Analysis of the embryonic stage of in vitro fertilization programs in patients with unexplained infertility
Objective: The purpose of the study was analysis of the embryonic stage of in vitro fertilization programs (IVF and ICSI) in patients with unexplained infertility compared to patients with tubo-peritoneal factor of infertility.Bachurin A.V., Kirakosyan E.V., Nazarenko N.A., Pavlovich S.V.
Materials and methods: We collected and analyzed information on embryonic stage of IVF programs in women with unexplained infertility (the study group consisting of 80 women), who underwent 113 IVF programs, and in women with tubo-peritoneal factor of infertility (the control group consisting of 30 patients), who underwent 41 IVF programs in the Departments of the Institute of Reproductive Medicine of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia in the period from 2019 to 2021. The comparison groups of patients were representative of the general population in qualitative and quantitative parameters, comparable in age, duration of infertility, ovarian reserve status, stimulation protocols and the number of IVF programs per woman.
Results: The blastulation rate was considered to be the endpoint in IVF programs, and it was significantly lower in the group of women with unexplained infertility. In-depth analysis of the embryonic stage of IVF programs showed, that low blastulation rate in unexplained infertility was mainly due to the fact that embryos stopped developing about three days after they were cultured. At the same time the morphological assessment showed that the quality of blastocysts was higher in the group of unexplained infertility compared to the group of tubo-peritoneal factor of infertility. Preimplantation genetic testing for aneuploidy (PGT-A) showed similar frequency of detection of euploid embryos.
Conclusion: Given the identified impairments of early embryonic development in unexplained infertility, it is appropriate to recommend the patients to undergo early use of IVF with good quality embryo (>3, AA, AB, BA) transfer on day 5 of culture without long-lasting preliminary examination and empirical treatment.
Keywords
The specialists refer unexplained infertility (UI) to the so-called diagnosis of exclusion due to the fact that in the process of medical examination of married couples the causes of infertility cannot be established [1–3].
According to different sources, the frequency of UI significantly varies and reaches 10-30% among infertile couples, and 10–17% among female infertility [4–6].
The specialists assess differently ovarian reserve status in women among married couples with UI. Some of them point out to low ovarian reserve parameters compared to the patients of similar age, who have tubo-peritoneal factor of infertility (TPFI), and others – to the absence of these differences [7–9].
The disagreement in the definition of the term «unexplained infertility» may be partly due to late admission of patients to in vitro fertilization (IVF) clinics, prolonged watch-and-wait strategy and empirical treatment, which are used because the cause of infertility is not clear [10, 11]. At the same time, undoubtedly that the most important factor limiting the effectiveness of IVF programs is a woman's age [1, 7].
The absence of obvious anatomical abnormalities and abnormal reproductive system physiology in partners – married couples, enabled the researchers to analyze the embryological parameters in IVF programs in patients with UI. However, there are practically no thorough researches that provide objective information about the quality of oocytes, their ability to fertilize, the features of embryogenesis in patients diagnosed with UI. This fact determined the necessity to carry out this study.
The purpose of the study was analysis of the embryonic stage of in vitro fertilization programs (IVF and ICSI) in patients with unexplained infertility compared to patients with tubo-peritoneal factor of infertility (TPFI).
Materials and methods
We collected and analyzed information on embryonic stage of IVF programs in women with UI (the study group consisting of 80 women), who underwent 113 IVF programs, and in women with TPFI (the control group consisting of 30 patients), who underwent 41 IVF programs in the Departments of the Institute of Reproductive Medicine of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia in the period from 2019 to 2021. The comparison groups of patients were representative of the general population in qualitative and quantitative parameters, comparable in age (the age of patients was below 35 years at the time when infertility was diagnoses; the mean age of women with UI was 31.69 years and with TPFI – 32.46 years), duration of infertility (not less than 3 years), ovarian reserve status average AMG level (average AMH levels: UI – 2.44 ng/Ml and TPBI – 2.34 ng/Ml), ovarian stimulation protocols with gonadotropin-releasing hormone (GnRH) antagonist (in UI group – 98.05% and TPFI group – 97.09%) and gonadotropin-releasing hormone agonist (in UI group – 1.95% and TPFI group – 2.91%), and the number of IVF programs per woman (in UI group – 1.41 and TPFI group – 1.37) [9].
