Clinical efficacy of in vitro fertilization in patients treated with melatonin during preconception care
Objective: To investigate the clinical efficacy of preconception care with exogenous melatonin for in vitro fertilization (IVF). Materials and methods: This nonrandomized controlled trial included 84 infertile women undergoing IVF and experiencing sleep disorders and elevated stress reactions. Patients in group 1 (n=39) received preconception care with melatonin; women in group 2 (n=45) did not receive melatonin. The effectiveness of melatonin was assessed based on the results of ovarian stimulation, comparative evaluation of melatonin concentrations in the follicular fluid, monitoring of the culture stage, determination of the genetic status of embryos, and pregnancy rate. Results: Use of melatonin in patients undergoing IVF programs was associated with a significantly higher number of oocyte-cumulus complexes and a higher fertilization rate. There were no statistically significant differences in melatonin levels in the follicular fluid in patients who received and did not receive melatonin. There was an association between marginal values of melatonin levels (less than 10‰ and more than 90‰) and the frequency of obtaining embryos of low morphological quality [risk difference, %=61.9 (15.91–74.99), p(χ2)<0.009]. Melatonin administration increased the probability of obtaining blastocysts of high morphological quality by 41.7% [risk difference 41.7%, p<0.001], reduced the probability (RR=0.29 (0.09–0.97)) of obtaining only low quality blastocysts by 3.37 times (1/0.29), reduced the risk (RR=0.52 (0.29–0.92)) of obtaining aneuploid blastocysts by almost 2 times (1/0.52) and increased the probability of pregnancy in the cryopreservation cycle by 2.5 times [OR=2.44 (1.17–5.11)]. Conclusion: The study findings suggest a positive role for exogenous melatonin administration on the clinical and biological outcomes of IVF. More studies investigating the effect of melatonin on reproductive function are needed to form a concept of preconception care with melatonin supplementation.Vakhlova O.S., Oboskalova T.A., Kvashnina E.V., Mukhlynina E.A.
Keywords
Infertility is a complex, multifactorial condition emerging from clinical, biological, genetic, and psychological factors. Sleep duration, psychological and physical stress may play a role in embryo implantation and pregnancy success [1]. Disruption of the internal biological clock (circadian rhythm) causes somnologic disorders, depressive conditions [2], and negatively affects reproductive function by inducing subfertility [3]. As a chronotherapy regulator of circadian rhythms, exogenous melatonin can be used as a biologically valuable substance with a high safety profile [4, 5]. In addition to the systemic effect of melatonin as a factor regulating circadian rhythm and synchronizing the sleep-wake cycle at night and day, its level in the follicular fluid correlates with the level of anti-Mullerian and follicle-stimulating hormones and may be a criterion of ovarian reserve [6–8]. Melatonin has been found to be involved in the folliculogenesis process. It is synthesized in follicles and diffuses into cumulus cells and oocytes, protecting them from oxidative damage [9]. A positive relationship has been established between melatonin concentration and follicle diameter [10]. This indole can reduce cell apoptosis and leukocyte recruitment in the area of follicular rupture [11], maintain oocyte morphology after ovulation, inhibit the intensity of postovulatory aging [12], and protect oocyte DNA from oxidative damage and cellular degeneration [13]. Melatonin has been found to have positive effects of on the quality by increasing fertilization potential, maintaining the specific location and level of proteins-regulators of fertilization [14], reducing reactive oxygen species, inhibiting apoptosis processes, both during fertilization and embryo culture [8]. The positive effect of melatonin on embryonic development is partially explained by its antioxidant properties that inhibit apoptosis among blastocysts, increasing the quality of implantation [14–17]. Understanding intrafollicular processes, determining their biochemical mechanisms of action on gamete quality, and, consequently, embryo quality, underlies the development of metabolic correction schemes to improve the clinical effectiveness of in vitro fertilization (IVF) programs. In this regard, several studies have considered the possibility of using exogenous melatonin as a metabolic component that improves fertility [18].
