Lipid profiling of follicular fluid and blood plasma as a method of predicting pregnancy in women undergoing assisted reproductive treatment
Fortygina Yu.A., Drapkina Yu.S., Makarova N.P., Kulakova E.V., Sysoeva A.P., Yushina M.N., Eldarov Ch.M., Chagovets V.V., Frankevich V.E., Kalinina E.A.
Background: Modifications of infertility treatment protocols in assisted reproductive technology (ART) programs based on the clinical and laboratory history of couples still have limitations in predicting their effectiveness. The search for minimally invasive markers of gamete quality and markers of the effectiveness of ART treatment is a relevant area at this stage of development of reproductive medicine.
Objective: To create a model for predicting pregnancy on the basis of the lipid profile of blood plasma and follicular fluid in patients undergoing ART treatment by means of liquid chromatography and mass spectrometry.
Materials and methods: The present study analyzed the clinical and anamnestic characteristics, as well as the lipid profile, of couples undergoing infertility treatment with standard ovarian stimulation in the ART department. Follicular fluid and blood plasma sampling followed by cryopreservation was performed on the day of puncture. The lipid composition of biological fluids was studied using liquid chromatography with tandem mass spectrometry. The lipid profile of the samples underwent analysis to develop OPLS-DA models.
Results: The study revealed statistically significant differences in the composition of follicular fluid and blood plasma depending on the outcome of the treatment in patients who had assisted reproduction. ROC curve analysis of the developed models showed that the model based on plasma lipid composition was more effective than the model based on follicular fluid (AUC=0.97, AUC=0.91, respectively).
Conclusion: The data of the present study are consistent with the results of world studies and confirm the potential of using the lipid composition of follicular fluid and blood plasma as markers of the effectiveness of ART programs.
Authors’ contributions: Makarova N.P., Chagovets V.V., Kalinina E.A. – developing the concept and design of the study; Sysoeva A.P., Fortygina Yu.A., Yushina M.N., Eldarov Ch.M., Chagovets V.V. – collecting and processing of the material, statistical processing of the data; Fortygina Yu.A., Drapkina Yu.S. – writing the text of the article; Kalinina E.A., Makarova N.P. Eldarov Ch.M., Kulakova E.V., Frankevich V.E. – editing the article.
Conflicts of interest: The authors declare that there are no conflicts of interest.
Funding: The study was carried out without sponsorship.
Ethical Approval: The study was approved by the Ethical Review Board of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia.
Patient Consent for Publication: The patients signed informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Fortygina Yu.A., Drapkina Yu.S., Makarova N.P., Kulakova E.V., Sysoeva A.P., Yushina M.N.,
Eldarov Ch.M., Chagovets V.V., Frankevich V.E., Kalinina E.A. Lipid profiling of follicular fluid and
blood plasma as a method of predicting pregnancy in women undergoing assisted reproductive treatment.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (12): 60-69 (in Russian)
https://dx.doi.org/10.18565/aig.2024.105
Keywords
Given the persistently high incidence of infertility in modern society, understanding the molecular mechanisms of embryonic development is an urgent and promising area of research that determines the rapid development of assisted reproductive technology (ART) programs [1]. One of the major challenges in the field of ART is to predict the effectiveness of infertility treatment in couples with different clinical and anamnestic characteristics. To date, a significant number of potential predictors of pregnancy in an ART program have been developed, including those using mathematical models [2]. It is possible to simplify the process of predicting the outcome of an ART program to the task of classifying patients into two groups: those who have conceived and those who have not. In clinical practice, such problems are most often solved by building logistic regression models, but in this case a limited number of parameters function as independent variables. At the same time, the organism is a complex biological system and its state depends on the balance of many variables. Significant advances in data processing, the development of multivariate analysis methods and algorithms, as well as improvements in machine learning (ML), make it possible to examine a whole range of variables together in terms of their correlation, therefore contributing to the development of a personalized approach in medicine [3]. ML is an artificial intelligence technology that has the capacity to ‘learn’ independently in order to formulate an answer to a given question. This process is based on algorithms that enable the computer to automatically analyze known data sets in advance. In Russia, such techniques are actively used to predict the pregnancy rate using the clinical data of the patients undergoing ART treatment [4].
However, a model based only on clinical data has limited predictive accuracy. Therefore, the investigation of additional markers to predict the likelihood of pregnancy in ART treatment programs will provide the timely guidance of the couple on the use of different treatment methods, adjust their expectations regarding the frequency of pregnancy, and appropriately manage financial resources from the Federal Compulsory Medical Insurance Fund [5]. In addition, the study of non-invasive or minimally invasive markers that are able to predict the efficacy of ART treatment and the quality of the obtained gametes has the potential to facilitate the preparation for an ART program in a more precise manner, which in turn could increase the effectiveness of treatment.
Minimally invasive markers such as small non-coding RNAs, telomerase enzyme, and molecular genetic markers in culture media, uterine aspirate, and follicular fluid have already been used in studies aimed at predicting treatment programs.
