A profile of autoantibodies to steroid hormones and steroidogenic enzymes in women with endometriosis
Menzhinskaya I.V., Pavlovich S.V., Melkumyan A.G., Chuprynin V.D.
Endometriosis is a chronic inflammatory disease that affects women of reproductive age and is characterized by the presence of a wide range of autoantibodies, including those directed against steroid hormones. Of particular scientific interest is the detection of autoantibodies to steroidogenic enzymes, which have been identified as a risk factor for premature ovarian insufficiency (POI).
Objective: To study the profile of autoantibodies to steroid and gonadotropic hormones, and steroidogenic enzymes, in women with endometriosis.
Materials and methods: Using enzyme immunoassay, serum antibodies to estradiol, progesterone, follicle-stimulating hormone, and steroidogenic enzymes (21-hydroxylase, aromatase, cholesterol side-chain cleavage enzyme) were determined in patients with ovarian and deep infiltrating endometriosis (n=45) and in women without endometriosis (n=20).
Results: In patients with endometriosis, in addition to the predominant antibodies to estradiol and progesterone (60% and 55.6%, respectively), antibodies (M and G) to steroidogenic enzymes were detected. The detection rate and odds of detecting these antibodies were higher (33.3%, OR=9.5) than those in the control group (5%). Combinations of several antibodies against enzymes were observed in patients with severe endometriosis.
Conclusion: In addition to the high prevalence of autoantibodies to steroid hormones, patients with endometriosis are highly likely to have antibodies to steroidogenic enzymes. The latter may contribute to the development of autoimmune POI and infertility. The study will continue with a larger sample size and broader range of laboratory and instrumental parameters.
Authors’ contributions: Menzhinskaya I.V., Pavlovich S.V. – conception and design of the study; Menzhinskaya I.V., Melkumyan A.G., Chuprynin V.D. – material collection and processing; Menzhinskaya I.V., Melkumyan A.G. – statistical analysis, drafting of the manuscript; Pavlovich S.V., Chuprynin V.D. – editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: The study was conducted in accordance with state assignment No. 122020900125-8, entitled "Development of a differentiated approach to the management of patients of reproductive age with different forms of endometriosis".
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' data sharing statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Menzhinskaya I.V., Pavlovich S.V., Melkumyan A.G., Chuprynin V.D. A profile of autoantibodies to steroid hormones and steroidogenic enzymes in women with endometriosis.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (11): 146-154 (in Russian)
https://dx.doi.org/10.18565/aig.2024.253
Keywords
Endometriosis is an estrogen-dependent chronic inflammatory disease that affects women of reproductive age and is characterized by the presence of ectopic endometrial tissue outside the uterine cavity [1–3]. This tissue contains glandular and stromal cells, nerve fibers, blood vessels, and immune cells. Ectopic endometrium is most commonly found in the ovaries, but it can also invade the pelvic peritoneum, uterosacral ligaments, fallopian tubes, and broad ligaments of the uterus. In rare cases, it can develop in atypical locations, both inside and outside the pelvic area [4]. Endometriosis presents with various symptoms, including dysmenorrhea, dyschezia, dysuria, dyspareunia, fatigue, and gastrointestinal discomfort, significantly impairing the quality of life of affected women because of severe chronic pelvic pain [5]. The diagnosis of endometriosis can be challenging owing to the prevalence of asymptomatic forms of the disease and the necessity for surgical confirmation.
Endometriosis is a common gynecological disease affecting approximately 10% of women of reproductive age [5] and is a leading factor contributing to female infertility [2]. Notably, 25–50% of women experiencing infertility are diagnosed with endometriosis, while 30–50% of patients with endometriosis encounter pregnancy-related problems [6]. A 2024 systematic review indicated that the prevalence of endometriosis among women with a normal ovulatory cycle and unexplained infertility, as well as normal semen analysis in their partners, is 44%, with the majority of endometriotic lesions classified as minimal (74%) [7].
The onset of endometriosis is linked to interrelated endocrine, inflammatory, immune, oxidative, and proangiogenic mechanisms; however, its pathogenesis remains unclear [5]. Endometriosis is associated with complex disorders of the immune system, including alterations in systemic and local immunity, as well as functional disorders of effector and antigen-presenting cells [3]. One potential cause of immune imbalance may be the abnormal expression of immune checkpoints, which along with their corresponding ligands, are responsible for maintaining autotolerance and modulating the initiation, duration, and strength of the immune response of effector cells in normal tissues to prevent damage [8].
