Reproductive function in patients with endocrine infertility
Objective: To analyze the restoration of reproductive function in endocrine infertility in women living in Altai Krai.Vostrikov V.V., Nazarenko T.A.
Materials and methods: The study included 1610 women with ovulation disorders out of 9325 who had sought counseling for infertility from April 27, 2001, to December 31, 2020. According to the classifications relevant during the study period, WHO (1973), and NICE (2013), all women were divided into three groups according to the nature of ovulatory dysfunction. The study analyzed gynecological and extragenital pathology among the groups and the influence of the type of ovulatory dysfunction on the choice of treatment strategy. The results of overcoming infertility in patients are presented.
Results: The anovulatory infertility rate in the entire cohort was 19.6%. The clinical differences among the three study groups were clarified. Information is presented on the restoration of the reproductive function of women with impaired ovulation in real clinical practice. The advantages and disadvantages of various management strategies, from expectant to ART, are presented. Algorithms have been developed to improve the efficiency of reproductive function in anovulatory infertility.
Conclusion: The success in the fulfillment of reproductive potential depends on the correct diagnosis of the causes of ovulation disorders and the choice of a rational way to overcome infertility, considering its clinical and pathogenetic forms. It is advisable to use assisted reproductive technologies when other treatments are ineffective or when multiple factors contribute to infertility. In a hypergonadotropic state, ART using donor oocytes is a rational way to overcome infertility.
Authors' contributions: Vostrikov V.V., Nazarenko T.A. – conception and design of the study; Vostrikov V.V. – data collection and analysis, statistical analysis, drafting of the manuscript; Nazarenko T.A. – editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: The findings were analyzed as part of a clinical study.
Acknowledgement: The authors would like to thank Elena A. Suprun and Elena A. Markova for their help and support in conducting the study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the Altai State Medical University (Ref. No: № 21 of 27.11.2017).
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Vostrikov V.V., Nazarenko T.A.
Reproductive function in patients with endocrine infertility.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (9): 98-106 (in Russian)
https://dx.doi.org/10.18565/aig.2023.14
Keywords
Infertility is currently becoming a global problem in medical, social, and demographic terms [1]. Ovulation failure is observed in 25–40% of all patients diagnosed with infertility [2–6]. Understanding the causes of ovulatory dysfunction is the basis for choosing a rational treatment strategy [5–7].
This study aimed to analyze the restoration of reproductive function in endocrine infertility in women living in Altai Krai.
Materials and methods
Study participants
The study analyzed clinical data from 1610 women who were evaluated for suspected endocrine infertility between April 27, 2001, and December 31, 2020, representing 19.6% of all women who sought medical attention for infertility.
Patients were examined at the Siberian Institute of Human Reproduction and Genetics LLC, a medical center specializing in the diagnosis and treatment of infertility.
To reflect the studied pathology, the database “Therapeutic strategy in overcoming female infertility associated with ovulation disorders” was designed and compiled (Certificate of State Registration No. 2021620486 dated 03/12/2021). The database, created on a spreadsheet platform, contained information about 77 signs: clinical manifestations, anamnesis, physical examination data, results of laboratory and instrumental methods, established clinical diagnosis, chosen treatment strategy, and treatment outcome [4]. The records of the patients were edited as information about changes in any of the 77 signs was received. All personal data of patients (full name, place of residence, and telephone number) were deleted after the completion of data collection.
Ovulation disturbance was confirmed by the lack of growth of the leading follicle during repeated ultrasound monitoring and low concentrations of progesterone in the supposed phase II of the cycle (less than 10 nmol/ml or 3.1 ng/ml).
After the initial consultation, 290 patients (18%) left the clinic and dropped out of the study. Fifty-one women (3.1%) were over 40 years old, all of whom were referred for assisted reproductive technology (ART) and did not participate in the study.
The mean age of those included in the study was 28.9 (4.4) years. Approximately 10% of the patients were over 35 years of age. The median duration of infertility was 36 months (range: 24–60 months).
According to the NICE classification (2013) [8] and the causes of ovulation disorders, WHO (1973) [9], patients were divided into three groups:
- I: Hypogonadotropic hypoestrogenic anovulation, n=23;
- II: normogonadotropic normoestrogenic anovulation, n=1100;
- III: Hypergonadotropic hypoestrogenic anovulation, n=140.
