khizroeva et al., 2018), the rate of APLA presence in patients with unsuccessful ART outcomes was higher in comparison with one in the patients who achieved pregnancy, 42.9% in the group of nonpregnant patients after ART program versus 19.1% in the group of pregnant women after ART [10]. Anti-β2-GP-1 (31.4%), anti-annexin V (24.7%) and anti-cardiolipin antibodies (8.9%) were detected more often. The authors suggest considering increased APLA titer as a temporary contraindication to performing ART programs.
In view of the contradictory results of the studies, the complex evaluation of hemostasis system in patients with persistence of APLAs continues to be relevant. In our research hemostasis system was evaluated with TD test. It was revealed that a higher level of APLAs before ovarian stimulation and on the day of TOR was associated with the development of hypercoagulation, though in 5 days after cessation of gonadotropin injections there was a reverse trend for the development of hypocoagulation. A higher level of APLAs after the stimulation was associated with spontaneous fibrin clotting that may be suggestive of a severe hypercoagulation; it may also cause the presence of procoagulant components in the plasma sample of a patient, namely microvesicles which are active clotting factors. Moreover, during the ovarian stimulation there was an association between TD parameters and the dose of injected gonadotropins.
Conclusion
Persistence of APLAs decreases the chances of pregnancy by 4.34 times (95% CI=1.04; 20.22). APLAs which have a negative influence on the pregnancy rate in ART programs include mainly the so-called noncriterial antibodies (anti-PS, anti-PE, anti-annexin V) with gradual conversion of IgM to IgG. APLA level has strong correlations with TD parameters showing hypercoagulation and subsequently developed hypocoagulation which in its turn can be one of pathogenetic mechanisms of implantation failures in ART programs. The development of hypocoagulation as the evidence of maximal association of APLAs and TD parameters in ART programs occurs after the ovarian stimulation and can be connected directly with the dose of the injected gonadotropins.
References
- Gardner D.K., Weissman A., Howles C.M., Shoham Z., eds. Textbook of assisted reproductive technologies. 3rd ed. London: Informa healthcare; 2009. 912 p.
- Bashiri A., Harlev A., Agarwal A., eds. Recurrent pregnancy loss : evidence-based evaluation, diagnosis and treatment. Springer; 2016. 208p.
- Гусина А.А., Гусина Н.Б. Наследственные тромбофилии и осложнения беременности. Репродуктивное здоровье. Восточная Европа. 2016; 4(6): 457-68. [Gusina A., Gusina N. Hereditary thrombophilia and placenta-mediated pregnancy complications. Reproductive health. Eastern Europe/ Reproduktivnoe zdorov’e. Vostochnaya Evropa. 2016; 4(6): 457-68. (in Russian).]
- Андреева М.Д., Капанадзе Д.Л., Самбурова Н.В. Акушерские и перинатальные исходы у пациенток с синдромом потери плода в анамнезе, генетическими и приобретенными формами тромбофилии. Акушерство, гинекология и репродукция. 2014; 8(4): 54-5. [Andreeva M.D., Kapanadze D.L., Samburova N.V. Akusherskie i perinatal’nye iskhody u patsientok s sindromom poteri ploda v anamneze, geneticheskimi i priobretennymi forami trombofilii. Obstetrics, Gynecology and Reproduction/Akusherstvo, ginekologiya i reproduktsiya. 2014; 8(4): 54-5. (in Russian).]
- Tong M., Viall C.A., Chamley L.W. Antiphospholipid antibodies and the placenta: a systematic review of their in vitro effects and modulation by treatment. Hum. Reprod. Update. 2015; 21(1): 97-118. https://dx.doi.org/10.1093/humupd/dmu049.
- Tan X., Yu Z., Sao J., Chen L., Shen Y., Ding J., Shi W. Association between in vitro fertilization outcomes and inherited thrombophilias: a meta-analysis. J. Assist. Reprod. Genet. 2016; 33(8): 1093-8. https://dx.doi.org/10.1007/s10815-016-0726-0.
- Абрамян Г.Р. Клиническое значение выявления антифосфолипидных антител и генетической тромбофилии у пациенток с неудачными попытками экстракорпорального оплодотворения. Практическая медицина. 2016; 3: 113-8. [Abramyan G.R. Clinical significance of detection of antiphospholipid antibodies and genetic thrombophilia in patients with failed attempts of in vitro fertilization. Prakticheskaya meditsina. 2016; (3): 113-8. (in Russian).]
- Di Nisio M., Ponzano A., Tiboni G.M., Guglielmi M.D., Rutjes A.W.S.,Porreca E. Effects of multiple inherited and acquired thrombophilia on outcomes of in-vitro fertilization. Thromb. Res. 2018; 167: 26-31.https://dx.doi.org/10.1016/j.thromres.2018.05.006.
- Ata B., Urman B. Thrombophilia and assisted reproduction technology-any detrimental impact or unnecessary overuse? J. Assist. Reprod. Genet. 2016; 33(10): 1305-10. https://dx.doi.org/10.1007/s10815-016-0771-8.
- Chighizola C.B., De Jesus G.R. Antiphospholipid antibodies and infertility. Lupus. 2014; 23(12): 1232-8. https://dx.doi.org/10.1177/0961203314529171.