Recently, endometriosis has been considered an autoimmune disease due to the polyclonal activation of B lymphocytes, presence of autoantibodies, high levels of cytokines, therapeutic responses to the introduction of immunomodulators, cell-mediated abnormalities, and associated autoimmune diseases [9, 10]. The presence of autoantibodies in patients with endometriosis has been documented in scientific literature since the 1980s. Various anti-endometrial antibodies (to tropomyosin 3, stomatin-like protein 2, and tropomodulin 3) are considered useful diagnostic markers for the early stages of endometriosis [3]. When comparing antibodies to α-enolase and CA-125, their diagnostic value for endometriosis is similar [11]. In addition to anti-endometrial antibodies, an association between endometriosis and antibodies to steroid and gonadotropic hormones, along with their potential diagnostic and pathogenetic value, has recently been demonstrated [12]. Currently, the research into autoimmune mechanisms in the pathogenesis of endometriosis is ongoing.
Given that endometriosis is associated with increased secretion of steroid hormones, polyclonal activation of B-lymphocytes, and high levels of autoantibodies to steroid hormones and often leads to infertility, there is significant scientific interest in studying the potential triggering of autoantibody production against the main steroidogenic enzymes of cytochrome P450. These include enzymes that cleave the side chain of cholesterol (P450scc, CYP11A1), aromatase (P450arom, CYP19A1), and 21-hydroxylase (P450c21, CYP21A2), which have recently been shown to be risk factors for premature ovarian insufficiency (POI) and have a high diagnostic value for POI [13]. Routine screening for antibodies to 21-hydroxylase is recommended by the European Society of Human Reproduction and Embryology (ESHRE) for each case of POI to identify the autoimmune form of the disease [14].
Based on the above considerations, this study aimed to investigate the profile of autoantibodies to steroid and gonadotropic hormones, as well as steroidogenic enzymes, in women with endometriosis.
Materials and methods
The study group included women aged 20–40 years with stage III–IV deep infiltrating endometriosis with or without ovarian involvement (group 1, n=45). The control group comprised women of reproductive age without endometriosis or other proliferative gynecological diseases (group 2, n=20). The diagnosis of endometriosis was based on laparoscopic identification of endometriosis foci and the results of histological examination of the obtained material, in accordance with the American Society for Reproductive Medicine (rASRM) classification. Blood samples for autoantibody testing were collected from women in the proliferative phase of their menstrual cycle prior to surgery.
Patients with oncological and autoimmune diseases, acute inflammatory diseases of the pelvic organs, infectious diseases, or those who had received hormonal or anti-inflammatory therapy within three months prior to the study were excluded.
Immunological investigations involved determining serum autoantibodies to steroidogenic enzymes using modifications of the indirect solid-phase enzyme-linked immunosorbent assay (ELISA), developed in the Laboratory of Clinical Immunology of the V.I. Kulakov NMRC for OG&P of the Ministry of Health of the Russian Federation [13]. Polystyrene microplates with high adsorption capacity, coated with recombinant human cytochrome P450 enzymes (CYP21A2, CYP11A1, and CYP19A1) from Cloud-Clone Corp. (USA), were utilized. This included conjugate of mouse monoclonal antibodies against human IgM and IgG with horseradish peroxidase, buffers, substrate-chromogenic solution, and stop reagent for ELISA (OOO HEMA, Russia). Antibodies to hormones (progesterone, estradiol, and follicle-stimulating hormone [FSH]) were determined using a modified ELISA, progesterone-BSA conjugates (OOO HEMA, Russia), estradiol-BSA (Sigma-Aldrich, USA), and a preparation of highly purified FSH from the human pituitary gland (Sigma-Aldrich, USA). The ELISA was performed as previously described [15]. Blood serum was analyzed at a 1:100 dilution. Optical density (OD) was measured using an Infinite F50 microplate reader (TECAN, Austria) at a wavelength of 450 nm. A result was considered positive if the average OD of the test sample exceeded the average OD of the negative control samples by more than two standard deviations (2δ).
Statistical analysis
Statistical analysis was conducted using Microsoft Office Excel 2010 and MedCalc v. 12. The normality of the distribution of continuous variables was evaluated using the Shapiro–Wilk W-test and Kolmogorov–Smirnov test. Continuous variables showing a normal distribution were expressed as the mean and standard deviation (M±δ), and a two-sample t-test was employed to compare the variables between the groups. Variables that were not normally distributed were presented as median (Me) and range (min; max), with the non-parametric Mann–Whitney U-test used for comparisons between groups. Categorical variables are presented as frequencies (n) with corresponding percentages (%), and differences between groups were assessed using the χ² test. Correlation analysis was conducted by calculating the Spearman's rank correlation coefficient (r). The relationship between risk factors and outcomes was assessed by calculating the odds ratio (OR) with 95% confidence interval (95% CI). Differences were considered statistically significant at p<0.05.