Methods for assessing target indicators
Methods for assessing target indicators included medical history, the degree of hirsutism was assessed according to the Ferriman-Gallwey scale, objective examination findings, general clinical investigations, laboratory and instrumental diagnostic methods.
The hormonal profile included concentrations of follicle-stimulating hormone (FSH), luteinizing hormone (LH), anti-Müllerian hormone (AMH), prolactin, thyroid-stimulating hormone, free thyroxine, testosterone from the 3rd to 5th day of the cycle, and progesterone on the 22nd to 25th day of the cycle. In patients with hirsutism or increased testosterone levels, the androgen profile was determined (total testosterone, albumin, sex steroid-binding globulin, dehydroepiandrosterone, androstenedione, 17-OH progesterone, cortisol).
Transvaginal ultrasound examination (multi-frequency sensor 6–9 MHz) was used to assess the size and echogenicity of the uterus, ovaries, antral follicle count, and thickness and structure of the endometrium.
Assessment of fallopian tube patency was performed in 862/1263 women (68.3%): in 395 by chromolaparoscopy and in 184 by hysterosalpingography), 162 patients underwent sonohysterosalpingography. In another 121 patients, medical documentation indicated the patency of the fallopian tubes without specifying the method. Tubal patency was not assessed in 297 women due to a lack of indications; another 104 women refused to participate in the study.
In patients with hypo- and hypergonadotropic amenorrhea, taking into account the high risk of chromosomal pathology, karyotyping and genetic consultation were performed. The “typical” form of gonadal dysgenesis 45X0 (Shereshevsky–Turner syndrome) was diagnosed in 9 women, the “erased” 45X0/46XX, in 2 and the “pure” form 46XY (Swyer syndrome) in 1 patient.
In patients with hyperprolactinemia, after obtaining medical history, instrumental studies of organs and systems involved in the regulation of prolactin secretion or metabolism (thyroid gland, kidneys, liver, pituitary gland) were performed. They included the specific gravity of macroprolactin, and the patients were consulted by an endocrinologist. Consultation with a neurosurgeon is recommended in cases of hyperprolactinemia.
Patients with comorbidities were referred to specialists, and measures for the treatment of infertility were implemented after compensation for the detected disease.
Patients undergoing intrauterine insemination or ART were examined in accordance with the Orders of the Ministry of Health of the Russian Federation (MH RF), which regulates the scope of examination when performing these technologies and is relevant at the time of treatment (Order of the Ministry of Health of the Russian Federation dated February 26, 2003, No. 67, valid until 2013 g., and Order of the Ministry of Health of the Russian Federation dated August 30, 2012, No. 107n, valid until December 31, 2020).
The study design is illustrated in Figure 1.
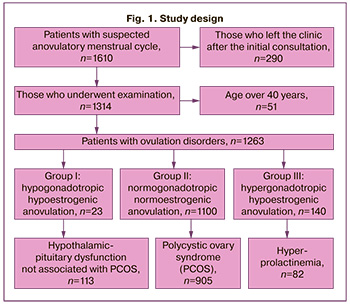
The clinically relevant endpoint of this study was pregnancy.
The study was reviewed and approved by the Research Ethics Committee of Altai State Medical University (Ref. No: № 21 of 27.11.2017). All the patients provided written informed consent to participate in the study.
Statistical analysis
Statistical analysis was performed using the Statistica 10 statistical package (StatSoft). Continuous variables were expressed as mean (M) and standard deviation) (SD). Categorical variables were reported as frequencies and percentages.
This study examined the statistical hypothesis of the presence of clinical and anamnestic differences among study groups I, II, and III.
Yates correction was used when the expected frequencies were between 5 and 10. Differences were considered statistically significant at p<0.05, where p was the probability of a type I error when testing the null hypothesis. Bonferroni correction was used for multiple comparisons.
Results
Majority of the patients (1010/1263 [80 %] ) reported menstrual dysfunction:742/1263 women (73.5%) had rare menstruation with a cycle delay from 10 days to 6 months, 236/1263 (23.4%) had amenorrhea, absence of independent menstruation for more than 6 months, and 32/1263 (3.1%) had heavy menstrual bleeding. A total of 253/1263 (20%) women had a normal menstrual cycle length of 23–36 days, with a mean of 28 (2.3) days, and anovulation was confirmed by ultrasound and progesterone blood tests.