Among the molecules most involved in the energy supply of cells, as well as those involved in plasma membrane formation, signaling molecules and regulators, lipids are particularly important [6]. Deficiency of lipid molecules, has a negative effect on steroidogenesis [7, 8]. However, excessive accumulation of intracellular lipids, such as fatty acids, may mediate the triggering of oxidative cell damage [7]. The results of studies have demonstrated that follicular fluid lipid composition correlates with embryo implantation in ART programs [9–14]. There was a significant positive association between a total number of monounsaturated fatty acids, especially oleic acid, and the number of mature oocytes and excellent quality embryos [9]. A recent study published in 2022 showed a high degree of correlation of blood lipid profile with the lipid composition of follicular fluid that acts as a marker of ovarian response to stimulation. Thus, lipid profiling of blood plasma can predict the quality of the reproductive material obtained before ovarian stimulation [15].
Previous studies have established an association between serum lipid profile and the likelihood of pregnancy. Elevated serum cholesterol levels in couples undergoing ART treatment have been associated with a poor likelihood of successful treatment outcome [16]. Increased serum triglyceride levels have also been observed in women with habitual miscarriage [17].
In view of the existing data on the influence of the lipid profile of follicular fluid and blood plasma on gamete quality, the aim of this study is to test the hypothesis of the prognostic significance of the lipid profile in case of pregnancy in infertile patients undergoing ovarian stimulation and to develop an appropriate mathematical model.
Materials and methods
The study was conducted at the Department of Assisted Technologies in the Treatment of Infertility in the Institute of Reproductive Medicine, as well as in the Laboratory of Metabolomics and Bioinformatics of the Academician V.I. Kulakov National Medical Research Centre for Obstetrics, Gynecology and Perinatology, Moscow, Russia.
The patients were included in the study after providing written informed consent for participation in the study and for storage of biological materials in the biobank. The patients had to meet the following inclusion criteria: age from 18 to 40 years, normal ovarian reserve and tuboperitoneal infertility factor in combination with normozoospermia or mild form of pathozoospermia.
The exclusion criteria were general contraindications indicated in the Order of the Ministry of Health of Russia No.803n dated July 31, 2020, ‘On the procedure for the use of assisted reproductive technologies, contraindications and restrictions to their use’, as well as the presence of common forms of extragenital endometriosis (III-IV degree), endometrial pathology, clinically significant uterine fibroid, polycystic ovary syndrome, uterine and vaginal malformations, history of ovarian surgery, karyotype disorders, severe forms of pathozoospermia, severe metabolic syndrome and class II–III obesity.
Stimulation of ovarian function was started from the 2nd–3rd day of the menstrual cycle with preparations of recombinant follicle-stimulating hormone (FSH), human menopausal gonadotropin (or a combination of these preparations); the patients were also given injection of gonadotropin-releasing hormone antagonist to prevent parasitic peak of luteinizing hormone (LH) (medication was administered when the maximum follicle reached 14 mm in diameter). Medications for triggering final oocyte maturation were administered when the maximum follicle size of 17 mm in diameter or more was detected. After 36 hours, transvaginal follicle puncture was performed under ultrasound guidance. On the day of transvaginal puncture, fasting blood plasma was collected from the patients before anesthetic management. All mature oocytes were fertilized with partner’s sperm using the intracytoplasmic sperm injection technique. Embryos were cultured in droplets of culture media in multigas incubators (Cook, Ireland). On the 5th day of culturing, all patients had selective transfer of the embryo. Post-transfer support was performed according to a standardized protocol. Embryos meeting morphological quality criteria were vitrified. In order to verify pregnancy, a blood test for beta-subunit human chorionic gonadotropin was performed 14 days after embryo transfer, and pelvic ultrasound was performed 21 days later. The groups were divided according to the presence/absence of pregnancy: group I ‘pregnancy +’ included 19 patients, group II ‘pregnancy -’ included 21 patients.
Method of lipid analysis
Lipids from follicular fluid and blood plasma of the patients were extracted using the Folch solution containing chloroform and methanol (2:1 v/v). This method of lipid extraction was applied as a result of multivariate analysis of previous studies carried out by a team of researchers on lipid extraction from various biological media. In the analysis of the material, a sample volume of 40 µl was taken and subjected to extraction twice, first with 480 µl of chloroform-methanol solution, then with 250 µl of the solution. The organic layer (containing lipids) was sampled twice: 150 μl was sampled first, then 300 μl was taken. The combined organic phase was dried in a stream of nitrogen in a thermostat and subsequently dissolved in 200 μl of acetonitrile-2-propanol mixture (1:1 v/v). Aliquots were analyzed in Dionex UltiMate 3000 liquid chromatograph (Thermo Scientific, Germany) with Maxis Impact qTOF mass spectrometric detector (Bruker Daltonics, Germany). Samples were separated at an elution rate of 35 µl/ml on Zorbax C18 column (150×2.1 mm, 5 µm, Agilent, USA) with two eluents (A is acetonitrile/water with a solution volume of 60:40 + 0.1% formic acid + 10 mmol/L ammonium formate; B is acetonitrile/isopropanol/water with a solution volume of 90:8:2 + 0.1% formic acid + 10 mmol/L ammonium formate) [18-21]. (A is acetonitrile/water with a solution volume of 60:40 + 0.1% formic acid + 10 mmol/L ammonium formate; B is acetonitrile/isopropanol/water with a solution volume of 90:8:2 + 0.1% formic acid + 10 mmol/L ammonium formate) [18-21].