Results
Blood serum from 45 patients with endometriosis and 20 women without endometriosis was analyzed by ELISA for class M and G antibodies to steroid hormones (estradiol and progesterone), FSH, and steroidogenic enzymes (21-hydroxylase (CYP21A2), aromatase (CYP19A1), and cholesterol side-chain cleavage enzyme (CYP11A1)). The diagnosis of endometriosis was confirmed by laparoscopic surgery, followed by histological examination of the obtained tissue.
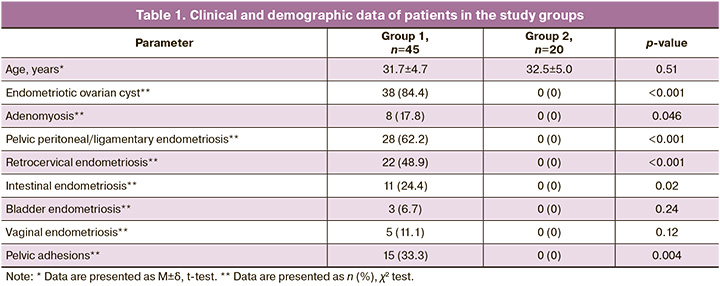
The ages of the patients in the two groups were comparable (Table 1). Most women in group 1 (38/45 (84.4%)) were diagnosed with ovarian endometriosis in combination with deep infiltrating endometriosis, while 7/45 (15.6%) had deep infiltrating endometriosis without ovarian involvement. Notably, 11/45 (24.4%) patients had previously undergone surgery to remove the endometriotic ovarian cysts. In 8/45 (17.8%) women, endometriosis was combined with adenomyosis, and 10/45 (22.2%) were diagnosed with infertility, including 5/45 (11.1%) with primary infertility and 5/45 (11.1%) with secondary infertility. Additionally, 42/45 (93.3%) of the patients had a regular menstrual cycle of 27.6±1.6 days, and 25/45 (55.6%) experienced painful periods. Oligomenorrhea was observed in two patients, and secondary amenorrhea was noted in one patient.
When serum antibody profiles were examined in patients with endometriosis, class M and G antibodies to steroid hormones (estradiol and progesterone), FSH, and steroidogenic enzymes (CYP11A1, CYP19A1, and CYP21A2) were detected (Fig. 1). In patients with endometriosis, class M and G antibodies to estradiol, progesterone, and CYP21A2 were found significantly more often (in 27/45 (60%), 25/45 (55.6%), and 9/45 (20%) patients, respectively) than in the control group (in which antibodies to estradiol were found in only 2/20 (10%) patients (p<0.05). Furthermore, antibodies to steroid hormones were detected more frequently in patients with endometriosis than antibodies to steroidogenic enzymes (6 (13.3%), 7 (15.6%), and 9 (20%), respectively; p<0.001), and FSH (8 patients (17.8%); p<0.001). Antibodies to steroid hormones were detected in 8/11 (72.7%) patients with a history of endometriotic cyst resection and in 22/34 (64.7%) patients without ovarian resection (p=0.63).
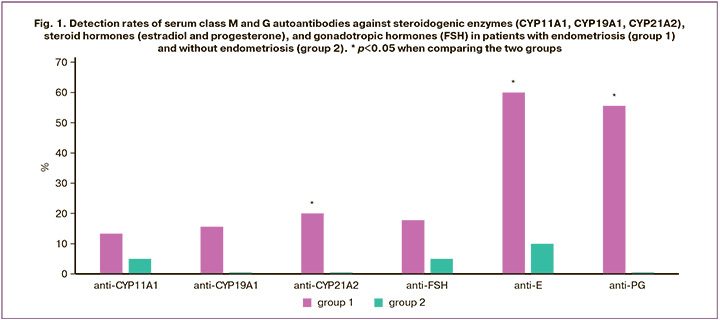
Serum antibodies (M, G) to steroidogenic enzymes were detected in 15/45 (33.3%) patients with endometriosis, significantly more often than in the control group (1/20 (5%) women; p=0.02). The likelihood of detecting these antibodies in patients with endometriosis was 9.5 times higher than that in women without endometriosis (OR=9.5 [1.16; 77.91]; p=0.04). Furthermore, antibodies against the CYP21A2 enzyme were detected more frequently in group 1 (9/45 (20%)) than in group 2 (0 (0%); p=0.03). In 8/45 (17.8%) patients with endometriosis, only an increase in IgM antibodies was observed, whereas 7/45 (15.6%) exhibited only IgG antibodies. Antibodies to only one steroidogenic enzyme were detected in 10/45 (22.2 %) women (four patients had IgM antibodies to CYP21A2, three had IgM antibodies to CYP19A1, and four had IgM or IgG antibodies to CYP11A1). Five of 45 (11.1%) patients had combinations of M or G antibodies to two enzymes (in three patients) or a combination of IgG antibodies to three enzymes (in two patients). In 11/45 (24.4%) patients, antibodies to steroidogenic enzymes were found in conjunction with antibodies to steroid hormones, whereas in 6/45 (13.3%) patients, they were combined with antibodies to FSH.
