Роль митохондрий в клетке
Качество гамет и эмбрионов является ключевым фактором успеха программ вспомогательных репродуктивных технологий (ВРТ). Основные методы оценки качества эмбриона в настоящее время представлены ранжированием по морфологии и результатам преимплантационного генетического тестирования. Тем не менее перенос эуплоидных эмбрионов хорошего и отличного качества по морфологии не гарантирует успешной имплантации и пролонгирования беременности. Поэтому исследователи активно разрабатывают возможные дополнительные методы изучения репродуктивного потенциала гамет и эмбрионов. Одним из важных факторов, влияющих на исход, авторы представляют адекватный энергетический метаболизм сперматозоидов, ооцит-кумулюсных комплексов (ОКК) и эмбрионов.
Митохондрии – единственные органеллы, имеющие собственную ДНК. Митохондриальная ДНК (мтДНК) представляет собой кольцевую двухцепочечную молекулу, кодирующую 13 субъединиц комплекса дыхательной цепи, участвующих в энергетическом метаболизме путем производства АТФ в клетке [1]. МтДНК содержит 37 генов. Кроме непосредственно участия в энергетическом метаболизме, митохондрия играет важную роль в поддержании кальциевого гомеостаза в клетке, участвует в процессах окисления жирных кислот и апоптозе. Митохондрии являются высокодинамичными органеллами, способными отвечать слиянием или делением, в зависимости от потребностей клетки в энергии [2]. Также и уровень мтДНК может
значительно различаться среди разных типов клеток, зависеть от потребности в энергии каждой клетки в изменяющихся условиях. Так, минимальный уровень мтДНК отмечен для сперматозоидов (несколько копий), а максимальные показатели – до сотен тысяч копий – зафиксированы для зрелых ооцитов [2, 3].
Уровень копийности мтДНК рассматривают как потенциальный биомаркер потенциала гамет к оплодотворению, развитию эмбриона и его имплантации.
мтДНК в сперматозоидах
Кроме выработки энергии, митохондрии участвуют в продукции активных форм кислорода (АФК). Предполагается, что оксидативный стресс, вызванный избытком АФК, может приводить к нарушению деметилирования в отцовском пронуклеусе и отрицательно влиять на развитие эмбриона [4]. В значительном количестве работ представлена связь между наличием мутаций и делеций (в том числе 4977 bp, 7345 bp, 7436 bp и 7599 bp) мтДНК и развитием астенозооспермии [5, 6]. Рядом исследователей показано, что повышение уровня мтДНК в сперматозоидах ассоциировано со снижением подвижности сперматозоидов, более низкой частотой фертилизации и снижением вероятности наступления беременности среди пар, не использующих контрацепцию [7–9].
В исследовании, включавшем 401 участника, повышение копийности мтДНК сперматозоидов было ассоциировано со снижением качества спермы [4]. Кроме того, 79 супружеских пар прошли лечение методами ВРТ с переносом эмбриона. Авторы не обнаружили зависимости между уровнем копийности мтДНК сперматозоидов и частотой клинической беременности или частотой живорождения.
Tiegs A.W. et al. в проспективном исследовании, включавшем 2062 уникальных образца спермы, также не обнаружили связи между уровнем мтДНК в сперматозоидах и частотой оплодотворения, хромосомным набором (эуплоидный/анеуплоидный) бластоцисты и частотой живорождения [10].
мтДНК в ооцит-кумулюсных комплексах
Взаимосвязь между уровнем мтДНК в ОКК и качеством ооцитов и эмбрионов изучается в ряде исследований [11–16].
Отечественные авторы оценили содержание мтДНК в 343 ооцитах, не оплодотворившихся в программах ВРТ. Ооциты разделили на 3 группы: 1-я – ооциты с цитоплазматическими дисморфизмами (n=126), 2-я – с экстрацитоплазматическими дисморфизмами (n=108), 3-ю группу составили ооциты без дисморфизмов (n=109). Исследователи показали снижение числа копий мтДНК для ооцитов с аномалиями строения. Авторы предположили возможное снижение энергетического потенциала подобных эмбрионов на этапах активного дробления [11].