Lipid identification was performed using the LipidMatch database, which contains the most comprehensive information on tandem mass spectra of more than 250,000 lipid species, ranked by intensity and molecular mass.
Statistical analysis
Statistical analysis of the data on clinical and anamnestic parameters was performed using STATTECH software. All variables were tested for normality of distribution using the Shapiro–Wilk test. Mean values (M) and standard deviation (SD) were calculated under normal data distribution. The medians (Me) and quartiles Q1 and Q3 were therefore calculated when the data distribution was other than normal. The Mann–Whitney test was used for pair-wise comparison between groups. Categorical data were described with absolute values and percentage, and comparisons were made using the Fisher’s exact test. The difference was considered statistically significant at p<0.05.
Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA) was used to classify patients according to the criteria of pregnancy presence/absence. Lipid profile was the independent variable, pregnancy criteria acted as the dependent variable. The quality of the developed models was assessed by constructing a ROC curve with determination of its area, sensitivity and specificity. The Variable Importance in Projection (VIP) analysis allowed us to identify the lipids of greatest importance in pregnancy that can be considered as non-invasive markers.
Results
All the selected patients were supposed to have minimal influence of external factors that could affect the outcome of in vitro fertilization (IVF) programs. These characteristics are shown in Table 1.
Since there is evidence in the literature on lipidome changes in patients of later reproductive period, patients younger than age of 40 years were included in the study. The analysis of the age of the patients in both groups showed no differences. The mean age in group I was 34 (4) years and it was 32 (4) years in group II. The mean age was not significantly different in males either, 34 (4) years and 33 (5) years in the groups, respectively.
The mean length of menstrual cycle was 28 days in both groups and menstrual bleeding was 5 days. Most patients did not exceed 13 years of age at menarche; however, patients in group I had the earliest (11 years) and latest (17 years) age of menarche. The age of sexual debut was comparable in both groups (18 years). Gynecological diseases, medical history, and serum hormone levels of women had no significant intergroup differences and were within the reference values. According to the obstetric history, patients with primary infertility predominated in both groups: 52.6% and 71.4% in groups 1 and 2, respectively. The duration of infertility was not statistically different in both groups. Combined infertility factor prevailed in both groups: 10 couples (52.6%) in group I and 14 couples (66.7%) in group II.
In the stimulated cycle, 19 out of 40 women (47.5%) had a clinical pregnancy at the end of the IVF treatment cycle, but 21 patients (52.5%) had a negative outcome.
Follicular fluid and serum samples obtained from patients with positive and negative outcomes were used to develop OPLS models for determining group membership based on the lipid profile of these samples.
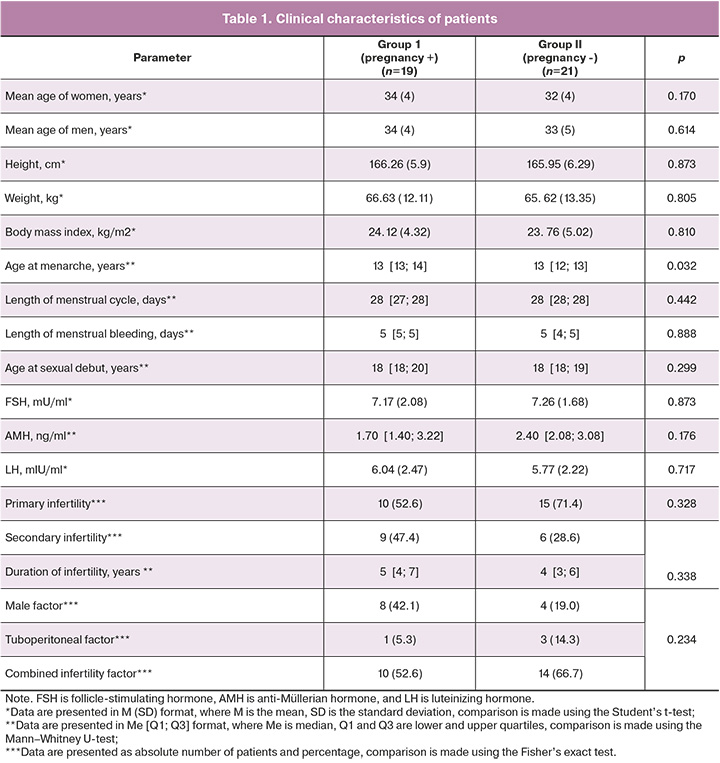
In the first stage, we constructed an OPLS-DA model where follicular fluid lipid levels were the independent variables and the outcome of the ART program was the dependent variable. It is worth noting that there are two clusters along the second principal component in the score plot (Fig. 1). These clusters correlate to samples obtained from the left and right ovaries.
The OPLS-DA model was constructed on the basis of the data from high-performance liquid chromatography with tandem mass spectrometry depending on the lipid profile in the sample. The points corresponding to samples obtained from patients who became pregnant were located in the second and fourth quadrants of the plot, while those corresponding to follicular fluid samples obtained from women with a negative outcome formed a cluster in the first and third quadrants.