Primary infertility was more common (877/1263, 69.4%) than secondary infertility (386/1263, 30.6%). In 101/386 (26.1%) women with secondary infertility, the pregnancy ended in childbirth. Moreover, in 17/386 (16.8%) women, endocrine infertility was associated with childbirth complications (placental abruption, severe preeclampsia, and antenatal fetal death). A history of pregnancy loss was reported by 149/386 (38.6%) women, and 27/386 (18.1%) had two or more miscarriages. 143/386 (37%) women had a history of medical abortions, while 26/386 (18.1%) had repeat abortions.
Assessment of the effectiveness of previous treatment during the initial consultation was possible in 491/1263 (38.8%) patients. Treatment aimed at normalizing the cycle using gestagens or oral contraceptives was previously administered to of 343/491 (69.8%) women. According to the medical records, almost one in five patients (98/491, 19.9%) had previously received biological supplements as their primary treatment. Drug stimulation of ovulation was performed in 260/491 (52.9%). Ovarian drilling to stimulate ovulation was used in 245/491 women (49.8%). Two-thirds of the patients, 327/491 (66.6%) had experience with several types of treatment. The time interval spent on treatment during the preliminary stage was 10 months (6–18 months). Failure to conceive was the reason for the repeated treatment.
Group I of ovulation disorders (according to WHO)
In 23/1263 (2.1%) women (median age 28.5 (26;31) years), FSH and estradiol levels indicated hypothalamic-pituitary hypogonadism (FSH concentration,1.8 (0.7;2.3) ME/l, LH, 1.5 (0.4;2.3) ME/l, estradiol less than 30 pg/ml). The median duration of infertility was 36 months (range: 24–55 months). The majority of patients (n=14) did not have spontaneous menstruation; in 9 patients, hypogonadism was associated with weight loss or stress. We did not find any cases of anosmia in the women with primary hypogonadotropic hypogonadism.
Only 3 women had a history of pregnancy that resulted in pregnancy loss. Weight deficiency was noted in five patients (21.7%), obesity or overweight in three (13%), and the rates of uterine development anomalies (such as uterine septum, bicornuate uterus, and saddle-shaped uterus) were comparable.
Group II of ovulation disorders (according to WHO)
Group II ovulation disorder (according to WHO) included 1100/1263 women (median age 28 (26;31) years) with the following hormone levels: FSH, 6.8 (4.8;8.9) IU/l; LH, 8.3 (6;12.8) ME/l; AMH, 5.1 (4.1, 5.7) ng/ml; and estradiol, 94.6 (42.3;69.1) pg/ml. The median duration of infertility was 36 (24;60) months.
The majority of the group, 1018/1100 (92.5%), had one of the four polycystic ovary syndrome (PCOS) phenotypes. Hyperprolactinemia was diagnosed in 82/1100 women (7.5%).
A detailed examination of 15 women with normogonadotropic anovulation revealed an uncompensated pathology of the thyroid gland, including in 8 cases – hypothyroidism (8 cases) and thyrotoxicosis (7 cases). Eleven patients had congenital adrenal hyperplasia. Three patients with ovulation disorders had epilepsy and two had brain diseases. Comorbidities dictated the treatment approach for these patients.
Most of the women had menstrual cycle disorders. A characteristic feature of the group was the frequency of detection of the clinical signs of hyperandrogenism (535/1100, 48.6%). In 268/535 women (50%), excess androgens manifested as hirustism and increased blood androgens; isolated hirsutism was diagnosed in 188/535 (35.1%), and hyperandrogenism without hirsutism in 79/535 (14.7%). Hyperandrogenism was absent in 547/1100 (49.7%) women in group II of the ovulation disorders (WHO).
Group III ovulation disorders (according to WHO)
Group III included 140 women (median age 32.2(28;36) years) with the following hormonal parameters: FSH, 29.1 (24.7;35.2) IU/l; LH, 14.2 (10.2;17.8) IU/l; AMH, 0.2 (0.1;0.3) ng/ml; estradiol, 23.7 (9.1;26.1) pg/ml. The median duration of infertility was 48 months (range: 24–87 months). Although the majority (91/140 [65 %] women) had primary infertility, 49/140 had a history of pregnancy:19 had a live birth, 22 had an abortion, and 18 had a miscarriage.
The causes of premature ovarian failure were divided into three main groups: iatrogenic (i.e., history of ovarian surgery, embolization of uterine fibroids, chemotherapy for cancer) (n=86), genetic, gonadal dysgenesis (n=13), and idiopathic and unspecified causes (n=41).