Antibodies to steroidogenic enzymes of classes M and/or G were detected in 13/38 (34.2%) patients with ovarian endometriosis, whereas only IgM antibodies were found in 2/7 (28.6%) patients with endometriosis without ovarian involvement. Antibodies to steroidogenic enzymes were detected in 4/11 (36.4%) patients with a history of endometriotic ovarian cyst resection and 9/27 (33.3%) patients without ovarian resection (p=0.86). Antibodies to steroidogenic enzymes were found in 5 of 10 (50%) patients with infertility (3 with primary infertility and 2 with secondary infertility) and in 10/35 (28.6%) patients without infertility (p=0.21). Additionally, 4 patients with infertility had class M or G antibodies to only one of the three steroidogenic enzymes (one patient had IgM to CYP21A2; one had IgG to CYP19A1; two had IgG to CYP11A1), while one patient had IgG antibodies to all three enzymes.
Table 2 shows the detection rates of class M and G antibodies to steroidogenic enzymes and hormones in patients with endometriosis and in the control group. In patients with endometriosis, the detection rates of class M and G antibodies to steroid hormones were significantly higher than those in the control group. Additionally, the likelihood of developing IgM and IgG antibodies to estradiol in patients with endometriosis was 11.5 times (OR=11.5 [1.4; 94.1]; p=0.02) and 8.6 times (OR=8.6 [1.04; 70.6]; p=0.046) greater, respectively. Furthermore, IgM antibodies to progesterone were 32.9 times higher (OR=32.9 [1.9; 578.5]; p=0.02) in women with endometriosis than in women without endometriosis. Antibodies to estradiol and progesterone of classes M and G were detected significantly more often than antibodies to steroidogenic enzymes and FSH (p<0.05), with the exception of anti-progesterone IgG (p=0.06 compared with anti-CYP19A1 IgG). In the control group, antibodies to steroidogenic enzymes and hormones were either not detected or identified at a low frequency (5%) in women without endometriosis.
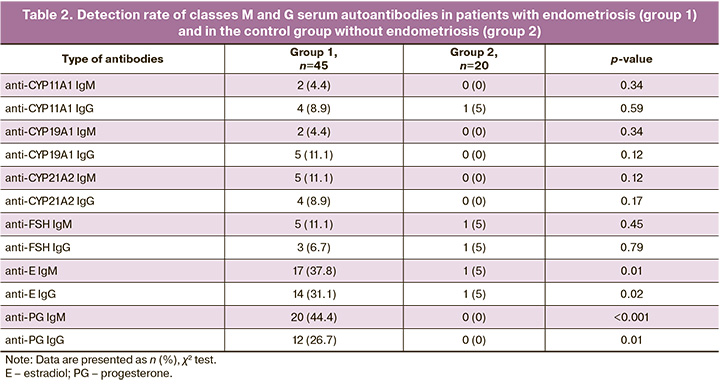
Table 3 presents the median levels of classes M and G autoantibodies in the endometriosis and control groups. The levels of autoantibodies (M, G) to steroid hormones (estradiol and progesterone) were significantly higher in patients with endometriosis than in the control group, whereas the levels of autoantibodies to steroidogenic enzymes and FSH did not differ significantly between the two groups (p>0.05).
Analysis of the correlation between the levels of autoantibodies to steroidogenic enzymes and hormones revealed a strong direct correlation between the levels of IgM and IgG antibodies to estradiol and progesterone (r≥0.90); between the levels of IgM antibodies to estradiol, progesterone, FSH, and IgM antibodies against CYP11A1 and CYP19A1 (r>0.7); and between the levels of IgM antibodies to FSH and CYP21A2 (r=0.73) (Fig. 2). A direct correlation was also found between the levels of IgG antibodies to FSH and IgG antibodies to CYP11A1 (r=0.78), CYP19A1 (r=0.35), and CYP21A2 (r=0.43). Furthermore, direct correlations were observed between IgM and IgG antibodies to different steroidogenic enzymes (r>0.7) and between IgM antibodies to FSH and estradiol, progesterone (0.73; 0.67).