This study aimed to investigate the clinical efficacy of preconception care with exogenous melatonin for in vitro fertilization (IVF).
Materials and methods
This non-randomized controlled trial included infertile women undergoing IVF [19]. In the first stage, a follow-up group was formed based on inclusion and non-inclusion criteria, as well as diagnosed sleep deficit and increased stress reactions, by psychological tests using a valid questionnaire. Baseline evaluation included clinical and anamnestic examination within the framework of the order of the Ministry of Health of Russia, 2020 [20], the increased level of stress, insufficient physiological need for sleep were revealed.
Inclusion criteria were age from 22 to 36 years, primary and secondary female tubal and unexplained infertility (N97.1 and N97.8 according to ICD-10 classification), sufficient ovarian reserve (Poseidon Group 1 and 2, 2016) [21], sleep disorders and increased levels of stress reactions. Non-inclusion criteria were aged more than 36 years, decreased ovarian reserve (Poseidon groups 3 and 4, 2016) [21], anovulatory infertility, male factor, and uterine factor infertility (N97.0; N97.4; N97.2 by ICD-10); women without somnologic and stress disorders and those who had contraindications to prescription of melatonin according to the instructions for its use.
At the second stage of the study, 84 infertile women were divided into Group 1 (n=39) and Group 2 (n=45). Patients in Group 1 received preconception care for 3 months under the MARS clinical protocol [22]: a month before starting ovarian stimulation, melatonin was started in a dose of 3 mg orally 40 minutes before bedtime. The use of exogenous melatonin was based on indications for the drug and informed voluntary consent. Group 2 did not receive melatonin during preconception care. The effectiveness of melatonin use was evaluated based on the results of the ovarian stimulation protocol, comparative evaluation of melatonin concentrations in the follicular fluid, monitoring of the embryo culture stage and determination of the genetic status of the embryos.
All women underwent an ovarian stimulation using gonadotropin-releasing hormone antagonists [23]. Melatonin in the follicular fluid was measured by ELISA. Morphological evaluation of oocyte fertilization were performed; the morphological quality of the embryos was determined according to the Gardner classification system [24]. Preimplantation genetic testing of embryos for aneuploidy was performed by Next Generation Sequencing (NGS).
At the third stage included analysis of pregnancy rates in the stimulation and in the cryopreservation cycles. In Group 1, the melatonin drug was discontinued ten days after embryo transfer. Human chorionic gonadotropin (β-hCG) levels were assessed on the 14th day after embryo transfer.
The study was reviewed and approved by the Research Ethics Committee of the Ural SMU(Ref. No. 8 of October 19, 2018).
Statistical analysis
Statistical analysis was performed using STATISTICA, version 10.0 (StatSoft Inc., Original Articles USA), MedСalc. Quantitative variables showing normal distribution were expressed as means (M) and standard deviation (SD); otherwise the median (Me) with 10‰, 25‰, 75‰, and 90‰ were reported. The normality of the distribution was tested by the Shapiro–Wilk test. Differences between groups were assessed using Student’s t-test for parametric and the Mann-Whitney test for non- parametric variables. Categorical variables were compared by Pearson’s χ2 test. When the expected frequencies were between 5 and 10, Yates' correction was used. The exact Fisher test was used when the expected frequency of one or more cells was less than five. Differences between groups were considered statistically significant at p<0.05. Relative risk with confidence intervals (RR, 95% CI) was used to assess the association; the absolute risk difference with 95% CI was used to assess the effect size [19, 25, 26].
Results
The mean age of patients was 31.9 (3.5), age at menarche was 13.2 (1.4), AMH index was 3.1 (1.9) ng/mL, FSH was 6.9 (1.4) mU/mL, mean body mass index was 23 (3.9) kg/m2. The menstrual cycle in women was normoponulating, with no differences between the study groups. Forty-four of 84 (52.4%) and 40/84 (47.6%) women had primary and secondary infertility, respectively. The duration of infertility was 5.3 (3.1) years and was comparable between the groups. Tubal factor infertility was diagnosed in 14/39 (36%) and 21/45 (47%), infertility of unspecified etiology in 25/39 (64%) and 24/45 (53%) in Groups 1 and 2, respectively.