Clinical and anamnestic differences between groups I–III of ovulation disorders
Tables 1 and 2 show the comparative rates of medical history of pathologies in the groups.
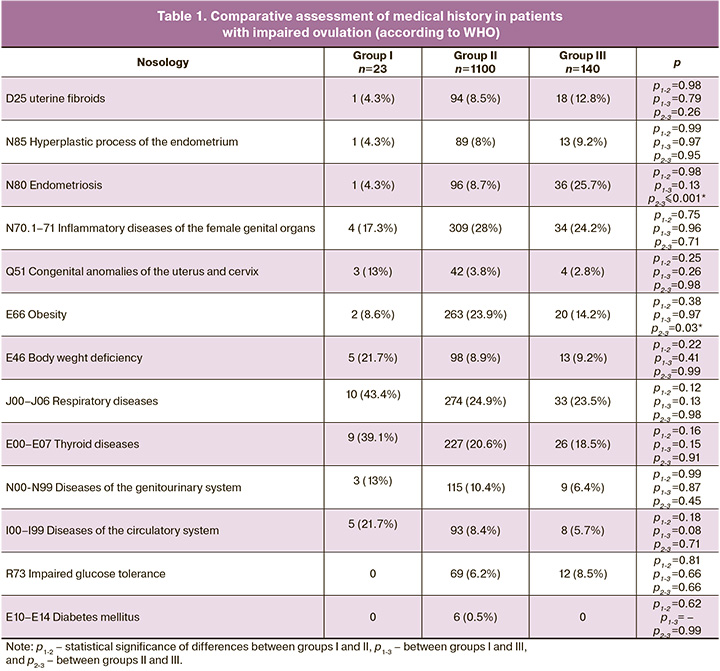
The statistical hypothesis of clinical and anamnestic differences between the study groups was confirmed for endometriosis (N80) and obesity (E66).
In the medical history of women in group III, endometriosis was more common than in the other groups, but these differences were statistically significant only in relation to group II (25.7% vs. 8.7%, p≤0.001).
In patients in group II, obesity was more common (23.9 %), and 8.6% and 14.2% in groups I and III, respectively; however, these differences were statistically significant only when compared with group III (p=0.03). Patients with diabetes mellitus were noted only in group II.
It should be noted that, in all cases, the identified comorbidities were in the compensation stage and were not the direct cause of ovulation disorders.
Data on reproductive outcomes
In 274/1263 (21.6%) patients included in the study and available for analysis of the structure and causes of ovulation disorders, the outcomes were not specified. All patients belonged to group II of ovulation disorders (according to the WHO).
Treatment strategies and results of fertility restoration
After baseline clinical evaluation, patients were recommended one of five main therapeutic models: complex correction of endocrine and metabolic disorders, as an independent method, and as preparation for the next stages: expectant management, stimulation of ovulation (medical and surgical), intrauterine insemination (IUI), and VRT. If the chosen model was ineffective, a transition to other methods of overcoming infertility was made.
A total of 726/1263 patients were administered drug ovarian stimulation. This group included 168 women in whom pregnancy did not occur during preparatory treatment, and 558 patients with anovulation who did not require preconception care. Depending on the state of the ovarian reserve, stimulation was used in 3 main protocols – “clomiphene,” “clomiphene+gonadotropins”, “gonadotropins.” Stimulation preceded IUI in 127 women. The treatment duration was 6–8 cycles. The pregnancy rate per stimulation cycle was 7.1% and 23.9% per woman.
The effectiveness of ART in restoring reproductive function in patients with endocrine infertility was 38.9%. The indication for ART was the presence of combined factors of infertility in of 124/1263 women (9.8%) (male factor in 44, fallopian tube pathology in 65, a combination of several causes of infertility in 15), and the ineffectiveness of previous treatment in 192 patients. The average number of ART programs per woman was 3.2 (range: 1–6). The pregnancy rate per attempt is 31.6 and 52.3% per woman.
The study showed that the mean age of women who achieved pregnancy was 28.4 (3.7) years versus 30.1 (4.5) years for those who remained infertile. The median duration of infertility in women with a successful pregnancy was 24 (18; 48) months, compared to 36 (24; 72) months in women with an unsuccessful pregnancy.
One of the problems revealed during the analysis was non-compliance with the administered prescriptions; a lack of adherence to treatment was noted in 268 (21.2%) women.