Exclusion criteria were: the age over 35 years; the presence of obvious reasons for infertility (absence of fallopian tubes, ovaries, the Mayer–Rokitansky–Küster–Hauser syndrome, Shereshevsky–Turner syndrome, azoospermia, oligoasthenoteratozoospermia grade 3–4); chromosomal abnormalities; hereditary syndromes, congenital malformations, including uterine anomalies; premature ovarian failure (POF); endocrine-metabolic syndrome, polycystic ovary syndrome (PCOS), anovulation, hypogonadotropic hypogonadism (HH); oncological diseases, atypical endometrial hyperplasia; endometrioid cyst, widespread endometriosis, multiple uterine fibroids, chronic endometritis; HIV, viral hepatitis; the presence of social reasons (no sex partner, preservation of reproductive material before treatment of oncological disease etc.).
The patients were examined and treated in F. Paulsen Scientific and Clinical Department of ART of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia [12]. For over 5 years experienced doctors and embryologists practiced their work methods, and the conditions in the laboratory of the Department were similar. Intracytoplasmic sperm injection (ICSI) was performed according to indications: failed or low rate (<20%) of fertilization in the previous IVF attempt, multiple IVF attempts in medical history [13]. Cultivation was carried out in single step culture medium in conditions of low oxygen levels (5% O2, 6% CO2, 89% N2). No more than two embryos were transferred into the uterus [12]. The average number of transferred embryos in UI group was 1.0 embryo per woman, in TPFI group – 1.2 embryo per woman.
The Gardner grading system was used for morphological assessment of the quality of blastocysts. According to the Istanbul consensus workshop on embryo assessment (2011) and the Vienna consensus meeting (2017), this grading system is currently a major embryological criterion for embryo quality because of its prognostic significance [14–17]. Evaluation of blastocysts was based on the analysis of trophectoderm cells, inner cell mass and the cavity size. The degree of blastocyst expansion or formation of the blastocyst cavity was assessed using grading scale (1–6 grades). The inner cell mass and trophectoderm were graded on a scale of ABC. This scoring system implies an unambiguous division of the blastocyst into trophectoderm and inner cell mass and their independent assessment, which are impossible for stage 1, and may be difficult for stage 2.
Statistical analysis
Statistical data processing was performed using absolute , relative and mean values, criteria to measure diversity of variation series, standardization method, parametric methods for assessment of research reliability: to measure representativeness errors, assess statistical significance of the difference in the results of the study (the t-failure criterion), calculate confidence interval for mean and relative values [18]. The Student's t-test was used to compare quantitative variables, and Student’s t-table was used for the critical values. The differences were considered statistically significant at p<0.05.
Results
The mean values were analyzed. Embryologic data of IVF programs in the groups and individual patients with UI and TPFI were assessed.
According to obtained mean values, the differences in the number of obtained mature oocytes between the groups were not statistically significant (Table). Despite the fact that ICSI was used as a fertilization technique most often in UI group (80.3%) compared to TPFI group (58.3%), there was no statistically significant difference between the average rates of fertilization in the groups (Table). Most important is that statistically the average blastulation rate in IVF programs was significantly low in UI group (Table).
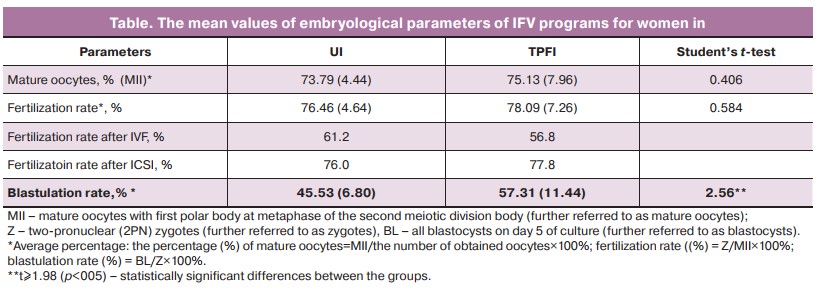
Reduction of blastulation rate in UI group occurred mainly because embryos stopped developing in the first 3 days of culture, inclusively (the percentage of stopping of the development in the first 3 days = 100% of embryo cleavage, where the percentage of embryo cleavage = E3/Z×100%, where E3 is embryo culture for 3 days). This occurred in UI group in 13,8% of cases versus 9% in TPFI group. There was no difference between UI group (44.7%) and TPFI group (44.3%) in the percentage of embryos that stopped developing on days 3–5 of culture (the percentage of stopping of the development on days 3–5 days of culture = the number of embryos that stopped developing E4-5/the number of E3×100%, where E4-5 – embryos on days 4–5 of culture). This confirms that reduction of blastulation in UI was because embryos stopped developing before 3 days of culture, inclusively.