More than half of the women, 45/84 (53.6%), did not use contraception from their sexual debut to the time of planning pregnancy. Seventy-four (88%) women had a history of bacterial and viral infections. The dominant benign proliferative diseases of the endometrium and myometrium were endometrial hyperplasia [27/84 (32.1%)] and polyposis [23/84 (27.4%)]. The proportion of surgical interventions on the pelvic organs was 41/84 (48.8%), the number of intra-abdominal operations per group of operated women (n=41) was 1.6 (1.0); the hysteroscopic intervention rate was 65/84 (77.4%), the number of hysteroscopic interventions was 2.1 (1.3).
Spontaneous pregnancy occurs in one woman in each group during preconception care. Ovarian stimulation was performed in 38/39 (97.4%) and 44/45 (97.8%) patients in Groups 1 and 2, respectively.
Antral follicle counts measured by transvaginal ultrasound at the start of stimulation did not differ between the groups. The number of oocyte-cumulus complexes (OCC) and fertilization rate in Group 1 tended to higher than in Group 2, but there were no statistically significant differences. One woman in Group 1 had vitrified oocytes. There were no differences on the fifth day of culture (Table 1).
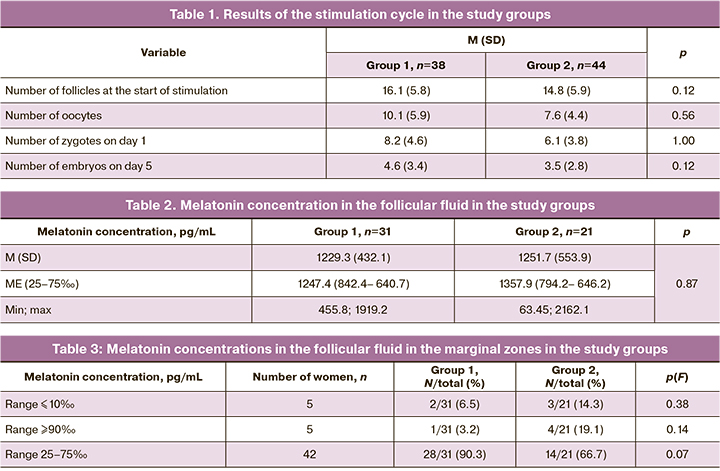
The melatonin concentration in the follicular fluid was 1238.3 (479.9) pkg/ml; there were no significant differences between Groups 1 and 2 (Table 2).
Melatonin levels in the follicular fluid were presented as percentile depending on the intake of exogenous melatonin. It was found that melatonin concentrations falling within the interval of more than 90‰ had a clear tendency to predominate in the group of women who did not take exogenous melatonin (p=0.058). Melatonin concentrations falling within the interval of normal values were significantly more frequently determined in Group 1 (p=0.03) (Table 3).
Values of melatonin less than 10‰ and more than 90‰ were associated with a high risk of developing low quality embryos. The attributive risk of developing embryos of low morphological quality was increased by 62% if a woman had intrafollicular melatonin levels that fell within the marginal value zones compared to those who had melatonin values in the range (Table 4).
