Use of in vitro fertilization programs to preserve reproductive material in patients with breast cancer
Biryukova A.M., Nazarenko T.A., Martirosyan Ya.O., Oshkina E.V.
Objective: This study aimed to refine methodologies for implementing assisted reproductive technologies (ART), specifically in vitro fertilization (IVF), to preserve reproductive material in patients diagnosed with breast cancer. A comparative analysis of various ovarian stimulation protocols was conducted along with an evaluation of the impact of incorporating aromatase inhibitors (AIs) or selective estrogen receptor modulators (SERMs) on folliculogenesis and oocyte yield.
Materials and methods: This study included 471 female patients, of whom 346 underwent IVF cycles for oocyte or embryo cryopreservation. Prior to initiating treatment, a comprehensive review of medical records provided by oncologists was conducted, detailing the oncological diagnosis, disease stage, and results of diagnostic imaging (CT, MRI, and PET-CT), as well as morphological and immunohistochemical analyses. Additionally, each patient received a consultation with an oncologist-mammologist on the day of enrollment, which included an evaluation of the patient’s reproductive health, ovarian reserve, and overall gynecological status. The data were integrated to develop a personalized treatment protocol.
Results: The mean age of patients seeking fertility preservation was 29.5 (6.8) years, with the majority (221/346 (63.9%)) being under 35 years of age. All patients exhibited preserved ovarian reserve at the time of enrollment. Of the 346 breast cancer patients, 133 (38.4%) initiated ovarian stimulation during the follicular phase using a conventional protocol, whereas 213 (61.6%) commenced stimulation in the luteal phase using a random-start protocol. This study demonstrated that treatment efficacy, as measured by the number of retrieved oocytes and mature oocytes, depended on the patient’s ovarian reserve and was independent of the menstrual cycle phase at which stimulation was initiated. The administration of letrozole at doses of 2.5 mg and 5 mg yielded comparable outcomes in oocyte retrieval; however, the 5 mg dose was associated with significantly lower preovulatory estradiol levels. In contrast, the inclusion of tamoxifen in gonadotropin-based stimulation protocols resulted in suboptimal outcomes, with a trend toward reduced oocyte yield and quality.
Conclusion: The implementation of IVF protocols in patients with breast cancer should be guided by a multidisciplinary approach that incorporates both oncological and reproductive parameters. The number of oocytes retrieved is primarily determined by the ovarian reserve and is unaffected by the timing of stimulation initiation during the menstrual cycle. Aromatase inhibitors are the preferred pharmacological agents for ovarian stimulation in this patient population, whereas the use of tamoxifen is associated with inferior outcomes, including higher preovulatory estradiol levels and reduced oocyte quantity and quality.
Authors' contributions: Biryukova A.M., Nazarenko T.A. – conception and design of the study, manuscript editing and final approval; Biryukova A.M., Martirosyan Ya.O., Oshkina E.V. – material collection, statistical analysis, drafting of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: Research 121040600410-7 Nazarenko T.A. "Solving the problem of infertility in modern conditions by developing a clinical and diagnostic model of infertile marriage and using innovative technologies in assisted reproduction programs".
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Biryukova A.M., Nazarenko T.A., Martirosyan Ya.O., Oshkina E.V. Use of in vitro fertilization programs
to preserve reproductive material in patients with breast cancer.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (4): 61-70 (in Russian)
https://dx.doi.org/10.18565/aig.2025.38
Keywords
Breast cancer is one of the most common malignancies affecting women of reproductive age. Treatment of this disease typically involves a combination of surgery, chemotherapy, endocrine therapy, and targeted therapy, each of which can significantly affect the reproductive function of patients [1]. Treatment-induced premature ovarian failure is frequently observed with gonadotoxic systemic therapies, particularly alkylating agents such as cyclophosphamide, which are commonly administered as part of (neo)adjuvant chemotherapy regimens [2]. Alkylating agents cause DNA damage that leads to follicular apoptosis and contributes to their high gonadotoxicity [3].
Approximately 50% of women of reproductive age who are diagnosed with breast cancer express a desire to become pregnant in the future. Unfortunately, studies have indicated low pregnancy rates in this group, ranging from 5% to 15%. This may be attributed to the effects of breast cancer and its treatment on a woman's fertility as well as concerns regarding the potential negative impact of pregnancy on the course of the disease [4].