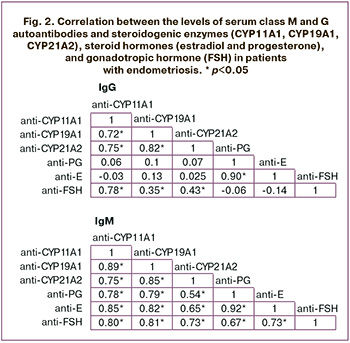
Discussion
This study investigated the profile of serum autoantibodies in reproductive-aged patients with ovarian and deep infiltrating endometriosis. In addition to assessing antibodies to steroid and gonadotropic hormones, we evaluated antibodies to key steroidogenic enzymes (CYP11A1, CYP19A1, and CYP21A2) in patients with endometriosis. The diagnosis of endometriosis was made according to the rASRM classification based on laparoscopic identification of endometriosis foci and conclusions from the histological examination of the obtained material.
In the study group, endometriotic ovarian cysts predominated in patients (80%), most often in combination with pelvic peritoneal endometriosis and ligaments (62.2%) and retrocervical endometriosis (48.9%). Other forms of endometriosis, such as endometriosis of the colon, bladder, and vagina, and adenomyosis, were diagnosed significantly less frequently. Moreover, patients with endometriosis often exhibited a pronounced pelvic adhesion process (33.3%). Primary or secondary infertility was diagnosed in 22.2% of the patients. Notably, most patients (93.3%) had regular menstrual cycles, while more than half experienced painful periods. Only two patients had oligomenorrhea and one had secondary amenorrhea.
Women with oncological and autoimmune diseases, acute inflammatory diseases of the pelvic organs, infectious diseases, and those who received hormonal and anti-inflammatory therapy three months prior to the study were excluded. The control group consisted of women of comparable age without endometriosis or other proliferative gynecological diseases, as determined by laparoscopy and hysteroscopy.
In examining the autoantibody profile in patients with endometriosis, we observed a higher detection rate and levels of serum autoantibodies (M, G) to progesterone and estradiol than in women without endometriosis and those with other specificities of antibodies. The likelihood of detecting antibodies (M, G) to estradiol and IgM antibodies to progesterone in patients with endometriosis was 11.5, 8.6, and 32.9 times higher, respectively, compared than in the control group, respectively. These findings are consistent with our previously obtained data, which demonstrated a broad range of serum autoantibodies and elevated levels of antibodies to endometrial antigens and steroid hormones in patients with endometriosis, particularly in cases of ovarian endometriosis, compared to women without endometriosis [15].
According to scientific literature, endometriosis is characterized by the polyclonal activation of B lymphocytes, dysfunction of T (Th1) and B lymphocytes, a shift in the Th1/Th2 balance towards a Th2-dependent response, induction and translocation of T regulatory cells, release of immunological and inflammatory factors, and increased production of autoantibodies [16, 17]. In patients with endometriosis, there is a sharp increase in the expression of the B-lymphocyte stimulator (BLyS), which is necessary for their proliferation and differentiation [18], as well as the transcription factor protein 6 B-cell lymphoma (BCL6), an important regulator of humoral immunity [19]. A recent systematic review showed that patients with endometriosis have increased presence and/or activation of B cells, along with excessive production of antibodies and pro-inflammatory cytokines [20]. In the present study, in addition to autoantibodies to steroid hormones, antibodies of classes M and G to key steroidogenic enzymes of human cytochrome P450 (CYP11A1, CYP19A1, CYP21A2) were detected in the blood serum of a significant proportion of patients with endometriosis (33.3%), and the likelihood of forming these antibodies in endometriosis was 9.5 times higher than that in women without endometriosis. Notably, antibodies to the CYP21A2 enzyme were detected significantly more often in patients with endometriosis than in the control group. Most frequently, antibodies to steroidogenic enzymes were found in combination with antibodies to steroid hormones, and less often, with antibodies to FSH.
The enzymes CYP11A1, CYP19A1, and CYP21A2 in women are responsible for the synthesis of steroid hormones in the ovaries (follicles and corpus luteum), placenta, adrenal glands, and adipose tissue [21]. Specifically, the enzyme cytochrome P450, which cleaves the side chain of cholesterol (P450scc, CYP11A1), catalyzes the first reaction in the steroid hormone biosynthesis pathway, converting cholesterol into pregnenolone, and is found both in the ovaries and adrenal glands. Aromatase (P450arom, CYP19A1) catalyzes the final stage of estrogen biosynthesis, converting androstenedione and testosterone into estrone and estradiol and is widely distributed in the ovaries, placenta, and adipose tissue. The adrenal cortex enzyme 21-hydroxylase (P450c21, CYP21A2) is involved in the synthesis of the steroid hormones aldosterone and cortisol from progesterone, catalyzing the conversion of progesterone and 17-hydroxyprogesterone.