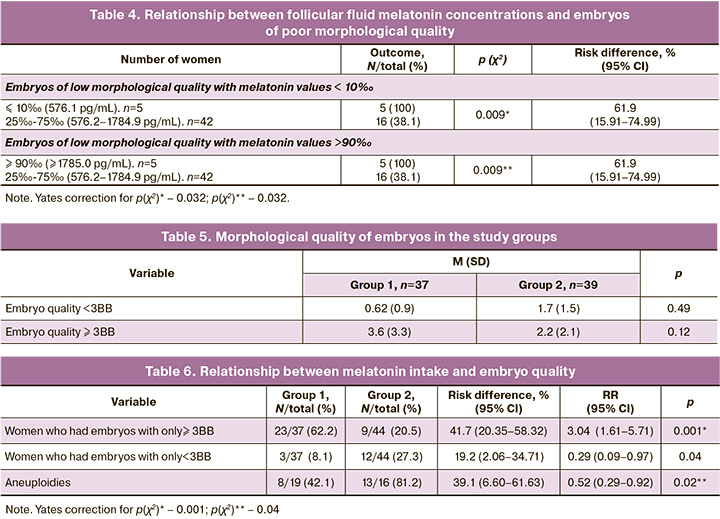
Five out of 44 (6.1%) women in Group 2 had embryos that were arrested in development. There were no cases of arrested embryonic development in Group 1. Therefore, there was a distinct tendency to an increase in the cases of complete arrest of embryonic development by day 5 of culture in the group of women who did not take melatonin preparations [5/44 (11.4%) vs. 0, respectively, p(F)≤0.06; p˃0.05].
In all women with complete embryonic arrest, the melatonin concentration fell within a range of more than 90‰ (5%, or 100%). At the same time, women who had melatonin values in the 25–75‰ range did not have complete embryonic arrest by day 5 of culture, which was significantly lower (p(χ2)=0.001).
Analysis of the embryological stage showed that the average number of embryos of low and high morphological quality had no significant differences in the study groups (Table 5).
However, women who received melatonin were more likely to have embryos of only high morphological quality (Table 6).
The likelihood of obtaining high-quality embryos increased by 41.7% (risk difference=41.7%, p<0.001) if melatonin was included in the preconception care. The number of women who had only low-morphological quality blastocysts was significantly lower in Group 1. The absolute risk of obtaining blastocysts of low morphological quality was lower by 39.1% (risk difference=39.1%, p<0.001), the likelihood (RR=0.29 (0.09–0.97)) of obtaining only low quality blastocysts was lower by 3.37 times (1/0.29) if a woman used melatonin (Table 6). Genetic pre-implantation tests of embryos was performed on 19 women in Group 1 and 16 in Group 2. The number of women who had aneuploid embryos was significantly higher in Group 2 than in Group 1 [3/16 (81.2%) and 8/19 (42.1%), respectively, p(χ2)=0.02]. The absolute risk of obtaining blastocysts of poor morphological quality was lower by 39.1% [risk difference=39.1% (6.60–61.63)]. The risk (RR=0.52 (0.29–0.92)) of obtaining aneuploid blastocysts was almost two times (1/0.52) lower with melatonin. The number of women who had euploid embryos was not significantly different between the study groups [17/19 (89.5%) and 13/16 (81.2%), p(χ2)=0.49]. The mean number (M (SD)) of euploid embryos obtained did not differ between Groups 1 and 2 [1.1 (0.5) and 1.6 (0.5), p=0.19].
The pregnancy rate in the ovarian stimulation cycle was 11/34 (32.4%) overall, 4/14 (28.5%) in Group 1, and 7/20 (35%) in Group 2, without statistically significant difference [p(F)≤0.73; ˃0.05]. Segmentation of the IVF cycle without embryo transfer was performed in 23/37 (62.2%) in Group 1 and in 20/40 (50%) in Group 2. The cumulative pregnancy rate in the cryopreservation cycle was 22/46 (47.8%). The embryo transfer in the cryocycle was performed with hormone replacement therapy. The mean endometrial thickness on the start day of gestagens was not significantly different between the comparison groups [9.6 (1.7) mm and 9.5 (1.37) mm, p=0.07]. However, the pregnancy rate in the cryo cycle was significantly higher in Group 1 (p (χ2) <0.008) (Table 7).
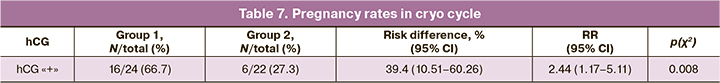
Therefore, melatonin preconception care with melatonin increased the likelihood of pregnancy almost 2.5-fold (OR=2.44 (1.17–.11)) and the absolute risk of pregnancy increased by 39% (risk difference, %=39.4% (39.15–39.65)).