Most studies suggest that women who have undergone breast cancer treatment can maintain fertility and achieve successful pregnancies [5]. Research on the effect of pregnancy on disease prognosis reveals that women who become pregnant after breast cancer tend to have better overall survival than those who do not, with a risk of death reduced by 41% [6, 7]. Similar findings were reported by Blakely L.J. et al. (2004), indicating that pregnancy following breast cancer treatment does not increase the risk of recurrence or mortality [8].
However, this reduced risk of death may be attributed to a selection bias known as the "healthy mother effect." Women who conceive after breast cancer treatment often feel healthier, leading to better prognosis than those who do not become pregnant. The impact of pregnancy on breast cancer prognosis, particularly hormone receptor status, remains a topic of scientific debate. Azim H.A. et al. (2013) investigated the influence of pregnancy on recurrence-free survival in women with breast cancer based on estrogen receptor (ER) status, concluding that pregnancy after ER-positive breast cancer does not decrease the risk of recurrence [9].
Medical professionals continue to debate the optimal duration for delaying pregnancy after the diagnosis and treatment of breast cancer. Some cohort studies suggest that women who wait two years or more post-treatment experience have improved survival rates [10]. Conversely, Nye L. et al. (2017) found no decrease in survival among premenopausal women with ER-positive breast cancer who became pregnant within five years of diagnosis [11]. While published studies show encouraging outcomes for pregnancies occurring more than two years after breast cancer diagnosis, achieving stable remission and receiving clearance to plan a pregnancy does not guarantee that pregnancy will be possible. In fact, loss and a significant decline in ovarian reserve occur in 80% of women, raising concerns about their ability to conceive [12].
Recent advances in reproductive medicine have introduced fertility preservation options for young women diagnosed with breast cancer. Methods for preserving fertility include the use of gonadotropin-releasing hormone (GnRH) agonists during chemotherapy, cryopreservation of oocytes or embryos prior to treatment, and cryopreservation of ovarian tissue. Future techniques, such as in vitro oocyte maturation of oocytes (IVM), promise to enhance fertility preservation options for breast cancer patients, allowing them to have children later [13].
International guidelines emphasize the necessity of counseling all cancer patients of reproductive age about the potential risks of premature ovarian failure and infertility, as well as the available fertility preservation options [12]. Preliminary cryopreservation of reproductive material from patients before gonadotoxic cancer treatment has become a standard practice worldwide [12, 14]. It is anticipated that ovarian stimulation will include aromatase inhibitors and direct antiestrogens in the stimulation protocol [12, 13]. However, the literature lacks criteria for selecting specific drugs and does not address their influence on folliculogenesis parameters, indicating the need for further research. The limited timeframe provided by oncologists necessitates ovarian stimulation from the moment patients seek treatment, disregarding the day of the menstrual cycle (random start). Despite numerous studies evaluating the effectiveness of random-start stimulation protocols, several issues remain unresolved [12].
These unresolved issues underscore the need for this study, which aims to refine approaches to IVF programs focused on preserving reproductive material in patients with breast cancer. This will be achieved through an analysis of the results from different ovarian stimulation protocols and an assessment of how aromatase inhibitors or selective estrogen receptor modulators included in the stimulation protocol affect the folliculogenesis parameters.
Materials and methods
The study was conducted from 2018 to 2024 at the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology, and Perinatology, Ministry of Health of the Russian Federation, Moscow, Russia (Director: Academician of the Russian Academy of Sciences, Professor G.T. Sukhikh). Patients were recruited from the Institute of Reproductive Medicine (Director: Professor T.A. Nazarenko), and Institute of Oncogynecology and Mammology (Director: Academician of the Russian Academy of Sciences, Professor L.A. Ashrafyan). Patient examinations, preservation of reproductive material, and treatment with assisted reproductive technologies were performed at the F. Paulsen Research and Educational Center for ART with the Clinical Department.