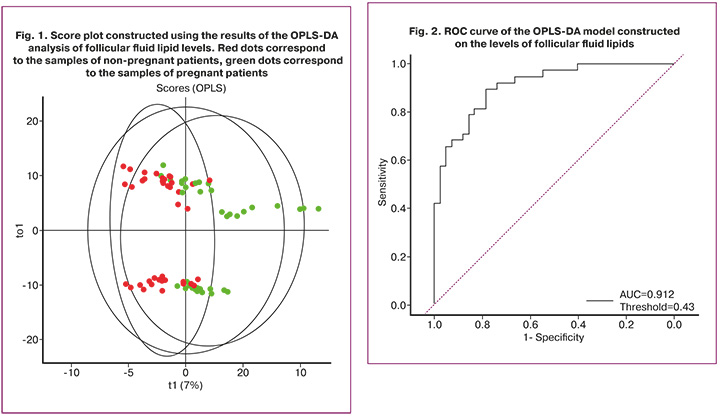
The result of ROC analysis of the OPLS-DA model is shown in Figure 2 and Table 2. The horizontal axis (x-axis) corresponds to the specificity of the test, the vertical axis (y-axis) corresponds to the sensitivity of the test. When pregnancy occurs, a model with high sensitivity gives the true result. In contrast, a model with high specificity is more likely to give a true result in the absence of pregnancy.
The area under the ROC curve of the OPLS-DA model that used follicular fluid samples was 0.912. Thus, this model can be used to classify the analyzed samples into one or another subgroup. The largest contribution to this model comes from 71 of the 235 identified follicular fluid lipids with VIP>1.0. Among the molecules of follicular fluid lipids with VIP>1.0 were Plasmenyl-PE (P-18:1/20:4), Plasmanyl-PC(O-18:1/20:5), OxPE (14:1(OOO)_22:6 (OOOO)), PC(16:0_18:0), PC(18:0_20:2), LPC(18: 0), which belong to the class of phospholipids, namely plasmalogens, glycerophosphoethanolamine, phosphatidylcholines, lysophosphatidylcholines.
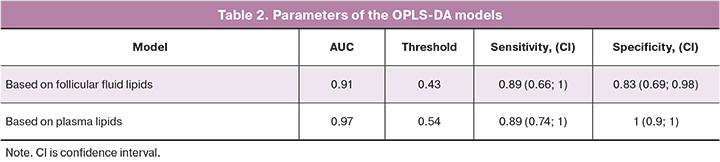
In the next step, a similar analysis was performed using the data obtained from the blood plasma samples (Fig. 3).
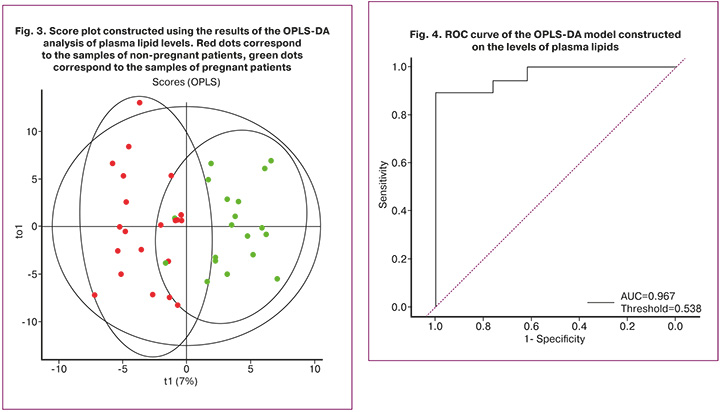
The plot shows the division of samples into two clusters according to the outcome of the ART treatment. Next, the ROC analysis of the model was performed to assess sensitivity and specificity (Fig. 4, Table 2).
Plasma lipid molecules with VIP>2.0 include SM(d16:1/20:0), SM(d18:2/20:0), which belong to the sphingomyelin class.
It is worth noting that AUC of the model generated using plasma lipids was higher than that of the model based on the data of follicular fluid lipidome (Table 2).
The method described in this article demonstrates high accuracy in predicting pregnancy on the basis of the lipids of follicular fluid and blood plasma. The results of the study may help to make infertility treatment and preparation for ART more individualized and personalized, and may help to understand the mechanisms of abnormalities in oocyte maturation that underlie the low fertility rate of prospective IVF patients.
Discussion
The assessment of the lipidomic profile of various biological fluids, including the lipidome of blood plasma and follicular fluid, is a promising direction in improving the effectiveness of ART treatment, as well as in predicting the IVF outcome [9–15]. It is worth noting that previous work has shown that levels of some plasma lipids correlate with lipids in follicular fluid. These molecules can be used for minimally invasive predictive assessment of the quality of oocytes and embryos during preparation for ovarian stimulation.
The study applied multivariate analysis including the use of the OPLS-DA models with sensitivity and specificity assessment using ROC analysis, based on the lipid composition of 235 identified lipids in follicular fluid and 230 lipids in plasma. The lipids that contributed most to the construction of the classification model were those of the plasmalogen, glycerophosphoethanolamine, phosphatidylcholine, lysophosphatidylcholine and sphingomyelin groups.