Antibodies against steroidogenic enzymes were detected in both patients with ovarian endometriosis and those with deep infiltrating endometriosis that did not involve ovaries. However, class G antibodies were found exclusively in cases of ovarian endometriosis. The frequency of detection of antibodies to steroid hormones and steroidogenic enzymes did not differ among the patients, regardless of whether they had previously undergone ovarian resection for endometriotic cysts.
In patients diagnosed with infertility, both primary and secondary antibodies to steroidogenic hormones were found in half of the cases. Additionally, in patients with endometriosis, individual antibodies to different enzymes (CYP21A2, CYP19A1, or CYP11A1) were detected. For a more effective detection of antibodies to steroidogenic enzymes, it is advisable to conduct all three tests, as relying on a single test for antibodies to CYP21A2, recommended by ESHRE as a routine test, is insufficient. This finding aligns with the recent research by Adamyan L.V. et al. [13]. It is also important to note that antibodies to CYP11A1 and CYP19A1 enzymes present in the ovaries are more specific for POI than antibodies to the adrenal cortex enzyme CYP21A2, which is included in the diagnostic criteria for Addison's disease. The increased formation of antibodies to steroid hormones and steroidogenic enzymes in patients with endometriosis is driven by the excessive production of estrogens in the ovaries, ectopic endometriotic foci, and peripheral adipose tissue, along with increased progesterone production by stromal cells in endometriotic foci [22]. Furthermore, reduced expression of 17-beta-hydroxysteroid dehydrogenase type 2 (17βHSD), which converts estradiol into estrone, is accompanied by elevated estradiol levels [23].
The combination of local estradiol biosynthesis in endometriotic foci and severe inflammation in the abdominal cavity creates an abnormal immune-endocrine microenvironment that promotes the growth and survival of cells in ectopic foci [3]. Analysis of clinical and medical history data from five patients with high levels of IgM or IgG antibodies to several steroidogenic enzymes revealed that all women had severe endometriosis with a long-term disease course, a combination of ovarian and deep infiltrating endometriosis, and a pronounced inflammatory and adhesive process (in four patients). Additionally, two patients had a history of repeated ovarian resections and two experienced primary or secondary infertility. The correlation observed between serum antibodies to steroids and gonadotropic (FSH) hormones and steroidogenic enzymes can be attributed to the polyclonal activation of B lymphocytes, a characteristic feature of endometriosis, as well as to the functional interaction between hormones and enzymes. Notably, there is a direct correlation between the levels of antibodies to steroidogenic enzymes (CYP11A1 and CYP19A1) and FSH, which stimulate folliculogenesis and the secretion of steroid hormones, gonadal peptides, and growth factors by the ovaries. FSH is known to be the main inducer of aromatase activity in granulosa cells; however, its stimulating effect depends on the modulating effects of various factors, including estradiol [24]. Using primary human cumulus cells, it was demonstrated that factors secreted by oocytes enhance the effect of FSH on the activity of the CYP19A1 promoter and the levels of mRNA and protein, promoting aromatase expression and increasing estradiol production [25]. It is important to note that increased production of autoantibodies to steroidogenic enzymes was observed in patients with endometriosis who had a regular menstrual cycle at the time of the study. However, it should be considered that autoantibodies typically appear several years before the clinical manifestations of autoimmune disease. Thus, it can be assumed that over time, as these autoantibodies reach high levels in patients with endometriosis, they may lead to disruptions in steroidogenesis and menstrual cycle irregularities, such as oligomenorrhea or amenorrhea, contributing to the development of the autoimmune form of primary ovarian insufficiency (POI) and infertility.
This hypothesis is confirmed and consistent with the results of a recent study by Adamyan et al., which demonstrated a high detection rate and level of serum autoantibodies to steroidogenic enzymes CYP11A1, CYP19A1, and CYP21A2 in patients with primary ovarian insufficiency (POI) and infertility, compared to a group of women without POI and proliferative gynecological diseases [13]. Autoantibodies against steroidogenic enzymes have been shown to be risk factors for POI, with their levels correlating with menstrual irregularities in women and a decrease in the number of antral follicles in the ovaries. Moreover, the probability of developing POI in seropositive patients was 26.8, 14.7, and 13.6 times higher than that in seronegative patients, respectively.