A total of 471 women were examined, of whom 346 underwent in vitro fertilization (IVF) to obtain and cryopreserve oocytes/embryos. Prior to treatment, medical documents provided by oncologists were analyzed. These documents contained a comprehensive description of the oncological disease, its stage, results of examinations including computed tomography, magnetic resonance imaging, positron emission tomography, and various morphological and immunohistochemical studies. Additionally, on the day of treatment, patients were consulted by an oncologist. During treatment, the state of the patient's reproductive system, ovarian reserve, and overall gynecological health were assessed. The accumulated data has enabled the development of individualized treatment algorithms. A total of 346 women provided permission to participate in the IVF program. The reasons for treatment refusal among 125 patients included severe disease requiring urgent treatment (52 patients), late reproductive age, reduced ovarian reserve, and inability to retrieve oocytes (50 women); 23 patients declined due to fear of worsening their condition or for financial reasons. Ovarian stimulation was performed in 133/346 (38.4%) women during the follicular phase of the cycle and in 213/346 (61.6%) during the luteal phase. In all cases, a protocol with a GnRH antagonist was employed; the dose of gonadotropins was tailored based on ovarian reserve parameters, ranging from 150 to 225 IU per day, with stimulation duration varying from 8 to 12 days. The patients were divided into the following subgroups:
- Patients with ER+ breast cancer and normal or reduced ovarian reserve, who received letrozole at a dose of 2.5 mg (n=215);
- Patients with ER+ breast cancer and high ovarian reserve, who received letrozole at a dose of 5 mg (n=71);
- Patients with ER+ breast cancer who received tamoxifen 20 mg/day (n=30);
- Triple-negative breast cancer patients who did not receive antiestrogen therapy (n=59).
The parameters assessed during ovarian stimulation included folliculogenesis, number of preovulatory follicles, number of oocytes retrieved, quality classification of oocytes, and assessment of oocyte maturity. If patients opted to fertilize the retrieved oocytes, the percentage of fertilization, blastocyst formation, and quality were evaluated.
Embryological assessment of the obtained material included analysis of oocyte quality, determination of oocyte maturity (MII, MI, GV stages), examination of mature oocyte dysmorphisms (polar body anomalies, vacuoles, smooth endoplasmic reticulum zones, necrotic areas of the cytoplasm, central granularity), assessment of the fertilization rate of mature oocytes, obtaining binuclear zygotes (2pn), culturing to the blastocyst stage, and evaluation of blastocyst quality.
The staging and classification of the underlying diseases included the following:
- TNM classification: For all cancer types, the TNM classification was utilized, considering the size and spread of the primary tumor (T), involvement of regional lymph nodes (N), and presence of distant metastases (M).
- Tumor differentiation (G1, G2, and G3) and histological tumor type.
All patients signed a specially developed informed consent form that outlined the risks of reproductive function loss associated with cancer treatment, specifics of the IVF program, and data regarding the safety of ovarian stimulation.
Statistical analysis
Descriptive statistical methods were employed to assess differences between the groups, including calculations of mean values, standard deviations, medians, and interquartile ranges. GraphPad software was used for statistical analysis of the results. Student's t-test, Mann–Whitney test, and Fisher's exact test were used to compare the indicators between groups.
Results
As part of the interdisciplinary consultation, 346 women received permission to undergo IVF to obtain and preserve reproductive material for delayed childbearing. All patients expressed a strong desire to preserve their reproductive material and were fully informed about the stages of upcoming treatment. Data regarding the staging of the oncological process in patients with breast cancer are presented in Table 1.
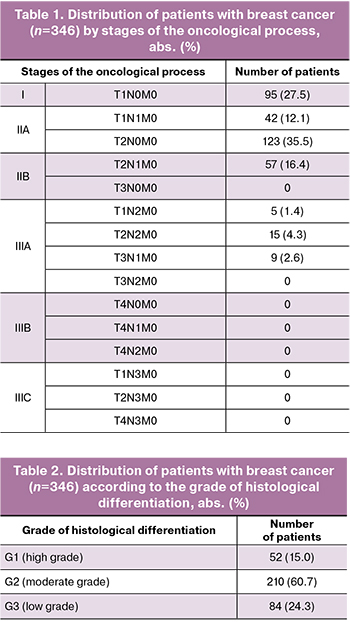
The data on the grade of histological differentiation of tumors in patients with breast cancer are presented in Table 2.