- Khizroeva J., Makatsariya A., Bitsadze V., Makatsariya N., Khamani N. In vitro fertilization outcomes in women with antiphospholipid antibodies circulation. J. Matern. Fetal Neonatal Med. 2018; Oct.12: 1-11. https://dx.doi.org/10.1080/14767058.2018.1535586.
- Di Rosa R., Ferrero S., Cifani N., Ferri L., Proietta M., Picchianti Diamanti A., Del Porto F. In vitro fertilization and autoimmunity: Evidence from an observational study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019; 234: 137-42. https://dx.doi.org/10.1016/j.ejogrb.2018.12.042.
- Менжинская И.В., Ванько Л.В. Патофизиологические механизмы развития акушерского антифосфолипидного синдрома. Акушерство и гинекология. 2018; 1: 5-12. [Menzhinskaya I.V., Vanko L.V. Pathophysiological mechanisms of obstetric antiphospholipid syndrome. Obstetrics and Gynecology/Akusherstvo i ginekologiya. 2018; (1): 5-12. (in Russian).]
- Akhtar M.A., Sur S., Raine-Fenning N., Jayaprakasan K,. Thornton J.G., Quenby S. Heparin for assisted reproduction. Cochrane Database Syst. Rev. 2013; (8): CD009452. https://dx.doi.org/10.1002/14651858.CD009452.pub2.
- Dugalic S., Petronijevic M., Stefanovic A., Stefanovic K., Petronijevic S.V., Stanisavljevic D. et al. Comparison of 2 approaches in management of pregnant women with inherited trombophilias: Prospective analytical cohort study. Medicine (Baltimore). 2019; 98(34): e16883. https://dx.doi.org/10.1097/MD.0000000000016883.
- Spratte J., Bornkessel F., Schütz F., Zygmunt M., Fluhr H. The presence of heparins during decidualization modulates the response of human endometrial stromal cells to IL-1β in vitro. J. Assist. Reprod. Genet. 2016; 33(7): 949-57. https://dx.doi.org/10.1007/s10815-016-0703-7.
- Приказ Минздрава России №107н От 30 августа 2013 г. «О порядке использования вспомогательных репродуктивных технологий, противопоказаниях и ограничениях к их применению». Доступно по: https://www.rosminzdrav.ru/documents/6787-Prikaz-Minzdrava-Rossii-107n [Prikaz Minzdrava Rossii No.107n on 30.08.2013. «O poryadke ispol’zovaniya vspomogatel’nykh reproduktivnykh tekhnologii, protivopokazaniyakh i ogranicheniyakh k ikh primeneniyu». Available at: https://www.rosminzdrav.ru/documents/6787-Prikaz-Minzdrava-Rossii-107n .(in Russian).]
- Gardiner C., Hills J., Machin S.J., Cohen H. Diagnosis of antiphospholipid syndrome in routine clinical practice. Lupus. 2013; 22(1): 18-25. https://dx.doi.org/10.1177/0961203312460722.
Received 22.11.2019
Accepted 29.11.2019
About the Authors
Elizaveta E. Kraevaya, junior researcher of the Department of Auxiliary Technologies in Infertility Treatment, Kulakov NMRC OGP, Ministry of Healthcare of the Russian Federation. Теl. +7(985) 294-03-18. E-mail: e_kraevaya@oparina4.ru 4 Oparin str, 117997, Moscow, RussiaNataliya V. Dolgushina, M.D., Ph.D., M.P.H., Head of R&D Department Kulakov NMRC OGP, Ministry of Healthcare of the Russian Federation.
Tel. +7(495) 438-49-77. E-mail: n_dolgushina@oparina4.ru 4 Oparin str, 117997, Moscow, Russia
Irina V. Menzhinskaya, Ph.D, senior Researcher of Department of Clinical Immunology, Kulakov NMRC OGP, Ministry of Healthcare of the Russian Federation.
Tel. +7(495)438-11-83. E-mail: i_menzinskaya@oparina4.ru 4 Oparin str, 117997, Moscow, Russia
Margarita A. Shpiluyk, researcher of the Laboratory of Clinical Immunology, Kulakov NMRC OGP, Ministry of Healthcare of the Russian Federation.
Теl. +7(926)686-31-17. E-mail: tambovtsevamr@mail.ru 4 Oparin str, 117997, Moscow, Russia
Olga S. Beznoschenko, clinical laboratory diagnostician of the Laboratory of Clinical Immunology, Kulakov NMRC OGP, Ministry of Healthcare of the Russian Federation.
Теl. +7(919)721-43-29. E-mail: o_beznoshchenko@oparina4.ru 4 Oparin str, 117997, Moscow, Russia
Liubov V. Krechetova, M.D., head of clinical immunology laboratory Kulakov NMRC OGP, Ministry of Healthcare of the Russian Federation.
Теl.+7-(495)-438-11-83. E-mail: k_l_v_@mail.ru 4 Oparin str, 117997, Moscow, Russia
For citation: Kraevaya Е.Е., Dolgushina N.V., Menzhinskaya I.V., Shpilyuk М.А., Beznoshchenko О.S., Krechetova L.V. Outcomes of assisted reproductive technologies in patients with persistence оf antiphospholipid antibodies.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2020; 4: 97-103. (In Russian).
https://dx.doi.org/10.18565/aig.2020.4.97-103