Discussion
This study showed that women with infertility, who participated in IVF programs, had a complicated gynecological history that included high rates of genital tract infections and pelvic surgeries, and proliferative endometrial diseases. These pathological conditions stimulate oxidative reactions, disrupt biological rhythms, and have a negative impact both on the psychological state and on the reproductive potential. The purpose of prescribing melatonin in preconception care was to evaluate its effect on the effectiveness of the clinical and biological outcomes of IVF. Data from available studies suggest that intrafollicular melatonin diffuses into cumulus cells [27] and oocytes, protecting them from oxidative damage [12], having a positive effect on ovulation processes and the early stages of embryogenesis [28]. In our study, there were no statistically significant differences in the concentrations of melatonin in the follicular fluid regardless the use of exogenous melatonin.
However, analysis of the percentile distribution of the concentration values revealed an association between marginal melatonin values (less than 10‰ and more than 90‰) and the frequency of obtaining embryos of poor morphological quality (61.9 (15.91–74.99), p(χ2)=0.009). Explaining this fact, we can assume that extremely low concentrations of endogenous melatonin in the follicular fluid could not prevent oxidative damage to the oocytes, while extremely high concentrations of intrafollicular melatonin contributed to pro-oxidative damage to the oocytes. At the same time, there was a distinct tendency to an increased incidence of arrested embryonic development by the fifth day of culture in the group of women who did not take melatonin preparations [5/44 (11.4%) and 0, respectively, p(F)≤0.06; p˃0.05]. It should be noted that extremely high levels of intrafollicular melatonin were detected only in the group of women who did not take it. This fact may be associated with the diagnosed sleep deficit and elevated stress levels in the women included in the study. It can be assumed that the additional melatonin supplementation does not so much increase its intrafollicular concentration as it regulates its physiological synthesis, thereby contributing to the maintenance of normal concentrations and determining timely nocturnal peaks. In this sense, the question of maintaining circadian synthesis of melatonin through its additional supplementation becomes relevant.
The positive effect of melatonin on embryonic development is partially explained by its antioxidant properties that allow for a reduction in apoptosis between blastocysts and, therefore, improve embryo development [14] and implantation quality [15]. This assumption is confirmed by the results of our study. We found that the use of melatonin in preconception care increased the likelihood of obtaining blastocysts of high morphological quality by 41.7% (risk difference=41.7%, p<0.001), reduced the likelihood [RR=0.29 (0.09–0.97)] of receiving only low quality blastocysts by a factor of 3.37 (1/0.29), reduced the risk [RR=0.52 (0.29–0.92)] of obtaining aneuploid blastocysts by almost 2 times (1/0.52), and increased the likelihood of pregnancy in the cryo cycle by 2.5 times [OR=2.44 (1.17–5.11)].
This study demonstrated a generally positive role of exogenous melatonin on the clinical and biological outcomes of IVF. Although this agent is increasingly used in clinical practice, more studies are needed investigating the effect of melatonin on reproductive function to form a concept of preconception care with melatonin supplementation.
Limitations
A limitation of this study was the lack of findings on somnological disorders in women and stress levels, which is a fragment of this study and is currently under analysis. Additionally, this study presented a single-factor analysis of the evaluation of efficacy portion of the exogenous melatonin. A multivariate analysis is needed to develop a predictive model for the success of IVF programs. A larger sample size is needed to analyze the association of marginal melatonin values, which is a further subject of this work.