At lower blastulation rate in UI group, there was no difference between UI group (55.3%) and TPFI group (55.7%) in the percentage of blastocyst development from embryos remaining in culture for 3 days (% of blastocysts = BL/number of E3×100%). This also confirms that the embryos stop developing before 3 days of culture inclusively in UI.
A higher quality of blastocysts was in group UI (66,7%) (good-quality blastocysts: >3, AA, AB, BA; the percentage of good-quality BL = the number of good-quality BL/BL×100%) versus TPFI group (45.8%), and there were more IVF programs with obtaining good-quality blastocysts versus TPFI group (65.5 and 43.9%), respectively.
In UI, the embryos were transferred before 3 days of culture inclusively less often versus TPFI [23.75% (on day 1 – 8.75%, on day 3 – 15%) and 30.6% (on day 1 – 5.6%, on day 3 – 25%), respectively]; and the blastocysts were transferred most often versus TPFI [68.75% (on day 5 – 68.75%) and 58.3% (on day 5 – 58.3%), respectively]. This may indirectly reflect the trend for embryo development in UI: after 3 days embryos developed to the blastocyst stage at a normal rate, and these blastocysts were often good-quality blastocysts.
Preimplantation genetic testing for aneuploidy (PGT-A) in UI (62.7%) was performed in more than half of cases and more often than in cases of TPFI (14.3%). It was to be expected in cases of unexplained infertility and unsuccessful attempts of getting pregnant naturally and using assisted reproductive technologies. However, according to the results of PGT-A, the frequency of euploid embryos did not differ between UI group (41.7%) and TPFI group (40%), i.e. aneuploidy in embryos is not a conditioning factor for unexplained infertility.
In UI group (57.5%), the cancellation rate of embryo transfer in IVF programs was higher than in TPFI group (34.1%) mainly due to fact that the patients underwent PGT-A (47.7%).
Despite the fact, that the rate of cryopreservation of embryos in IVF programs was higher in UI group (68.1%) versus TPFI group (53.7%), there were no differences between the groups in the number of cryopreserved embryos per IVF program (UI – 3.66 and TPFI – 3.36) and in the rate of cryopreserved blastocysts (the percentage of Cryo BL = Cryo BL/BL; UI – 70% and TPFI – 66.4%).
Integral indicator of blastocysts utilization rate [utilization rate = (transferred BL + Cryo BL)/Z×100%] was lower in UI group (40%) versus TPFI group (44%). This reflects decreased blastulation rate in UI group, all other conditions being equal [17].
Discussion
Decreased blastulation rate and high rate of embryos that stopped developing before 3 days of culture in IVF programs indicate impaired early embryo development and reflect regularity of this process in unexplained infertility. This can be regarded as a possible cause of infertility in UI, and, therefore, additional tests that are often recommended for married couples and long-term empiric treatment cannot be justified. As has been previously shown, statistically, the average duration of sexual activity without using contraception with purpose to become pregnant was significantly higher in UI group – 5.65 years than in TPFI group – 4.75 years [9].
It is believed that embryonic genome activation (EGA) occurs by the four-cell stage. For this reason, it is probable that embryo stops developing at cleavage stage (on days 1–3) due to oocyte factor, and impaired embryo development between morula compaction and blastocyst formation (on days 4–6) occurs due to embryo genome [19].
Normal fertilization rate, decreased blastulation rate due to higher incidence of stopping of embryonic development up to 3 days of culture and, at the same time, high quality of obtained blastocysts explain the pathogenesis of unexplained infertility. Conception in these married coupled occurs with normal frequency, the embryos stop developing most often up to 3 days of culture, blastocysts are produced less often, but they are good-quality blastocysts. For this reason, pregnancy may occur, but the time for getting pregnant may be prolonged. At the same time, the age and increasing age-related comorbidity of patients with UI lead to an additional restriction of realization of their reproductive function. IVF reduces the time to obtain good-quality blastocysts and, accordingly, to become pregnant, i.e. this is a pathogenetic approach to unexplained infertility treatment technique. All this proves that it is reasonable that the patients with UI should undergo IVF as early as possible with good-quality embryo transfer (>3, AA, AB, BA) on day 5 of culture.
No differences between UI and TPFI groups in the rates of euploid embryos detected by PGT-A indicate that routine PGT-A in cases of unexplained infertility is inexpedient. This increases the time before embryo transfer and, accordingly, for getting pregnant. Moreover, trophectoderm biopsy procedure may reduce the implantation rate of IFV due to a possible effect of invasive intervention on proper development and function of the placenta [20, 21].