Ogino M. et al. изучали уровень мтДНК в клетках кумулюса и периферической крови в зависимости от качества эмбрионов. Авторы показали сильную корреляционную связь между мтДНК в ОКК и клетках периферической крови (r=0,900; p<0,0001). Эмбрионы хорошего качества по морфологии имели более высокие показатели мтДНК в ОКК, из которых они были получены, в сравнении с эмбрионами плохого качества (p<0,0001). Однако для показателей мтДНК в клетках периферической крови в зависимости от качества эмбрионов статистически значимой разницы не было обнаружено [12].
Некоторые авторы оценивали уровень мтДНК ОКК в зависимости от степени зрелости ооцита (GV, MI, MII). Одной группой обнаружено повышение мтДНК ОКК для ооцитов GV в сравнении с ооцитами MI. Для ОКК ооцитов MI и MII статистически значимой разницы по количеству мтДНК не было найдено [13]. Другая группа исследователей отметила выраженное нарушение энергетического метаболизма митохондрий среди клеток кумулюса незрелых ооцитов [14].
Отечественные исследователи изучали уровень мтДНК в 454 образцах клеток кумулюса 67 пациенток позднего репродуктивного возраста. Показана отрицательная корреляционная связь между копийностью мтДНК и возрастом пациенток. Авторы не зафиксировали статистически значимой взаимосвязи между уровнем мтДНК и зрелостью ооцита, частотой оплодотворения, качеством эмбриона и его плоидностью [15].
В другом исследовании оценивали уровень мтДНК в 452 образцах ОКК 62 пациенток. Авторы не обнаружили взаимосвязи между уровнем мтДНК в клетках кумулюса и степенью зрелости ооцитов или успешностью их оплодотворения. Но существовала статистически значимая взаимосвязь между повышением мтДНК в ОКК и хорошим и отличным качеством эмбрионов по морфологии (p=0,006). Авторы предложили модель для прогнозирования возможности получения эмбрионов хорошего и отличного качества в зависимости от мтДНК в ОКК (AUC=0,618). Прогностическая точность предложенной модели возрастала при дополнительном учете индекса массы тела пациенток и их статуса курения (AUC=0,806; 95% ДИ 0,719–0,869) [16].
мтДНК в клетках эмбриона
Рядом отечественных и зарубежных авторов проведены работы, исследующие клиническую и биологическую значимость количества мтДНК у эмбрионов. Оценивали уровень мтДНК в клетках эмбрионов в зависимости от морфологической оценки качества эмбриона, плоидности, потенциала к имплантации, частоты наступления беременности и частоты живорождения.
Fragouli E. et al. показали повышение мтДНК для анеуплоидных эмбрионов в сравнении с эуплоидными (p=0,025). Анализ клинических исходов переноса эуплоидных бластоцист (n=131) выявил повышение потенциала к имплантации для эмбрионов с более низкими показателями мтДНК (p=0,007) [3]. В последующем аналогичном исследовании авторы подтвердили полученные результаты и снова продемонстрировали повышение частоты наступления беременности при переносе эуплоидных бластоцист со сниженным уровнем мтДНК [17].
Diez-Juan A. et al. также отметили снижение шансов на имплантацию для эуплоидных эмбрионов 3-х и 5-х суток развития в зависимости от повышения уровня мтДНК [18]. Другие авторы показали снижение частоты наступления беременности, ассоциированное с повышением мтДНК в клетках трофэктодермы (ТФЭ) (p<0,05) [2].
Отечественные авторы представили аналогичные результаты. Копийность мтДНК в ТФЭ анеуплоидных бластоцист была статистически значимо выше, чем эуплоидных. Авторы определили пороговый уровень мтДНК (0,004 о.е.), превышение которого с чувствительностью 76,8% и специфичностью 74,9% предсказывало отрицательный исход переноса эуплоидного эмбриона [19].
Напротив, иные результаты представил авторский коллектив Treff N. et al. Их исследование включало 187 пациентов с одновременным переносом двух эуплоидных эмбрионов мужского и женского пола, которые в 69 случаях закончились рождением одного живого плода. Не было продемонстрировано какой-либо взаимосвязи между копийностью мтДНК в клетках ТФЭ эуплоидных эмбрионов и их потенциалом к имплантации [20].
Эти результаты повторили Victor A.R. et al. В исследование было включено 1396 эмбрионов, прошедших биопсию с целью преимплантационного генетического тестирования на анеуплоидии. Ученые не обнаружили статистически значимой разницы между уровнем мтДНК эмбрионов в зависимости от их хромосомного набора (эуплоидный/анеуплоидный), возраста матери и частоты успешной имплантации [21].