Conclusions
The administration of melatonin in IVF programs had no effect on the number of OCCs obtained or the fertilization rate. There were no statistically significant differences in follicular fluid melatonin levels in patients receiving and not receiving exogenous melatonin. An association was found between marginal values of melatonin levels (less than 10‰ and more than 90‰) and the rates of obtaining embryos of low morphological quality [risk difference, %=61.9 (15.91–74.99)), p(χ2)<0.009]. We found that melatonin administration increased the likelihood of obtaining high quality embryos by 41.7% [risk difference=41.7%, p<0.001], reduced the likelihood [RR=0.29 (0.09–0.97)] of obtaining only low-quality blastocysts by 3, 37 times (1/0.29), reduced the risk [RR=0.52 (0.29–0.92)] of obtaining aneuploid blastocysts by almost 2 times (1/0.52), and increased likelihood of pregnancy in the cryo cycle by 2.5 times [OR=2.44 (1.17–5.11)].
References
- Willis S.K., Hatch E.E., Wise L.A. Sleep and female reproduction. Curr. Opin. Obstet. Gynecol. 2019; 31(4): 222-7. https://dx.doi.org/10.1097/gco.0000000000000554.
- Ohdo Sh., Koyanagi S., Matsunaga N. Chronopharmacological strategies focused on chrono-drug discovery. Pharmacol. Ther. 2019 Jun 5. https://dx.doi.org/10.1016/j.pharmthera.2019.05.018.
- Honma A., Revell V.L., Gunn P.J., Davies S.K., Middleton B., Raynaud F.I., Skene D.J. Effect of acute total sleep deprivation on plasma melatonin, cortisol and metabolite rhythms in females. Eur. J. Neurosci. 2020; 51(1): 366-78. https://dx.doi.org/10.1111/ejn.14411.
- Zhao X., Wang D., Wu Z., Pan B., Yang H., Zeng C. et al. Female reproductive performance in the mouse: effect of oral melatonin. Molecules. 2018; 23(8): pii: E1845. https://dx.doi.org/10.3390/molecules23081845.
- Бурчаков Д.И., Кузнецова И.В. Мелатонин в репродуктивной медицине: можно ли улучшить качество ооцитов? Эффективная фармакотерапия. 2017; 35: 96-101. [Burchakov D.I., Kuznetsova I.V. Melatonin in reproductive medicine for the improvement of oocytes quality. Effective Pharmacotherapy. 2017; 35: 96-101. (in Russian)].
- Khan S.N., Shaeib F., Najafi T., Kavdia M., Gonik B., Saed G.M. et al. Diffused intra-oocyte hydrogen peroxide activates myeloperoxidase and deteriorates oocyte quality. PLoS One. 2015; 10(7): e0132388. https://dx.doi.org/10.1371/journal.pone.0132388.
- Amaral F.G.D., Andrade-Silva J., Kuwabara W.M.T., Cipolla-Neto J. New insights into the function of melatonin and its role in metabolic disturbances. Expert Rev. Endocrinol. Metab. 2019; 14(4): 293-300. https://dx.doi.org/10.1080/17446651.2019.1631158.
- Tong J., Sheng S., Sun Y., Li H., Li W.P., Zhang C., Chen Z.J. Melatonin levels in follicular fluid as markers for IVF outcomes and predicting ovarian reserve. Reproduction. 2017; 153(4): 443-51. https://dx.doi.org/10.1530/REP-16-0641.
- Reiter R.J., Tan D.X., Manchester L.C., Paredes S.D., Mayo J.C., Sainz R.M. Melatonin and reproduction revisited. Biol. Reprod. 2009; 81(3): 445-56. https://dx.doi.org/10.1095/biolreprod.108.075655.
- Yang M., Tao J., Chai M., Wu H., Wang J., Li G. et al. Melatonin improves the quality of inferior bovine oocytes and promoted their subsequent IVF embryo development: mechanisms and results. Molecules. 2017; 22(12); pii: E2059. https://dx.doi.org/10.3390/molecules22122059.
- Tordjman S., Chokron S., Delorme R., Charrier A., Bellissant E., Jaafari N., Fougerou C. Melatonin: pharmacology, functions and therapeutic benefits. Curr. Neuropharmacol. 2017; 15(3): 434-43. https://dx.doi.org/10.2174/1570159x14666161228122115.