Phosphatidylcholines belong to the class of phospholipids and in addition to their primary function of membrane stability, they are also intracellular signaling molecules and perform both metabolic and structural functions [22]. Wang J. et al. showed that an increase in the level of phosphatidylcholine in the follicular fluid leads to impaired embryo development on day 3 of culturing due to the negative effect of arachidonic acid, the main metabolite of this class of lipids, on dividing cells [23]. Furthermore, the study by Chen Z. et al. demonstrated that phosphatidylcholines function as significant markers of quality, adequate fertilization, and oocyte potential for subsequent development [24]. Thus, the data obtained in the present study are consistent with the findings in the literature on the correlation between establishing pregnancy and the presence of phospholipids in the follicular fluid.
Sphingomyelins and glycerophospholipids also contribute greatly to determining embryo quality by having an indirect effect on the oocyte. The study by Liu L. et al. showed that changing levels of these lipids lead to a decrease in the frequency of oocyte fertilization due to the regulation of proliferative responses, inhibition of cell growth, differentiation, ageing and motility, as well as apoptosis levels [25].
The knowledge of embryonic fatty acid metabolic processes provides the opportunity to influence the fatty acid composition of the culture media, thus promoting embryo development. Fatty acid composition has been shown to vary with the developmental stage. Thus, four-day-old embryos have increased concentrations of unsaturated acids, such as linoleic and oleic acids, and decreased concentrations of common saturated acids. These acids regulate the processes of endo/exocytosis, modulation of ion channels, and gene expression [13, 14]. The increased need for saturated fatty acids in preimplantation embryos has the capacity to impact the outcomes of reproductive technology programs.
Plasmalogens are another important group of lipids that may have a positive effect on the outcome of the ART treatment, according to the literature and the results of the present study. Plasmalogens, defined as phospholipids with a simple ester bond, have been shown to play a significant role in oocyte aging processes, as reported in the study [22]. Chao de la Barca J.M. et al. showed that the profile of these molecules differs among patients with normal ovarian reserve and women with reduced AMH and antral follicle counts. Shehadeh A. et al. found that there was also a statistically significant increase in the pregnancy rate in patients with reduced plasma triglyceride levels and increased levels of membrane lipids (phospholipids and sphingolipids) [12].
High-density lipoproteins (HDL) and low-density lipoproteins (LDL) play an important role in cholesterol transport to the ovarian tissue, as they deliver cholesterol molecules required for steroid hormone synthesis in theca and granulosa cells [17]. In addition, changes in endometrial lipid levels have been shown to reduce susceptibility and receptivity and result in repeated failures of embryo implantation in ART programs [26, 27]. Cai W.Y. et al. analyzed plasma lipidome in relation to pregnancy establishment in ART program. The study found that higher HDL levels were associated with the production of more mature oocytes. Moreover, changes in the levels of LDL, total cholesterol and triglycerides affect not only the pregnancy rate but also the rate of live births and reproductive losses [17].
Thus, the obtained data reflect the possibility of using the lipidome of follicular fluid and blood plasma as markers of the effectiveness of ART treatment. The obtained results are consistent with the findings of the literature and can be used to develop a diagnostic test system with various mathematical techniques to improve the accuracy of diagnosing the cause of infertility and treatment prognosis. It should be noted that plasma lipidome showed higher accuracy in predicting clinical pregnancy than follicular fluid lipid profile. Predicting pregnancy in women undergoing ART treatment using plasma is more promising in terms of the practical application of the obtained data. Additionally, the contribution of various lipid groups to the ultimate result differed significantly in the analyzed biological samples. Investigating the complex relationships of lipid profile of blood plasma, follicular fluid, and pregnancy rate in association with the analysis of different biological entities using several mathematical methods of data processing, including ML, seems to be an extremely promising task for future research.
Conclusion
Increasing the predictive capacity of the effectiveness of ART treatment requires an integrative approach to finding better models for predicting a positive outcome using additional non-invasive markers with high diagnostic accuracy. In order to determine changes in the lipid profile in patients with different program outcomes, a high-performance liquid chromatography method combined with tandem mass spectrometry was used. This method confirmed the association between pregnancy establishment and a specific lipid profile. Changes in the lipid profile of blood plasma and follicular fluid depending on the pregnancy rate in the ART program make it possible to consider these markers as promising predictors of the effectiveness of infertility treatment. An important and promising area for further studies is the search for possible interventions to influence the lipid composition of follicular fluid for improving the quality of gametes, obtained embryos and the outcomes of ART programs. In addition, the analysis of the lipid profile of various biological objects makes it possible to take a more differentiated approach to the choice of a preparation strategy for IVF programs and provides an opportunity to study additional mechanisms of oocyte damage in patients seeking infertility treatment.
References
- Фролова Н.И., Белокриницкая Т.Е., Анохова Л.И., Богомазова Т.В. Эпидемиология и причины бесплодия у девушек в возрасте 18-25 лет как характеристика демографического потенциала популяции. Репродуктивное здоровье детей и подростков. 2015; 3: 19-25. [Frolova N.I., Belokrinitskaya T.E., Anokhova L.I., Bogomazova T.V. Epidemiology and causes of infertility in young female adults aged 18-25 years as a demographic characteristic of the population. Reproductive health of children and adolescents. 2015; 3: 19-25. (in Russian)].