Conclusion
This paper presents the data on reduced blastulation rate in IVF programs in patients with unexplained infertility. In-depth analysis of the embryonic stage of IVF programs showed, that low blastulation rate in unexplained infertility was mainly due to stopping of embryoinic development up to 3 days of culture. At the same time, the morphological assessment showed that the quality of blastocysts was higher in UI group compared to TPFI group; and preimplantation genetic testing for aneuploidy (PGT-A) showed a similar frequency of detection of euploid embryos.
The obtained results suggest that it is inexpedient to recommend the married couple with UI to undergo expanded diagnostic testing and empirical treatment, and early IVF with good-quality embryo transfer (>3, AA, AB, BA) on day 5 of culture is necessary. All this dictates the need for further scientific search for molecular mechanisms that cause impairment of early stages of embryogenesis in patients with UI.
References
- Siristatidis C., Pouliakis A., Sergentanis T.N. Special characteristics, reproductive, and clinical profile of women with unexplained infertility versus other causes of infertility: a comparative study. J. Assist. Reprod. Genet. 2020; 37(8): 1923-30. https://doi.org/10.1007/s10815-020-01845-z.
- Practice Committee of the American Society for Reproductive Medicine. Electronic address: asrm@asrm.org; Practice Committee of the American Society for Reproductive Medicine. Evidence-based treatments for couples with unexplained infertility: a guideline. Fertil. Steril. 2020; 113(2): 305-22. https://dx.doi.org/10.1016/j.fertnstert.2019.10.014.
- ACOG Committee. Infertility workup for the women’s health specialist: ACOG Committee Opinion, Number 781. Obstet. Gynecol. 2019; 133(6): e377-84. https://dx.doi.org/1097/AOG.0000000000003271.
- Wang R., Danhof N.A., Tjon-Kon-Fat R.I., Eijkemans M.J.C., Bossuyt P.M.M., Mochtar M.H. et al. Interventions for unexplained infertility: a systematic review and network meta-analysis. Cochrane Database Syst. Rev. 2019; 9(9): CD012692. https://dx.doi.org/10.1002/14651858.CD012692.pub2.
- Cariati F., D’Argenio V., Tomaiuolo R. The evolving role of genetic tests in reproductive medicine. J. Transl. Med. 2019; 17(1): 267. https://dx.doi.org/10.1186/s12967-019-2019-8.
- Berek J.S., Novak E., Berek D.L. Berek & Novak’s gynecology. 16th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2019: 942-1000.
- Yücel B., Kelekci S., Demirel E. Decline in ovarian reserve may be an undiagnosed reason for unexplained infertility: a cohort study. Arch. Med. Sci. 2018; 14(3): 527-31. https://dx.doi.org/10.5114/aoms.2016.58843.
- Bosch E., Alviggi C., Lispi M., Conforti A., Hanyaloglu A.C., Chuderland D. et al. Reduced FSH and LH action: implications for medically assisted reproduction. Hum. Reprod. 2021; 36(6): 1469-80. https://dx.doi.org/1093/humrep/deab065.
- Kirakosyan E.V., Nazarenko T.A., Bachurin A.V., Pavlovich S.V. Clinical characteristics and embryological parameters in IVF programs for women with unexplained infertility. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 5: 83-90. (in Russian). https://dx.doi.org/10.18565/aig.2022.5.83-90.
- Buckett W., Sierra S. The management of unexplained infertility: an evidence-based guideline from the Canadian Fertility and Andrology Society. Reprod. Biomed. Online. 2019; 39(4): 633-40. https://dx.doi.org/10.1016/j.rbmo.2019.05.023.
- Abrahami N., Izhaki I., Younis J.S. Do young women with unexplained infertility show manifestations of decreased ovarian reserve? J. Assist. Reprod. Genet. 2019; 36(6): 1143-52. https://dx.doi.org/1007/s10815-019-01467-0.
- Ministry of Health of the Russian Federation. Clinical Recommendations. Female infertility. 2021: 81. (in Russian).
- Order of the Ministry of Health of Russia dated 31.07.2020 N 803n "On the procedure for the use of assisted reproductive technologies, contraindications and restrictions on their use" (in Russian).
- Gardner D.K., Schoolcraft W.B. In vitro culture of human blastocysts. In Jansen R., Mortimer D., eds. Toward reproductive certainty: fertility and genetics beyond 1999: The plenary proceedings of the 11th world congress. CRC Press; 1999: 378-88.
- Gardner D.K., Schoolcraft W.B. Culture and transfer of human blastocysts. Curr. Opin. Gynecol. 1999; 11(3): 307-11. https://dx.doi.org/10.1097/00001703-199906000-00013.
- Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum. Reprod. 2011; 26(6): 1270-83. https://dx.doi.org/10.1093/humrep/der037.
- ESHRE Special Interest Group of Embryology and Alpha Scientists in Reproductive Medicine. The Vienna consensus: report of an expert meeting on the development of ART laboratory performance indicators. Reprod. Biomed. Online. 2017; 35(5): 494-510. https://dx.doi.org/1016/j.rbmo.2017.06.015.
- Kucherenko V.Z., red. Application of statistical analysis methods for the study of public health and health care: a textbook. M.: GEOTAR-Media; 2011. 256p. (in Russian).
- Sfakianoudis K., Maziotis E., Karantzali E., Kokkini G., Grigoriadis S., Pantou A. et al. Molecular drivers of developmental arrest in the human preimplantation embryo: A systematic review and critical analysis leading to mapping future research. Int. J. Mol. Sci. 2021; 22(15): 8353. https://dx.doi.org/10.3390/ijms22158353.
- Cimadomo D., Capalbo A., Ubaldi F.M., Scarica C., Palagiano A., Canipari R. et al. The impact of biopsy on human embryo developmental potential during preimplantation genetic diagnosis. Biomed. Res. Int. 2016; 2016: 7193075. https://dx.doi.org/10.1155/2016/7193075.
- Zacchini F., Arena R., Abramik A., Ptak G.E. Embryo biopsy and development: the known and the unknown. Reproduction. 2017; 154(5): R143-8. https://dx.doi.org/10.1530/REP-17-0431.
Received 26.05.2022
Accepted 13.07.2022
About the Authors
Alexey V. Bachurin, embryologist, Head of the Laboratory of Clinical Embryology, Scientific and Clinical Department of ART named after F. Paulsen, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, a_bachurin@oparina4.ru,https://orcid.org/0000-0002-3768-7657, Akademika Oparina str., 4, Moscow, 117997, Russia.
Evgeniya V. Kirakosyan, Ph.D. student, Department of Obstetrics, Gynecology, Perinatology and Reproductology, I.M. Sechenov First Moscow State Medical University, Ministry of Health of the Russian Federation (Sechenov University); Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, +7(916)574-79-63, evgeniya.kirakosyan@mail.ru, https://orcid.org/0000-0002-6021-2449, Akademika Oparina str., 4, Moscow, 117997, Russia.
Tatyana A. Nazarenko, Dr. Med. Sci., Professor, Director of the Institute of Reproductive Medicine, Academician V.I. Kulakov National Medical Research Center
for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, +7(495)531-44-44, t_nazarenko@oparina4.ru,
https://orcid.org/0000-0002-5823-1667, Akademika Oparina str., 4, Moscow, 117997, Russia.
Stanislav V. Pavlovich, Ph.D., Academic Secretary, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology,
Ministry of Health of the Russian Federation; Professor, Department of Obstetrics, Gynecology, Perinatology and Reproductology, I.M. Sechenov First Moscow State Medical University, Ministry of Health of the Russian Federation (Sechenov University), +7(495)438-20-88, s_pavlovich@oparina4.ru,
https://orcid.org/0000-0002-1313-7079, Akademika Oparina str., 4, Moscow, 117997, Russia.
Corresponding author: Evgeniya V. Kirakosyan, evgeniya.kirakosyan@mail.ru
Authors’ contributions: Bachurin A.V., Kirakosyan E.V., Nazarenko T.A., Pavlovich S.V. – the concept and design of the study, published data analysis, editing the article; Bachurin A.V., Kirakosyan E.V. – material collection and processing, statistical data processing; Kirakosyan E.V. – writing the article.
Conflicts of interest: The authors declare that they have no conflicts of interest.
Funding: The study was carried out without any sponsorship.
Ethical Approval: The protocol of the study was approved by the Academic Council (extract from the Order No. 4070/OP-32 of September 30, 2020) and the local Ethics Committee (extract from Protocol No.33-20 of November 25, 2020) of I.M. Sechenov First Moscow State Medical University, Ministry of Health of the Russian Federation; and by the Ethics Committee for biomedical researches (extract from Protocol No. 11 of November 12, 2020) of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation.
Patients’ Consent for Publication: All patients provided informed consent for participation in the study and publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Bachurin A.V., Kirakosyan E.V., Nazarenko N.A., Pavlovich S.V. Analysis of the embryonic stage of in vitro fertilization programs in patients with unexplained infertility.
Akusherstvo i Gynecologia/Obstetrics and Gynecology. 2022; 9: 81-86 (in Russian)
https://dx.doi.org/10.18565/aig.2022.9.81-86