- Wang T., Gao Y.Y., Chen L., Nie Z.W., Cheng W., Liu X. et al. Melatonin prevents postovulatory oocyte aging and promotes subsequent embryonic development in the pig. Aging (Albany NY). 2017; 9(6): 1552-64. https://dx.doi.org/10.18632/aging.101252.
- Choi D. Potency of melatonin in living beings. Dev. Reprod. 2013; 17(3):149-77. https://dx.doi.org/10.12717/DR.2013.17.3.149.
- Dai X., Lu Y., Zhang M., Miao Y., Zhou C., Cui Z., Xiong B. Melatonin improves the fertilization ability of post-ovulatory aged mouse oocytes by stabilizing ovastacin and Juno to promote sperm binding and fusion. Hum. Reprod. 2017; 32(3): 598-606. https://dx.doi.org/10.1093/humrep/dew362.
- Tian X., Wang F., Zhang L., Ji P., Wang J., Lv D. et al. Melatonin promotes the in vitro development of microinjected pronuclear mouse embryos via its antioxidative and anti-apoptotic effects. Int. J. Mol. Sci. 2017; 18(5): pii: E988. https://dx.doi.org/10.3390/ijms18050988.
- Бакшеев В.И., Коломоец Н.М. Мелатонин: место в системе нейрогуморальной регуляции у человека. Часть 2. Клиническая медицина. 2011; 89(2): 8-13. [Baksheev V.I., Kolomoets N.M. Melatonin: its role in the system of neurohumoral regulation in man. Part 2. Klinicheskaya medicina/Clinical medicine. 2011; 89(2): 8-13. (in Russian)].
- Eryilmaz O.G., Devran A., Sarikaya E., Aksakal F.N., Mollamahmutoğlu L., Cicek N. Melatonin improves the oocyte and the embryo in IVF patients with sleep disturbances, but does not improve the sleeping problems. J. Assist. Reprod. Genet. 2011; 28(9): 815-20. https:/dx.doi.org/10.1007/s10815-011-9604-y.
- Genario R., Morello E, Bueno A.A., Santos H.O. The usefulness of melatonin in the field of obstetrics and gynecology. Pharmacol. Res. 2019; 147: 104337. https://dx.doi.org/10.1016/j.phrs.2019.104337.
- Кельмансон И.А. Принципы доказательной педиатрии. СПб.: Фолиант; 2004. 240с. [Kelmanson I.A. Principles of evidence-based pediatrics. St. Petersburg: Foliant; 2004. 240 p. (in Russian)].
- Приказ Министерства здравоохранения РФ от 31 июля 2020 г. № 803н "О порядке использования вспомогательных репродуктивных технологий, противопоказаниях и ограничениях к их применению". [Order of the Ministry of Health of the Russian Federation dated July 31, 2020 No. 803n "On the procedure for using assisted reproductive technologies, contraindications and restrictions on their use." (in Russian)].
- Humaidan P., Alviggi C., Fischer R., Esteves S.C. The novel POSEIDON stratification of 'Low prognosis patients in Assisted Reproductive Technology' and its proposed marker of successful outcome. F1000Res. 2016; 5: 2911. https://dx.doi.org/10.12688/f1000research.10382.1.
- Прегравидарная подготовка. Клинический протокол Междисциплинарной ассоциации специалистов репродуктивной медицины (МАРС). М.: StatusPraesens; 2020. 128 с. [Pre-Gravidary Preparation. Clinical Protocol of the Interdisciplinary Association of Reproductive Medicine Professionals (MARS). М.: StatusPraesens; 2020. 128 p. (in Russian)].
- Felberbaum R., Diedrich K. Ovarian stimulation for in-vitro fertilization/intracytoplasmic sperm injection with gonadotrophins and gonadotrophin-releasing hormone analogues: agonists and antagonists. Rev. Hum. Reprod. 1999; 14(Suppl. 1): 207-21. https://dx.doi.org/10.1093/humrep/14.suppl_1.207.