- Драпкина Ю.С., Калинина Е.А., Макарова Н.П., Мильчаков К.С., Франкевич В.Е. Искусственный интеллект в репродуктивной медицине: этические и клинические аспекты. Акушерство и гинекология. 2022; 11: 37-44. [Drapkina Yu.S., Kalinina E.A., Makarova N.P., Milchakov K.S., Frankevich V.E. Artificial intelligence in reproductive medicine: ethical and clinical aspects. Obstetrics and Gynecology. 2022; (11): 37-44 (in Russian)]. https://dx.doi.org/10.18565/aig.2022.11.37-44.
- Ившин А.А., Багаудин Т.З., Гусев А.В. Искусственный интеллект на страже репродуктивного здоровья. Акушерство и гинекология. 2021; 5: 17-24. [Ivshin A.A., Bagaudin T.Z., Gusev A.V. Artificial intelligence on guard of reproductive health. Obstetrics and Gynecology. 2021; (5): 17-24 (in Russian)]. https://dx.doi.org/10.18565/aig.2021.5.17-24.
- Драпкина Ю.С., Макарова Н.П., Татаурова П.Д., Калинина Е.А. Поддержка врачебных решений с помощью глубокого машинного обучения при лечении бесплодия методами вспомогательных репродуктивных технологий. Медицинский совет. 2023; (15): 27-37. [Drapkina J.S., Makarova N.Р., Tataurova P.D., Kalinina E.A. Deep machine learning applied to support clinical decision-making in the treatment of infertility using assisted reproductive technologies. Medical Council. 2023; (15): 27-37. (in Russian)].https://dx.doi.org/10.21518/ms2023-368.
- Гусев А.В., Морозов С.П., Кутичев В.А., Новицкий Р.Э. Нормативно-правовое регулирование программного обеспечения для здравоохранения, созданного с применением технологий искусственного интеллекта, в Российской Федерации. Медицинские технологии. Оценка и выбор. 2021; (1): 36-45. [Gusev A.V., Morozov S.P., Kutichev V.A., Novitsky R.E. Legal regulation of artificial intelligence software in healthcare in the Russian Federation. Medical Technologies. Assessment and Choice. 2021; (1): 36-45.(in Russian)]. https://dx.doi.org/10.17116/medtech20214301136.
- Комедина В.И., Юренева С.В., Чаговец В.В., Стародубцева Н.Л. Особенности липидного состава сыворотки крови женщин в период менопаузального перехода. Акушерство и гинекология. 2022; 6: 90-97. [Komedina V.I., Yureneva S.V., Chagovets V.V., Starodubtseva N.L. Changes in serum lipid profile during the menopausal transition. Obstetrics and Gynecology. 2022; (6): 90-7 (in Russian)]. https://dx.doi.org/10.18565/aig.2022.6.90-97.
- Бурдули А.Г., Кициловская Н.А., Сухова Ю.В., Ведихина И.А., Иванец Т.Ю., Чаговец В.В., Стародубцева Н.Л., Франкевич В.Е. Фолликулярная жидкость и исходы программвспомогательных репродуктивных технологий (обзор литературы). Гинекология. 2019; 21(6): 36-40. [Burduli A.G., Kitsilovskaya N.A., Sukhova Y.V., Vedikhina I.A., Ivanets T.Y., Chagovets V.V., Starodubtseva N.L., Frankevich V.E. Follicular fluid and assisted reproductive technology programs outcomes (literature review). Gynecology. 2019; 21(6): 36-40. (in Russian)]. https://dx.doi.org/10.26442/20795696.2019.6.190663.
- Tokareva A.O., Chagovets V.V., Kononikhin A.S., Starodubtseva N.L., Nikolaev E.N., Frankevich V.E. Comparison of the effectiveness of variable selection method for creating a diagnostic panel of biomarkers for mass spectrometric lipidome analysis. J. Mass Spectrom. 2021; 56(3): e4702.https://dx.doi.org/10.1002/jms.4702.
- Zarezadeh R., Mehdizadeh A., Leroy J.L.M.R., Nouri M., Fayezi S., Darabi M. Action mechanisms of n-3 polyunsaturated fatty acids on the oocyte maturation and developmental competence: Potential advantages and disadvantages. J. Cell. Physiol. 2019; 234(2): 1016-29. https://dx.doi.org/10.1002/jcp.27101.
- DeBose-Boyd R.A. Significance and regulation of lipid metabolism. Semin. Cell. Dev. Biol. 2018; 81:97. https://dx.doi.org/10.1016/j.semcdb.2017.12.003.
- Ding Y., Jiang Y., Zhu M., Zhu Q., He Y., Lu Y. et al. Follicular fluid lipidomic profiling reveals potential biomarkers of polycystic ovary syndrome: A pilot study. Front. Endocrinol. (Lausanne). 2022; 13: 960274.https://dx.doi.org/10.3389/fendo.2022.960274.