In most cases, the women did not follow the recommendations to change their lifestyle: weight gain in case of weight loss, 40 (14.9%); weight loss in case of obesity or overweight, 72 (26.8%); oocyte donation in case of hypergonadotropic hypogonadism, and 63 (23.5%). In addition, we noted cases of refusal to follow recommendations with the subsequent use of irrational methods of overcoming infertility, including ovarian drilling in seven women and IUI in five patients with ovulation disorders caused by hypogonadotropic or hypergonadotropic hypogonadism.
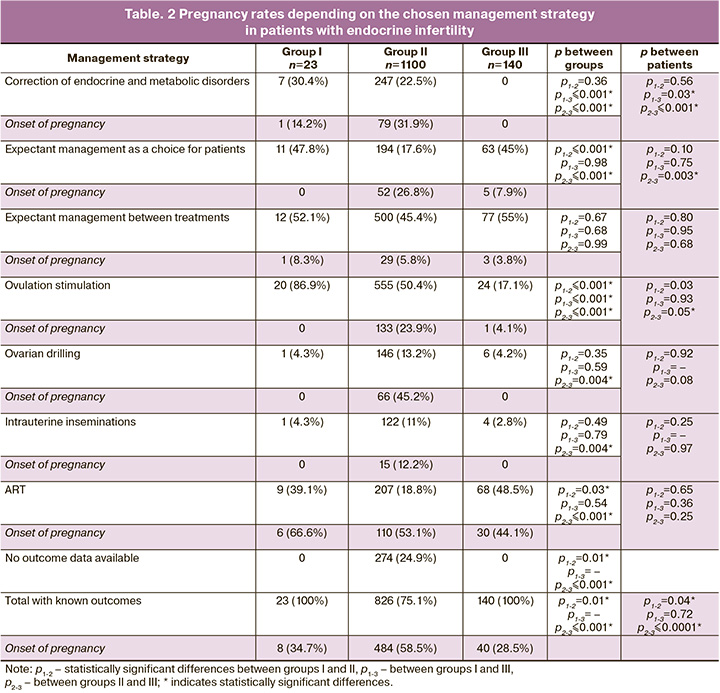
Table 2 provides information on the pregnancy rates in the groups, depending on the management model.
Of the seven women in group I, who followed the weight gain recommendation, spontaneous pregnancy occurred in one (14.2%). In another patient, spontaneous pregnancy occurred while awaiting ART. Stimulation of ovulation, administered to 20 women in group I, proved ineffective. The use of clomiphene citrate recommended by Gordon et al. (2017) in patients with sufficient estrogen levels [10], also proved ineffective in all stimulation protocols. Ovulation was achievable with the administration of urinary gonadotropins at a dose of at least 225 IU per day with a stimulation duration of 12 (2.5) days; however, this did not lead to pregnancy. ART was the most effective way to overcome infertility in group I.
Recommendations for the correction of endocrine-metabolic disorders (lifestyle changes, diet, exercise, insulin sensitizers), recommended for 385 women in group II with obesity or overweight, insulin resistance, and hypertension, were followed by 247 patients, which contributed to pregnancy in 79 (31, 9%) patients. These were predominantly young women (mean age 28.2 (4.3) years) with a short history of the disease, 24 months (18;36), who were clearly aware of the need to modify their lifestyle.
During medical correction of the pathology of the thyroid gland, ovulation was restored in 8 out of 13 women with the specified results. Pregnancy due to compensation for thyroid function occurred in 3 women (23%).
Spontaneous pregnancy occurred in 2 of the 11 women with congenital dysfunction of the adrenal cortex; another 3 patients achieved pregnancy during ART.
In five women, ovulation disorders were associated with treatment of the underlying disease (epilepsy, brain pathology). Correction of antiepileptic treatment contributed to spontaneous pregnancy in one woman; 1 more pregnancy occurred when ovulation was stimulated by clomiphene.
During treatment of hyperprolactinemia, ovulation was restored in 50 women (83.3%). Spontaneous pregnancy occurred in 16 (26.7%) women, and in 4 women, pregnancy was induced by clomiphene. In another 10 women, childbearing was achieved by ovarian drilling (n=1), IUI (n=1), and ART (n=8).
Ovulation stimulation, expectant management, and ovarian drilling in group III patients were considered incorrect management strategies. All 140 patients assigned to group III were recommended to donate oocytes to achieve pregnancy. 93 programs were performed on 68 patients. The pregnancy rate per treatment attempt was 32.2%, and 44.1% per woman.