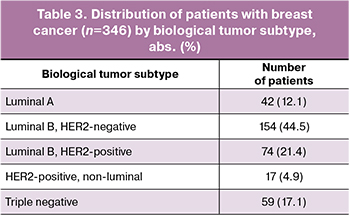
The molecular classification of breast cancer was based on immunohistochemical research and included several subtypes, which are determined by the presence or absence of estrogen (ER), progesterone (PR), and HER2/neu receptors. The typing results are presented in Table 3. The most frequently diagnosed subtype was the luminal B HER2-negative subtype (30.1%).
The mean anti-Müllerian hormone (AMH) level in patients was 2.5 (1.3) ng/ml. In 173/346 patients (50.0%), the AMH level was higher than 2.0 ng/ml, in 104/346 (30.1%) the value was in the range of 1.0–2.0 ng/ml, and in 69 (19.9%) patients it was lower than 1.0 ng/ml. The average level of follicle-stimulating hormone (FSH) was 6.3 (2.8) mIU/ml. In 142/346 patients (41.0%), FSH levels were <5.0 mIU/mL, in 104/346 (30.1%) they were in the range of 5.0–7.5 mIU/mL, and in 100/346 patients (28.9%) they were >7.5 mIU/mL.
The mean antral follicle count was 10.8 (7.0). In 139/346 patients (40.2%), the antral follicle count was >15, in 104/346 (30.1%) patients, it was in the range of 10–15, and in 103/346 patients (29.8%), the antral follicle count was <10.
Thus, most patients were characterized by a preserved ovarian reserve at the time of presentation for fertility preservation.
Features of ovarian stimulation and embryological outcomes in patients with breast cancer based on the day of stimulation initiation
Of the 346 patients with breast cancer, 133 (38.4%) began ovarian stimulation in the follicular phase following the classical protocol, whereas 213 (61.6%) started in the luteal phase according to the random-start protocol.
Table 4 presents the comparative characteristics of IVF programs in patients with breast cancer who underwent ovarian stimulation during the follicular and luteal phases of the menstrual cycle.
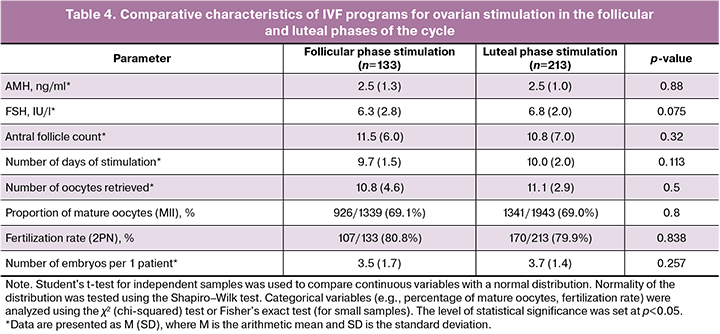
The stimulation duration averaged 9 (8–10) days, and the total gonadotropin dose was 2,350 IU (2,000–2,900 IU). The stimulation protocol was selected based on the state of the ovarian reserve and phase of the menstrual cycle at the time of the patient's visit. The average number of oocytes per patient in the breast cancer group was 10 (6–14), with a total of 3,282 oocytes obtained. Of these, 2,267 (69.1%) were mature (MII), 365 (11.1%) were in the MI stage, 496 (15.1%) were in the GV stage, and 154 (4.7%) were degenerative. Embryogenesis parameters showed that the number of binuclear zygotes (2PN) was 751 (80.8%), the total number of blastocysts was 434 (57.8%), the average number of blastocysts per patient was 2.5 (1.5–4), the number of excellent quality blastocysts was 219 (29.9%), and the average number of excellent quality blastocysts per patient was 2 (1–3).
There were no differences in the induced cycle parameters when stimulation was initiated during the follicular and luteal phases of the menstrual cycle.
Results of ovarian stimulation and embryological outcomes in patients with breast cancer, depending on the use of an aromatase inhibitor or a selective estrogen receptor modulator
At this stage of the study, all patients were divided into 4 subgroups:
- Patients with ER+ breast cancer and normal or reduced reserve, who received letrozole at a dose of 2.5 mg – 215 patients (62.1%);
- Patients with ER+ breast cancer and high ovarian reserve, who received letrozole at a dose of 5 mg – 71 patients (20.5%);
- Patients with ER+ breast cancer who received tamoxifen 20 mg/day – 30 patients (8.7%);
- Triple-negative breast cancer patients who did not receive antiestrogen therapy – 59 patients (17.1%).