Conclusion
Endometriosis is characterized by the prevalence of serum autoantibodies to steroid hormones (estradiol and progesterone) and an increased likelihood of detecting autoantibodies (M and G) to steroidogenic enzymes (CYP11A1, CYP19A1, and CYP21A2), which are found in ovarian and deep infiltrating endometriosis. The use of a panel of three enzymes enhances the efficiency of detecting antibodies to steroidogenic enzymes. Combinations of antibodies to several enzymes have been observed in patients with severe endometriosis. It is believed that antibodies to steroidogenic enzymes in patients with endometriosis may contribute to the development of autoimmune POI and infertility. The study will continue with an expanded sample size and analysis of laboratory and instrumental parameters while controlling for possible confounding factors.
References
- Taylor H.S., Kotlyar A.M., Flores V.A. Endometriosis is a chronic systemic disease: clinical challenges and novel innovations. Lancet. 2021; 397(10276): 839-52. https://dx.doi.org/10.1016/S0140-6736(21)00389-5.
- Becker C.M., Bokor A., Heikinheimo O., Horne A., Jansen F., Kiesel L. et al.; ESHRE Endometriosis Guideline Group. ESHRE guideline: endometriosis. Hum. Reprod. Open. 2022; 2022(2): hoac009. https://dx.doi.org/10.1093/hropen/hoac009.
- Gajbhiye R.K. Endometriosis and inflammatory immune responses: Indian experience. Am. J. Reprod. Immunol. 2023; 89(2): e13590. https://dx.doi.org/10.1111/aji.13590.
- Smolarz B., Szyłło K., Romanowicz H. Endometriosis: epidemiology, classification, pathogenesis, treatment and genetics (review of literature). Int. J. Mol. Sci. 2021; 22(19): 10554. https://dx.doi.org/10.3390/ijms221910554.
- Cuffaro F., Russo E., Amedei A. Endometriosis, pain, and related psychological disorders: unveiling the interplay among the microbiome, inflammation, and oxidative stress as a common thread. Int. J. Mol. Sci. 2024; 25(12): 6473. https://dx.doi.org/10.3390/ijms25126473.
- Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: a committee opinion. Fertil. Steril. 2012; 98(3): 591-8. https://dx.doi.org/10.1016/j.fertnstert.2012.05.031.
- Van Gestel H., Bafort C., Meuleman C., Tomassetti C., Vanhie A. The prevalence of endometriosis in unexplained infertility: a systematic review. Reprod. Biomed. Online. 2024; 49(3): 103848. https://dx.doi.org/10.1016/j.rbmo.2024.103848.
- Suszczyk D., Skiba W., Pawłowska-Łachut A., Dymanowska-Dyjak I., Włodarczyk K., Paduch R. et al. Immune checkpoints in endometriosis – a new insight in the pathogenesis. Int. J. Mol. Sci. 2024; 25(11): 6266. https://dx.doi.org/10.3390/ijms25116266.
- Greenbaum H., Galper B.L., Decter D.H., Eisenberg V.H. Endometriosis and autoimmunity: can autoantibodies be used as a non-invasive early diagnostic tool? Autoimmun. Rev. 2021; 20(5): 102795. https://dx.doi.org/10.1016/j.autrev.2021.102795.
- Shigesi N., Kvaskoff M., Kirtley S., Feng Q., Fang H., Knight J.C. et al. The association between endometriosis and autoimmune diseases: a systematic review and meta-analysis. Hum. Reprod. Update. 2019; 25(4): 486-503. https://dx.doi.org/10.1093/humupd/dmz014.
- Nisenblat V., Bossuyt P.M., Shaikh R., Farquhar C., Jordan V., Scheffers C.S. et al. Blood biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst. Rev. 2016; 2016(5): CD012179. https://dx.doi.org/10.1002/14651858.CD012179.
- Menzhinskaya I.V., Pavlovich S.V., Melkumyan A.G., Chuprynin V.D., Yarotskaya E.L., Sukhikh G.T. Potential significance of serum autoantibodies to endometrial antigens, α-enolase and hormones in non-invasive diagnosis and pathogenesis of endometriosis. Int. J. Mol. Sci. 2023; 24(21): 15578. https://dx.doi.org/10.3390/ijms242115578.
- Adamyan L.V., Menzhinskaya I.V., Antonova A.A., Tonoyan N.M., Sukhikh G.T. Diagnostic value of autoantibodies against steroidogenic enzymes and hormones in infertile women with premature ovarian insufficiency. Int. J. Mol. Sci. 2024; 25(12): 6545. https://dx.doi.org/10.3390/ijms25126545.
- Webber L., Davies M., Anderson R., Bartlett J., Braat D., Cartwright B. et al.; European Society for Human Reproduction and Embryology (ESHRE) Guideline Group on POI. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum. Reprod. 2016; 31(5): 926-37. https://dx.doi.org/10.1093/humrep/dew027.