An algorithm for the management of patients with anovulatory infertility was developed (Fig. 2).
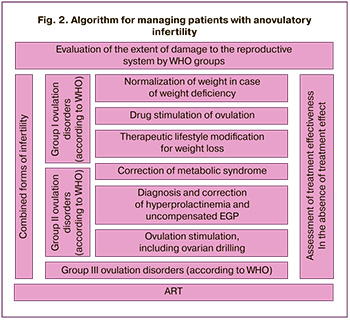
Discussion
The incidence of anovulatory forms of infertility among residents of the Altai Territory was 19.6% of all those who sought medical attention for infertility, which generally corresponds to literature data ranging from 20 to 40% [2–7].
Normalization of the cycle with gestagens and contraceptives and a history of biologically active supplement use in 491 women (38.8%) did not contribute to the onset of pregnancy and, in our opinion, delayed the use of rational methods to overcome infertility.
As expected, hypogonadotropic hypogonadism (group I) was rare (2.1 %). ART proved to be an effective management strategy for overcoming infertility in women in group I. Normalization of weight, compensation, or elimination of stress led to pregnancy in 2/9 (22.2%) women. Expectant management and ovarian drilling are ineffective. It is believed that ovulation in group I patients can be achieved with pulsating therapy with gonadotropin-releasing hormone and gonadotropin administration [11]. The high cost of ovulation stimulation was a limiting factor in our patients' adherence to repeated ovulation stimulation.
Traditionally, the largest group of women with impaired ovulation was represented by patients with PCOS, 1018/1100 (92.5%), which has been confirmed by other researchers [12–14]. The effectiveness of therapeutic lifestyle modifications for ovulation disorders caused by normogonadotropic hypogonadism has been noted by many authors [10, 14–17]. Our data confirm this hypothesis. Spontaneous pregnancy occurred with weight loss and correction of endocrine metabolic disorders in 79 patients (31.9%).
Ovulation stimulation is the first-line treatment for women in group II [2, 3, 5, 10, 11, 14, 18, 19]. Clomiphene remains the first-line drug; in cases of resistance to clomiphene, the use of gonadotropins is recommended. Surgical drilling occupies its niche in the treatment of impaired ovulation and is indicated for clomiphene resistance in group II patients with ovulation disorder (according to WHO) [3, 6, 19, 20]. According to Bordewijk et al. (2020), the probability of a live birth after stimulating ovulation with clomiphene was 42%, and after ovarian drilling, it ranged from 28 to 40% [20]. In our study, the use of ovarian drilling in 146 women in group II with repeated failures of ovulation stimulation contributed to pregnancy in 66 women (45.2%). However, if we consider previously ineffective surgical interventions on the ovaries in 235 women, the pregnancy rate (66 of 381 patients) is only 17.3%, which more accurately reflects the therapeutic potential of ovarian drilling in PCOS.
It is believed that only 25% of premature ovarian failure cases have a known etiology [21]. In our study, 86/140 (61.4%) patients assigned to group III indicated iatrogenic interventions that potentially affected the ovarian reserve, and in 15 women ovarian surgery was performed for PCOS. The optimal way to overcome infertility in group III is oocyte donation.
Despite the lack of adherence to medical recommendations in 268 patients, spontaneous pregnancies occurred in 57 women (21.2%). According to the WHO definition, adherence to treatment is defined as the degree to which the patient’s behavior complies with the doctor’s recommendations (diet, lifestyle changes, and taking medications) [22]. Spontaneous onset of pregnancy characterizes endocrine infertility as a reversible condition, with the likelihood of spontaneous restoration of ovulation. Spontaneous pregnancy without treatment was more likely in women assigned to group II than in those assigned to III (26.8% vs. 7.9 %, p=0.002).
Indications for ART were combined forms of infertility, diagnosed in 124/1263 (9.8%), hypergonadotropic hypogonadism as an indication for oocyte donation in 140 women (11%), and the ineffectiveness of other methods of treating endocrine infertility performed during the year in 192 patients (15.2%). Only 284/456 (62.2%) women with these indications resorted to ART. Pregnancy was observed in 146 participants. The cumulative pregnancy rate was 51.4%.
Without the use of ART, pregnancy occurred in 385/807 women (47.7%) who did not have absolute indications for ART.