The duration of stimulation ranged from 8 to 11 days, and there were no statistically significant differences between the groups (p=0.132). Patients receiving 5 mg letrozole had a slightly shorter stimulation duration, whereas those in the tamoxifen group had a longer stimulation duration, probably due to differences in the pharmacodynamics of the drugs (Table 5). There were no statistically significant differences in the initial and total doses of gonadotropins required to achieve an adequate response to ovarian stimulation, according to the use of letrozole or tamoxifen.
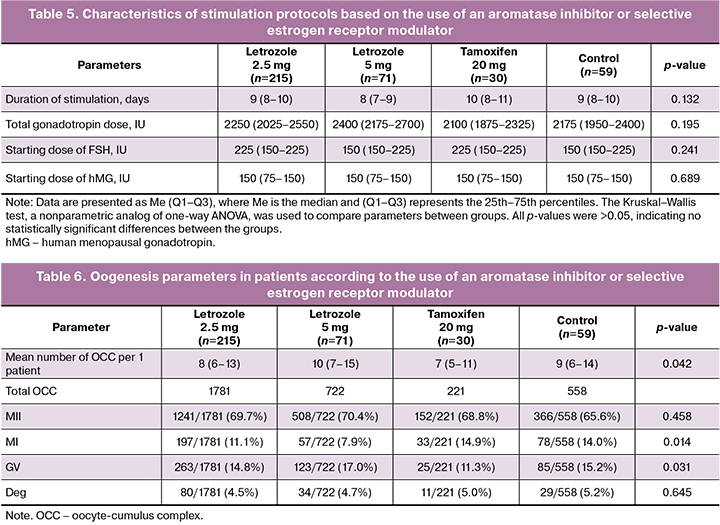
The highest number of oocytes retrieved per patient was in the letrozole 5 mg group, with a median of 10 (7–15) oocytes per patient (Table 4). The lowest number of oocytes was retrieved in the tamoxifen group, with 7 (5–11) oocytes per patient. The median number of oocytes in the control group was 9 (6–14), while the letrozole 2.5 mg group had a median of 8 (6–13).
These differences were statistically significant (p=0.042). The percentage of mature oocytes (MII) was comparable between the groups, ranging from 65.6% in the control group to 70.4% in the letrozole 5 mg group, although the differences were not statistically significant (p=0.458). The percentage of oocytes at the MI stage was significantly higher in the tamoxifen group (14.9%) and the control group (14.0%) than in the groups using with 2.5 mg (11.1%) and 5 mg (7.9%) (p=0.014). The percentage of oocytes at the GV stage was significantly higher in the letrozole 5 mg group (17.0%) than in the other groups (p=0.031). The percentage of degenerative oocytes did not differ significantly between the groups and ranged from 4.5 to 5.2% (p=0.645).
The results of this study showed that the use of letrozole at doses of 2.5 mg and 5 mg in ovarian stimulation protocols in patients with breast cancer allows a comparable number of mature oocytes (MII) to be obtained compared to the control group. The letrozole groups were characterized by a lower proportion of oocytes in the MI stage and a slightly higher proportion of oocytes in the GV stage, which may indicate slower follicular maturation with this drug. Degenerative changes in oocytes did not show significant differences between the groups. Oogenesis parameters are shown in Table 6.
The number of successfully fertilized oocytes varied from 40 to 54.4%, with the highest rate recorded in the letrozole 2.5 mg group (54.4%) and the lowest in the tamoxifen group (40.0%), but the difference was not statistically significant (p=0.173). The oocyte fertilization rate was higher in the control group (51.9%), but slightly lower in the letrozole 5 mg (39.8%) and letrozole 2.5 mg (38.0%) groups. These differences were not statistically significant (p=0.124). The number of binuclear zygotes (2PN) was significantly higher in the control group (96.3%) than in the letrozole (76.5% and 77.2%) and tamoxifen (77.3%) groups (p=0.025). The total number of blastocysts was slightly higher in the control group (68.3%) than in the letrozole and tamoxifen groups (53.7% and 58.8%, respectively); however, the differences were not statistically significant (p=0.373). The number of excellent quality blastocysts was higher in the tamoxifen group (63.3%) and letrozole 5 mg group (57.6%), while in the control group it was lower (42.4%). However, the differences between the groups were not statistically significant (p=0.352). Embryogenesis parameters are presented in Table 7.