- Менжинская И.В., Мелкумян А.Г., Павлович С.В., Чупрынин В.Д., Кречетова Л.В. Сравнение профиля сывороточных аутоантител у женщин при разных формах эндометриоза. Акушерство и гинекология. 2023; 8: 78-85. [Menzhinskaya I.V., Melkumyan A.G., Pavlovich S.V., Chuprynin V.D., Krechetova L.V. Comparison of the profile of serum autoantibodies in women with different forms of endometriosis. Obstetrics and Gynecology. 2023; (8): 78-85 (in Russian)]. https://dx.doi.org/10.18565/aig.2023.105.
- Maksym R.B., Hoffmann-Młodzianowska M., Skibińska M., Rabijewski M., Mackiewicz A., Kieda C. Immunology and immunotherapy of endometriosis. J. Clin. Med. 2021; 10(24): 5879. https://dx.doi.org/10.3390/jcm10245879.
- Zhang T., De Carolis C., Man G.C.W., Wang C.C. The link between immunity, autoimmunity and endometriosis: a literature update. Autoimmun. Rev. 2018; 17(10): 945-55. https://dx.doi.org/10.1016/j.autrev.2018.03.017.
- Hever A., Roth R.B., Hevezi P., Marin M.E., Acosta J.A., Acosta H. et al. Human endometriosis is associated with plasma cells and overexpression of B lymphocyte stimulator. Proc. Natl. Acad. Sci. U S A. 2007; 104(30): 12451-6. https://dx.doi.org/10.1073/pnas.0703451104.
- Louwen F., Kreis N.N., Ritter A., Friemel A., Solbach C., Yuan J. BCL6, a key oncogene, in the placenta, pre-eclampsia and endometriosis. Hum. Reprod. Update. 2022; 28(6): 890-909. https://dx.doi.org/10.1093/humupd/dmac027.
- Riccio L.G.C., Baracat E.C., Chapron C., Batteux F., Abrão M.S. The role of the B lymphocytes in endometriosis: a systematic review. J. Reprod. Immunol. 2017; 123: 29-34. https://dx.doi.org/10.1016/j.jri.2017.09.001.
- Miller W.L., Auchus R.J. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 2011; 32(1): 81-151. https://dx.doi.org/10.1210/er.2010-0013.
- Bulun S.E., Yilmaz B.D., Sison C., Miyazaki K., Bernardi L., Liu S. et al. Endometriosis. Endocr. Rev. 2019; 40(4): 1048-79. https://dx.doi.org/10.1210/er.2018-00242.
- Han S.J., Hawkins S.M., Begum K., Jung S.Y., Kovanci E., Qin J. et al. A new isoform of steroid receptor coactivator-1 is crucial for pathogenic progression of endometriosis. Nat. Med. 2012; 18(7): 1102-11. https://dx.doi.org/10.1038/nm.2826.
- Савина В.А., Потин В.В., Тарасова М.А. Роль ароматазы в патогенезе первично-овариальной недостаточности. Журнал акушерства и женских болезней. 2010; 59(6): 85-93. [Savina V.A., Potin V.V., Tarasova M.A. The role of aromatase in the pathogenesis of primary ovarian deficiency (literature review). Journal of Obstetrics and Women's Diseases. 2010; 59(6): 85-93.(in Russian)].
- Hobeika E., Armouti M., Kala H., Fierro M.A., Winston N.J., Scoccia B. et al. Oocyte-secreted factors synergize with FSH to promote aromatase expression in primary human cumulus cells. J. Clin. Endocrinol. Metab. 2019; 104(5):1667-76. https://dx.doi.org/10.1210/jc.2018-01705.
Received 15.10.2024
Accepted 12.11.2024
About the Authors
Irina V. Menzhinskaya, Dr. Med. Sci., Leading Researcher, Laboratory of Clinical Immunology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 4, Oparina str., Moscow, Russia, 117997, +7(495)438-11-83, i_menzinskaya@oparina4.ruStanislav V. Pavlovich, PhD, Scientific Secretary, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 4, Oparina str., Moscow, Russia, 117997; Professor, Department of Obstetrics, Gynecology, Perinatology and Reproductology, Institute of Vocational Education,
I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia, +7(495)438-52-25, s_pavlovich@oparina4.ru
Arika G. Melkumyan, Obstetrician-Gynecologist, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 4, Oparina str., Moscow, Russia, 117997, +7(495)438-52-25, dr.melkumyan@gmail.com
Vladimir D. Chuprynin, PhD, Head of the Department of Surgery, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 4, Oparina str., Moscow, Russia, 117997, +7(495)438-35-75, v_chuprynin@oparina4.ru