Исследование Klimczak A. et al., включавшее 1510 бластоцист, выявило более высокое содержание мтДНК для эмбрионов низкого качества по морфологии в сравнении с эмбрионами удовлетворительного и хорошего качества. Однако при анализе только эуплоидных бластоцист (n=717) статистически значимой разницы по уровню мтДНК в указанных группах не было выявлено. Копийность мтДНК эмбрионов также не отличалась в зависимости от успеха имплантации и частоты наступления беременности [22].
По данным отечественных авторов, уровень мтДНК в клетках ТФЭ эуплоидных эмбрионов (n=244) не отличался в зависимости от исхода программ ВРТ [23]. Более высокие показатели мтДНК в клетках ТФЭ имели эмбрионы соответствующей категории отличного качества по морфологии.
мтДНК в отработанной культуральной среде
Наличие у эмбриона высокопористой мембраны «zona pellucida», проницаемой для ДНК, цитоплазматическое расположение мтДНК в клетке, а также ее повышенная копийность, в сравнении с геномной ДНК (гДНК), предполагает более высокую эффективность детекции именно мтДНК в отработанной культуральной среде (ОКС) – среде, полученной после культивирования в ней эмбриона [1].
В мировой литературе в настоящее время представлено незначительное количество работ, изучающих мтДНК в ОКС. Результаты и характеристики основных исследований, опубликованных по указанной теме, отражены в таблице. Всего отобрано 8 статей, вышедших в печать с 2013 по 2022 гг. Объем выборок составил от 53 до 800 образцов, метод генетического исследования в 7 случаях – полимеразная цепная реакция (PCR), и в 1 случае – секвенирование следующего поколения (NGS). Метод оплодотворения во всех исследованиях – интрацитоплазматическая инъекция сперматозоида (ИКСИ). Объем капли культивирования составил 20–25 мкл, объем собранного образца в большинстве случаев (5 из 8) – 20 мкл.
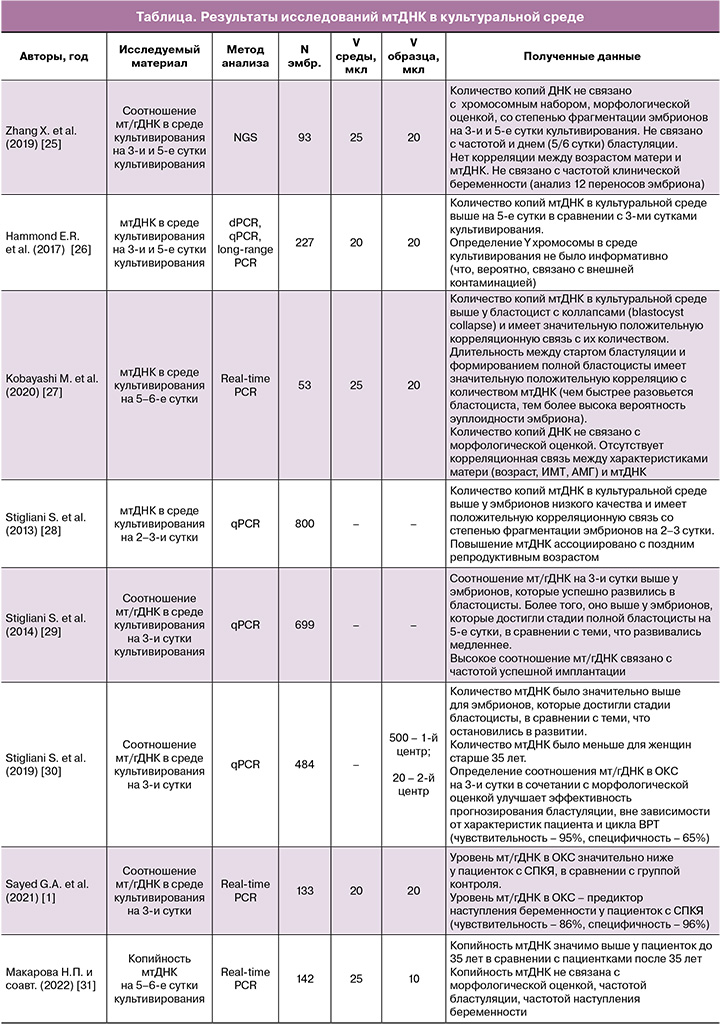
Следует отметить, что точность определения внеклеточной мтДНК в ОКС зависит от ряда факторов, влияющих на возможную контаминацию образцов [24]. К таким факторам относят: метод оплодотворения (чаще выполняется ИКСИ), условия культивирования эмбриона (объем капли, время культивирования, наличие индивидуальной капли), условия работы с образцом (использование шапочек, масок, халатов и перчаток), условия коллекции полученных образцов (использование индивидуальных насадок-фильтров).