- Shehadeh A., Bruck-Haimson R., Saidemberg D., Zacharia A., Herzberg S., Ben-Meir A. et al. A shift in follicular fluid from triacylglycerols to membrane lipids is associated with positive pregnancy outcome. FASEB J. 2019; 33(9): 10291-9. https://dx.doi.org/10.1096/fj.201900318RR.
- Довгань А.А., Ахмедова З.Ф., Сысоева А.П., Зингеренко Б.В., Романов Е.А., Силачев Д.Н., Макарова Н.П., Калинина Е.А. Внеклеточные везикулы фолликулярной жидкости: клинические аспекты и молекулярная биология. Акушерство и гинекология. 2023; 6: 38-43. [Dovgan A.A., Akhmedova Z.F., Sysoeva A.P., Zingerenko B.V., Romanov E.A., Silachev D.N., Makarova N.P., Kalinina E.A. Extracellular vesicles in follicular fluid: clinical aspects and molecular biology. Obstetrics and Gynecology. 2023; (6): 38-43 (in Russian)]. https://dx.doi.org/10.18565/aig.2022.320.
- Романов А.Ю., Эльдаров Ч.М., Фролова А.М., Макарова Н.П., Бобров М.Ю., Долгушина Н.В. Влияние контролируемой механической микровибрации на метаболомный профиль сред культивирования эмбрионов человека пятых суток развития. Акушерство и гинекология. 2020; 11: 131-8. [Romanov A.Yu., Eldarov Ch.M., Frolova A.M., Makarova N.P.,Bobrov M.Yu., Dolgushina N.V. Influence of controlled mechanical microvibration on embryo metabolomic profile. Obstetrics and Gynecology. 2020; (11): 131-8 (in Russian)]. https://dx.doi.org/10.18565/aig.2020.11.131-138.
- Núñez Calonge R., Guijarro J.A., Andrés C., Cortés S., Saladino M., Caballero P. et al. Relationships between lipids levels in blood plasma, follicular fluid and seminal plasma with ovarian response and sperm concentration regardless of age and body mass index. Rev. Int. Androl. 2022; 20(3):178-88.https://dx.doi.org/10.1016/j.androl.2021.02.004.
- Jamro E.L., Bloom M.S., Browne R.W., Kim K., Greenwood E.A., Fujimoto V.Y. Preconception serum lipids and lipophilic micronutrient levels are associated with live birth rates after IVF. Reprod. Biomed. Online. 2019; 39(4): 665-73. https://dx.doi.org/10.1016/j.rbmo.2019.06.004.
- Cai W.Y., Luo X., Chen E., Lv H., Fu K., Wu X.K. et al. Serum lipid levels and treatment outcomes in women undergoing assisted reproduction: a retrospective cohort study. Front. Endocrinol. (Lausanne). 2021; 12: 633766.https://dx.doi.org/10.3389/fendo.2021.633766.
- Chagovets V., Kononikhin A., Tokoreva A., Bormotov D., Starodubtseva N., Kostyukevich Y. et al. Relative quantitation of phosphatidylcholines with interfered masses of protonated and sodiated molecules by tandem and Fourier-transform ion cyclotron resonance mass spectrometry. Eur. J. Mass Spectrom. (Chichester). 2019; 25(2): 259-64. https://dx.doi.org/10.1177/1469066718799992.
- Юрова М.В., Чаговец В.В., Франкевич В.Е., Стародубцева Н.Л., Хабас Г.Н., Павлович С.В. Дифференциация серозных новообразований яичников на основании масс-спектрометрического анализа липидного профиля сыворотки крови: пилотное исследование. Акушерство и гинекология. 2021; 9: 107-19. [Iurova M.V., Chagovets V.V., Frankevich V.E., Starodubtseva N.L., Khabas G.N., Pavlovich S.V. Differential diagnosis of serous ovarian tumors using mass spectrometry-based serum lipid profiling: a pilot study. Obstetrics and Gynecology. 2021; (9): 107-19 (in Russian)]. https://dx.doi.org/10.18565/aig.2021.9.107-119.
- Юрова М.В., Франкевич В.Е., Павлович С.В., Чаговец В.В., Стародубцева Н.Л., Хабас Г.Н., Ашрафян Л.А., Сухих Г.Т. Диагностика серозного рака яичников высокой степени злокачественности Iа–Iс стадии по липидному профилю сыворотки крови. Гинекология. 2021; 23(4): 335-40. [Iurova M.V., Frankevich V.E., Pavlovich S.V., Chagovets V.V., Starodubtseva N.L., Khabas G.N., Ashrafyan L.A., Sukhikh G.T. Diagnosis of Ia-Ic stages of serous high-grade ovarian cancer by the lipid profile serum of blood serum. Gynecology. 2021; 23(4): 335-40 (in Russian)]. https://dx.doi.org/10.26442/20795696.2021.4.200911.