When comparing the pregnancy rate in patients with impaired ovulation, the total effectiveness of all treatment strategies and the effectiveness of ART were comparable (47.7% versus 51.4%, p=0.41), considering the low probability of pregnancy without ART in combined forms of infertility and premature ovarian failure syndrome, when the therapeutic potential of other treatment methods has been exhausted.
The present study has some limitations. Given the small size of groups I and III, the risk of potential systematic errors cannot be excluded, and the data obtained cannot be projected onto the entire population. The used classification of the causes of ovulation disorders, proposed in 1973, currently does not fully reflect the complexity of the problem, which may be overcome by the FIGO classification (2022) approved as a world standard [23].
Conclusion
Anovulatory infertility is a favorable form of infertility for the restoration of reproductive function when the correct form of the disorder is diagnosed and an appropriate management strategy is chosen to achieve pregnancy. In cases where there is combined damage to the reproductive organs, or when treatment proves ineffective for a year, the use of ART may be considered. Hypergonadotropic hypogonadism, which involves impaired ovulation, indicates a need for oocyte donation.
An analysis of patient management strategies at the primary level of medical organizations in the Altai Krai revealed obvious shortcomings. Empirical treatment without adequate examination and identification of the clinical and pathogenic forms of disorders has resulted in delayed and unjustified treatments.
References
- Хвыля-Олинтер Н.А. Демографическое состояние современной России. Социум и власть. 2015; 4: 15-23. [Сhvilia-Olinter N.A. The demographic situation in Russia. Society and Power. 2015; (4): 15-23. (in Russian)].
- Назаренко Т.А. Синдром поликистозных яичников (современные подходы к диагностике и лечению бесплодия). 2-е изд. М.: МЕДпресс-информ; 2008. 207с. [Nazarenko T.A. Polycystic ovary syndrome. Mosсow: MEDpress-inform; 2008. 207p. (in Russian)].
- Назаренко Т.А. Эндокринные факторы женского и мужского бесплодия. Принципы гормонального лечения. М.: МИА; 2017. 132с. [Nazarenko T.A. Endocrine factors of female and male infertility. Moscow: MIA; 2017. 132p.(in Russian)].
- Востриков В.В., Бельницкая О.А., Кравцова Е.С., Белов В.М. К вопросу формирования баз данных в диагностике и лечении бесплодия. Бюллетень медицинской науки. 2021; 2: 4-12. [Vostrikov V.V., Bel'nickaya O.A., Kravcova E.C., Belov V.M. On the issue of database formation in the diagnosis and treatment of infertility. Bulletin of Medical Science. 2021; (2): 4-12.(in Russian)]. https://dx.doi.org/10.31684/25418475_2021_2_4.
- National Institute for Health and Clinical Excellence: Guidance. Fertility: assessment and treatment for people with fertility problems. National Collaborating Centre for Women’s and Children’s Health (UK). London: Royal College of Obstetricians & Gynaecologists; 2013. Available at:https://pubmed.ncb.nlm.gov Accessed 13.01.2023.
- Homburg R. Management of infertility and prevention of ovarian hyperstimulation in women with polycystic ovary syndrome. Best Pract. Res. Clin. Obstet. Gynaecol. 2004; 18(5): 773‐88. https://dx.doi.org/10.1016/j.bpobgyn.2004.05.006.
- Malcolm C.E., Cumming D.C. Does anovulation exist in eumenorrheic women? Obstet. Gynecol. 2003; 102(2): 317-8. https://dx.doi.org/10.1016/s0029-7844(03)00527-1.
- National Institute for Health and Care Excellence (NICE). Fertility problems: assessment and treatment. Clinical guideline CG156. NICE and RCOG; 2013.
- WHO-Scientific-Group. Agents stimulating gonadal function in the human. Report of a WHO scientific group. World Organ. Tech. Rep. Ser. 1973;514:1-30.
- Gordon C.M., Ackerman K.E., Berga S.L., Kaplan J.R., Mastorakos G., Misra M. et al. Functional hypothalamic amenorrhea: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2017; 102(5): 1413-9.https://dx.doi.org/10.1210/jc.2017-00131.
- Boehm U., Bouloux P.M., Dattani M.T., de Roux N., Dode C., Dunkel L. et al. Expert consensus document: European Consensus Statement on congenital hypogonadotropic hypogonadism – pathogenesis, diagnosis and treatment. Nat. Rev. Endocrinol. 2015; 11(9): 547-64. https://dx.doi.org/10.1038/nrendo.2015.112.