Обсуждение
Известно, что эмбрионы человека наследуют митохондрии по материнской линии, в то время как митохондрии сперматозоидов подвергаются разрушению после оплодотворения [2, 7]. Учитывая, что репликация мтДНК отсутствует у эмбрионов первых трех суток развития, энергетический потенциал эмбриона на данном этапе зависит от количества и качества митохондрий, привнесенных ооцитом. Активация митохондриального генома происходит примерно на стадии 4–8 бластомеров через 72 ч после оплодотворения и усиливается на стадии бластоцисты [1, 3, 32].
Учитывая высокую активность энергетического метаболизма во время фолликулогенеза, концентрация мтДНК повышается в ооцитах, а затем постепенно снижается в делящихся клетках эмбриона [14]. Согласно теории «горлышка от бутылки» («mtDNA bottleneck theory»), суммарное количество мтДНК ооцита сохраняется после оплодотворения и распределяется между бластомерами при каждом их делении до момента полноценной активации митохондриального генома эмбриона. Таким образом, высокое содержание мтДНК в ооцитах и клетках кумулюса связано с более высоким потенциалом к развитию и имплантации эмбриона [7, 16].
Согласно другой теории «тихого эмбриона» («quiet embryo hypothesis») [3, 23], бластоцисты хорошего качества с высоким потенциалом к имплантации испытывают меньший «стресс» и содержат меньшее количество мтДНК в клетках внутренней клеточной массы и трофэктодермы. На стадии бластоцисты потребность в энергии возрастает с учетом продолжающегося развития эмбриона и активной дифференциации клеток, подготовки к имплантации. Повышение концентрации мтДНК у эмбрионов плохого качества связывают с их возрастающей потребностью к выработке энергии в условиях развивающегося клеточного стресса [3, 18, 23, 33]. Отмечено также, что анеуплоидные эмбрионы содержат повышенное количество мтДНК в сравнении с эуплоидными [33]. Кроме того, в литературе предложена гипотеза компенсаторного повышения уровня мтДНК, связанного с мутациями в митохондриальном геноме, одним из проявлений которых отмечено снижение частоты имплантации эмбрионов [2, 23].
Данной теории не противоречат результаты последних исследований, оценивающих уровень мтДНК в ОКС. Так, высокий уровень мтДНК в культуральной среде чаще отмечался у эмбрионов пациенток молодого возраста (до 35 лет) [30, 31], эмбрионов с более высоким потенциалом к бластуляции и имплантации [29, 30]. Повышение уровня мтДНК культуральной среды было ассоциировано с повышением частоты клинических беременностей [1]. Однако в литературе также встречаются и противоположные данные [25, 28].
Таким образом, с одной стороны, согласно данным литературы, можно отметить тенденцию, определяющую повышенный потенциал к развитию, имплантации и наступлению клинической беременности для бластоцист с пониженным содержанием мтДНК в клетках ТФЭ и внутриклеточной массы и повышенным содержанием мтДНК в ОКК и ОКС.
С другой стороны, среди исследователей остаются разные мнения относительно эффективности определения мтДНК в качестве дополнительного способа оценки качества гамет и эмбрионов [34]. Представленные исследования в большинстве случаев являются одноцентровыми, когда на полученные результаты оказывают влияние условия и организация работы в эмбриологической и генетической лабораториях отдельного центра. Кроме того, количество работ, оценивающих именно репродуктивные исходы циклов ВРТ на основании учета уровня мтДНК, крайне ограничено. Размеры выборок в опубликованных работах также незначительные.
Заключение
Таким образом, вопрос возможности рекомендации широкого применения оценки мтДНК тем или иным способом остается нерешенным. Для ответа на него требуется проведение дополнительных крупных хорошо спланированных исследований.