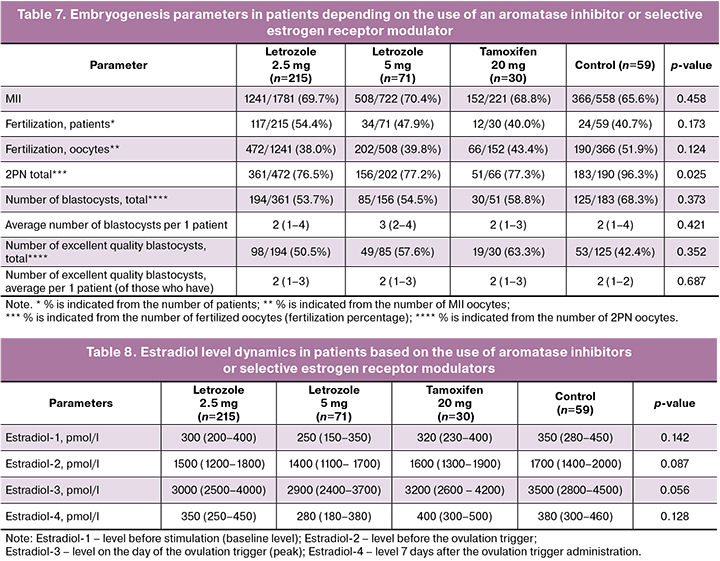
The dynamics of estradiol levels in the study groups indicated that by the time of ovulation trigger administration, estradiol levels were somewhat lower in the groups using letrozole, reflecting its inhibitory effect on estrogen production. In contrast, the control and tamoxifen groups exhibited higher estradiol levels due to the lack of a direct effect of these drugs on reducing estrogen production. Although the differences between the groups did not reach statistical significance, there was a tendency for lower estradiol levels in the groups receiving letrozole, particularly in the 5 mg group. The dynamics of the estradiol levels are presented in Table 8.
The results of the study demonstrated that incorporating aromatase inhibitors or selective estrogen receptor modulators into the ovarian stimulation protocol did not significantly reduce the quantity or quality of oocytes obtained, fertilization rate, or number of good-quality blastocysts compared to the control group. However, a significantly lower number of oocytes was observed in protocols that included tamoxifen. Additionally, a significant difference was noted in the higher pre-ovulatory levels of estradiol in ovarian stimulation protocols that incorporated tamoxifen. This finding is crucial for treatment safety, as the goal is to prevent supraphysiological concentrations of estrogens.
Discussion
Patients with breast cancer who sought fertility preservation prior to oncological treatment participated in a multidisciplinary consultation. Among these, 125 of 471 patients were denied treatment due to oncological indications, which included the presence of disseminated metastases, the need for prompt treatment, reproductive characteristics, reduced ovarian reserve, the inability to obtain oocytes, and, at the women's request, opting not to undergo treatment. Our experience, along with the opinions of other specialists, underscores the necessity of a consultative discussion for each patient to determine the indications and contraindications for ovarian stimulation [13]. The 346 women included in the study exhibited several clinical and laboratory parameters, indicating preserved ovarian reserve and active reproductive age. The mean age of the patients was 29.5 years, highlighting the importance of addressing fertility concerns in women at this stage of life. Analysis of the molecular characteristics of breast cancer within the study revealed that the most common subtypes were luminal B HER2-negative and luminal A. These findings align with international studies, which indicate that women with these cancer subtypes are more likely to seek fertility preservation, as they have higher chances of successful treatment and preservation of reproductive function [13]. The hormonal status of the patients was characterized by high and normal ovarian reserve. The AMH level exceeded 2.0 ng/ml in half of the patients, suggesting a strong potential for preserving fertility. The data obtained indicate that ovarian stimulation in breast cancer patients demonstrates high efficiency despite the specific challenges associated with this population. Application of both the classical stimulation protocol in the follicular phase and the random-start protocol initiated in the luteal phase resulted in the same number of mature oocytes. This suggests that the random-start protocol, which allows immediate stimulation without waiting for the onset of a new cycle, is as effective as the traditional approach, as supported by the findings of other studies [15–17].