- Фортыгина Ю.А., Макарова Н.П., Драпкина Ю.С., Новоселова А.В., Гамисония А.М., Чаговец В.В., Франкевич В.Е., Калинина Е.А. Сравнительный анализ липидного профиля крови и фолликулярной жидкости женщин, проходящих лечение бесплодия методами вспомогательных репродуктивных технологий. Акушерство и гинекология. 2024; 4: 93-102. [Fortygina Yu.A., Makarova N.P., Drapkina Yu.S., Novoselova A.V., Gamisonia A.M., Chagovets V.V., Frankevich V.E., Kalinina E.A. Comparative analysis of blood and follicular fluid lipid profiles in women undergoing infertility treatment with assisted reproductive technologies. Obstetrics and Gynecology. 2024; (4): 93-102 (in Russian)]. https://dx.doi.org/10.18565/aig.2024.62.
- Фортыгина Ю.А., Макарова Н.П., Непша О.С., Лобанова Н.Н., Калинина Е.А. Роль липидомных исследований в репродукции человека и исходах программ лечения бесплодия методами вспомогательных репродуктивных технологий. Акушерство и гинекология. 2022; 10: 14-20. [Fortygina Yu.A., Makarova N.P., Nepsha O.S., Lobanova N.N., Kalinina E.A. The role of lipidomic studies in human reproduction and in the outcomes of infertility treatment programs using assisted reproductive technologies. Obstetrics and Gynecology. 2022; (10): 14-20 (in Russian)]. https://dx.doi.org/10.18565/aig.2022.10.14-20.
- Wang J., Zheng W., Zhang S., Yan K., Jin M., Hu H. et al. An increase of phosphatidylcholines in follicular fluid implies attenuation of embryo quality on day 3 post-fertilization. BMC Biol. 2021; 19(1): 200. https://dx.doi.org/10.1186/s12915-021-01118-w.
- Chen Z., Wu Y., Nagano M., Ueshiba K., Furukawa E., Yamamoto Y. et al. Lipidomic profiling of dairy cattle oocytes by high performance liquid chromatography-high resolution tandem mass spectrometry for developmental competence markers. Theriogenology. 2020; 144: 56-66.https://dx.doi.org/10.1016/j.theriogenology.2019.11.039.
- Liu L., Yin T.L., Chen Y., Li Y., Yin L., Ding J. et al. Follicular dynamics of glycerophospholipid and sphingolipid metabolisms in polycystic ovary syndrome patients. J. Steroid Biochem. Mol. Biol. 2019; 185: 142-9.https://dx.doi.org/10.1016/j.jsbmb.2018.08.008.
- de la Barca J.M.C., Boueilh T., Simard G., Boucret L., Ferré-L'Hotellier V., Tessier L. et al. Targeted metabolomics reveals reduced levels of polyunsaturated choline plasmalogens and a smaller dimethylarginine/arginine ratio in the follicular fluid of patients with a diminished ovarian reserve. Hum. Reprod. 2017; 32(11): 2269-78. https://dx.doi.org/10.1093/humrep/dex303.
- Li J., Gao Y., Guan L., Zhang H., Chen P., Gong X. et al. Lipid profiling of peri-implantation endometrium in patients with premature progesterone rise in the late follicular phase. J. Clin. Endocrinol. Metab. 2019; 104(11): 5555-65. https://dx.doi.org/10.1210/jc.2019-00793.
Received 08.08.2024
Accepted 20.08.2024
About the Authors
Yulia A. Fortygina, PhD student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, yu_fortygina@oparina4.ru, https://orcid.org/0000-0002-1251-0505Yulia S. Drapkina, PhD, Researcher at the Department of IVF named after Professor B.V. Leonov, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, yu_drapkina@oparina4.ru, https://orcid.org/0000-0002-0545-1607
Natalya P. Makarova, PhD, Leading Researcher at the Department of IVF named after Professor B.V. Leonov, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, np_makarova@oparina4.ru,
https://orcid.org/0000-0003-1396-7272
Elena V. Kulakova, Dr. Med. Sci., Senior Researcher at the Department of IVF named after Professor B.V. Leonov, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, E-mail: e_kulakova@oparina4.ru,
https://orcid.org/0000-0002-4433-4163
Anastasiia P. Sysoeva, PhD, Embryologist at the Department of IVF named after Professor B.V. Leonov, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, sysoeva.a.p@gmail.com,
https://orcid.org/0000-0002-6502-4498
Marina N. Yushina, Junior Researcher at the Laboratory of Metabolomics and Bioinformatics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, gmarinanikolaevna@gmail.com
Chupalav M. Eldarov, Senior Researcher at the Laboratory of Metabolomics and Bioinformatics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, chup4lav@yandex.ru, https://orcid.org/0000-0003-4027-6469
Vitaly V. Chagovets, PhD (in Physics and Mathematics), Head of the Laboratory of Metabolomics and Bioinformatics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, vvchagovets@gmail.com,
https://orcid.org/0000-0002-5120-376X
Vladimir E. Frankevich, Dr. Sci. (in Physics and Mathematics), Head of the Department for Systems Biology in Human Reproduction, Academician V.I. Kulakov National
Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, v_frankevich@oparina4.ru,
https://orcid.org/0000-0002-9780-4579
Elena A. Kalinina, Dr. Med. Sci., Professor, Head of the Department of IVF named after Professor B.V. Leonov, Academician V.I. Kulakov National Medical Research Center
for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, e_kalinina@oparina4.ru,
https://orcid.org/0000-0002-8922-2878