- Gardner D.K., Schoolcraft W.B. In Vitro culture of human blastocyst. In: Jansen R., Mortimer D., eds. Towards reproductive certainty: infertility and genetics beyond. Carnforth: Parthenon Press; 1999: 377-88.
- Реброва О.Ю. Статистический анализ медицинских данных. Применение пакета прикладных программ STATISTICA. M.: МедиаСфера; 2006. 187с. [Rebrova O.Yu. Statistical analysis of medical data. Application of the STATISTICA application package. M.: MediaSphere; 2006. 187p. (in Russian)].
- Гланц С. Медико-биологическая статистика. M.: Практика; 1999. 262с. [Glantz S. Medical and biological statistics. M.: Practice; 1999. 262p. (in Russian)].
- Kim M.K., Park E.A., Kim H.J., Choi W.Y., Cho J.H., Lee W.S. et al. Does supplementation of in-vitro culture medium with melatonin improve IVF outcome in PCOS? Reprod. Biomed. Online. 2013; 26(1): 22-9. https://dx.doi.org/10.1016/j.rbmo.2012.10.007.
- Zheng M., Tong J., Li W.P., Chen Z.J., Zhang C. Melatonin concentration in follicular fluid is correlated with antral follicle count (AFC) and in vitro fertilization (IVF) outcomes in women undergoing assisted reproductive technology (ART) procedures.al. Gynecol. Endocrinol. 2018; 34(5): 446-50. https://dx.doi.org/10.1080/09513590.2017.1409713.
Received 15.02.2022
Accepted 30.05.2022
About the Authors
Olesya S. Vakhlova, Postgraduate Student, Obstetrician-Gynecologist at the Department of Obstetrics and Gynecology, Ural State Medical University, Ministry of Healthcare of the Russian Federation, 620028, Russian Federation, Ekaterinburg, Repin str., 3; Partus Center for Reproductive Disoders, 620026, Russian Federation, Ekaterinburg, Belinsky str., 61, +7(982)639-26-74, dr.vakhlova@mail.ru, https://orcid.org/0000-0001-5069-8177Tatyana A. Oboskalova, Dr. Med. Sci., Professor, Head of the Department of Obstetrics and Gynecology, Ural State Medical University, Ministry of Healthcare
of the Russian Federation, +7(912)283-76-31, oboskalova.tat@yandex.ru, https://orcid.org/0000-0003-0711-7896, 620028, Russian Federation, Ekaterinburg, Repin str., 3.
Elena V. Kvashnina, PhD, Chief Physician, Partus Center for Reproductive Disoders, +7(902)870-27-89, doctor.kvashnina@gmail.com, https://orcid.org/0000-0002-1251-2420, 620026, Russian Federation, Ekaterinburg, Belinsky str., 61.
Elena A. Mukhlynina, PhD (Bio), Senior Researcher, Head of the Collective Use Center, Institute of Immunology and Physiology – Ural branch of the RAS,
+7(902)877-12-12, elena.mukhlynina@yandex.ru, https://orcid.org/0000-0002-5159-4465, 620049, Russian Federation, Yekaterinburg, Pervomayskaya str., 106.
Corresponding author: Olesya S. Vakhlova, dr.vakhlova@mail.ru
Authors' contributions: Vakhlova O.S., Oboskalova T.A., Kvashnina E.V. – conception and design of the study, manuscript editing; Vakhlova O.S., Mukhlynina E.A. – material collection and analysis; Mukhlynina E.A. – immunohistochemical study; Vakhlova O.S. – statistical analysis, drafting of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was approved by the Research Ethics Committee of the Ural SMU(Ref. No. 8 of October 19, 2018).
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Vakhlova O.S., Oboskalova T.A., Kvashnina E.V., Mukhlynina E.A. Clinical efficacy of in vitro fertilization in patients treated with melatonin during preconception care.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 6: 67-74 (in Russian)
https://dx.doi.org/10.18565/aig.2022.6.67-74