- Lizneva D., Suturina L., Walker W., Brakta S., Gavrilova-Jordan L. et al. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil. Steril. 2016; 106(1): 6-15. https://dx.doi.org/10.1016/j.fertnstert.2016.05.003.
- Bozdag G., Mumusoglu S., Zengin D., Karabulut E., Yildiz B.O. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum. Reprod. 2016; 31(12): 2841-55.https://dx.doi.org/10.1093/humrep/dew218.
- Teede H.J., Misso M.L., Costello M.F., Dokras A., Laven J., Moran L. et al. International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum. Reprod. 2018; 33(9): 1602-18. https://dx.doi.org/10.1093/humrep/dey256.
- Mumusoglu S., Yildiz B.O. Polycystic ovary syndrome phenotypes and prevalence: differential impact of diagnostic criteria and clinical versus unselected population. Curr. Opin.Endocr. Metab. Res. 2020; 12: 66-71.https://dx.doi.org/10.1016/j.coemr.2020.03.004.
- Lizneva D., Kirubakaran R., Mykhalchenko K., Suturina L., Chernukha G., Diamond M.P. et al. Phenotypes and body mass in women with polycystic ovary syndrome identified in referral versus unselected populations: systematic review and meta-analysis. Fertil. Steril. 2016; 106(6): 1510-20.е2.https://dx.doi.org/10.1016/j.fertnstert.2016.07.1121.
- Awoke M.A., Earnest A., Joham A.E., Hodge A.M., Teede H.J., Brown W.J. et al. Weight gain and lifestyle factors in women with and without polycystic ovary syndrome. Hum. Reprod. 2022; 37(1): 129-41. https://dx.doi.org/10.1093/humrep/deab239.
- Lawrenz B., Melado L., Fatemi H.M. Ovulation induction in anovulatory infertility is obsolete. Reprod. Biomed. Online. 2023; 46(2): 221-4.https://dx.doi.org/10.1016/j.rbmo.2022.08.102.
- Balen A.H., Morley L.C., Misso M., Franks S., Legro R.S., Wijeyaratne C.N. et al. The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance. Hum. Reprod. Update. 2016; 22(6): 687-708.https://dx.doi.org/10.1093/humupd/dmw025.
- Bordewijk E.M., Ng K.Y.B., Rakic L., Mol B.W.J., Brown J., Crawford T.J. et al. Laparoscopic ovarian drilling for ovulation induction in women with anovulatory polycystic ovary syndrome. Cochrane Database Syst. Rev. 2020; 2(2): CD001122. https://dx.doi.org/10.1002/14651858.CD001122.pub5.
- Vabre P., Gatimel N., Moreau J., Gayrard V., Picard-Hagen N., Parinaud J. et al. Environmental pollutants, a possible etiology for premature ovarian insufficiency: a narrative review of animal and human data. Environ. Health. 2017; 16(1): 37. https://dx.doi.org/10.1186/s12940-017-0242-4.
- World Health Organization. Adherence to long-term therapies: evidence for action. WHO Library Cataloguing-in-Publication Data. Geneva:WHO; 2003.
- Munro M.G., Balen A.H., Cho S., Critchley H.O.D., Díaz I., Ferriani R. et al.; FIGO Committee on Menstrual Disorders and Related Health Impacts, and FIGO Committee on Reproductive Medicine, Endocrinology, and Infertility. The FIGO ovulatory disorders classification system. Int. J. Gynaecol. Obstet. 2022; 159(1): 1-20. https://dx.doi.org/10.1002/ijgo.14331.
Received 22.01.2023
Accepted 29.08.2023
About the Authors
Viacheslav V. Vostrikov, PhD, Аssociate Professor at the Department of Obstetrics and Gynecology, Altai State Medical University, Ministry of Health of Russia,wkoctar@mail.ru, https://orcid.org/0000-0002-5567-2758, 656038, Russia, Barnaul, Lenin Ave., 40.
Tatyana А. Nazarenko, Dr. Med. Sci., Director of the Institute of Reproductive Medicine, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, t.nazarenko@oparina4.ru, https://orcid.org/0000-0002-5823-1667, 117997, Russia, Moscow, Ac. Oparin str., 4.
Corresponding author: Viacheslav V. Vostrikov, wkoctar@mail.ru