The average number of mature oocytes per patient was 10, which was comparable to the results observed in non-cancer patient populations. The proportion of mature oocytes was 69.1%, which is consistent with studies demonstrating the efficacy of ovarian stimulation in women with preserved ovarian reserves. This highlights the significance of an individualized approach to ovulation stimulation, considering both ovarian reserve status and the urgency of initiating chemotherapy [15].
An analysis of the patients' marital status revealed that 45% did not have a regular partner; these women cryopreserved an average of 8.4 (1.9) eggs per patient. Women in stable relationships were advised to split the material if they had more than 15 oocytes, fertilize some, and cryopreserve the remainder. Those with fewer than 10 oocytes were advised to fertilize and cryopreserve embryos.
Embryological analysis yielded satisfactory results; more than half of the zygotes developed to the blastocyst stage, which is a favorable indicator for this patient group. This is particularly important because successful blastocyst formation is crucial for cryopreservation and the subsequent use of embryos in assisted reproductive technology programs after completing treatment for the primary disease. However, it is essential to note that cryopreserving one or two blastocysts may not be sufficient to achieve successful pregnancy posttreatment. These findings align with studies that emphasize the necessity of cryopreserving a larger number of embryos to enhance the likelihood of successful conception in the future [18].
Currently, it is widely accepted that aromatase inhibitors or selective estrogen receptor modulators should be incorporated in stimulation protocols for hormone-dependent tumors. Most specialists adhering to this recommendation have not investigated the effects of these drugs on folliculogenesis within the ovarian stimulation protocol. From our perspective, this is a critical issue, as these drugs operate via different mechanisms. Aromatase inhibitors block the conversion of androgens to estrogens, thereby lowering estrogen concentrations, whereas selective estrogen receptor modulators compete with endogenous estrogens at the receptor level. Tamoxifen, a selective estrogen receptor modulator, exhibits both anti-estrogenic and weak estrogenic effects. It is believed that the antiestrogenic effects of tamoxifen are realized in breast tissue, whereas its estrogenic effects may lead to the persistence of follicular cysts in the ovaries and endometrial hyperplasia [19]. Among the patients who were consulted, we also included women who were receiving tamoxifen as prescribed by their oncologist. Some women had endometrial hyperplasia, which precluded their inclusion in the study. Consequently, the decision to include aromatase inhibitors or selective estrogen receptor modulators in the stimulation protocol is fundamental. Equally important are the quantity and quality of the retrieved oocytes and the preovulatory estradiol levels in the stimulation protocols. The data indicated that patients receiving letrozole at doses of 2.5 and 5 mg did not differ in the number of oocytes or the stages of their maturation, while fewer oocytes were retrieved when tamoxifen was prescribed. The highest number of oocytes was obtained from patients receiving letrozole 5 mg, although these were women with a high ovarian reserve and a tendency toward hyperstimulation. No significant differences were observed in the quality of the retrieved oocytes, fertilization rates, or blastocyst formation rates; however, there was a trend toward improved results with the inclusion of aromatase inhibitors in the protocol compared with tamoxifen. Significant differences were noted in the preovulatory estradiol levels, which were lower when letrozole was included in the stimulation protocol. Letrozole has been shown to reduce estrogen levels during ovarian stimulation, which is particularly important for patients with ER-positive breast cancer, making the use of aromatase inhibitors in stimulation protocols preferable for this group. No differences were detected when prescribing letrozole at doses of 2.5 and 5 mg; however, for patients with reduced ovarian reserve, a dose of 2.5 mg is adequate to normalize elevated concentrations of preovulatory estradiol.
Conclusion
The selection of breast cancer patients for IVF programs with pre-cryopreservation of oocytes for delayed childbearing should occur only after a multidisciplinary consultation, considering the patient's oncological and reproductive characteristics as well as the woman's personal wishes and understanding of the upcoming treatment. If the ovarian reserve is preserved, stimulation can be performed at any phase of the menstrual cycle, and the number and quality of oocytes obtained using different stimulation protocols are comparable. It is advisable to incorporate aromatase inhibitors into the stimulation protocol as they significantly reduce preovulatory estradiol levels, whereas tamoxifen results in estradiol levels similar to those in the control group. In cases of high ovarian reserve and a tendency toward hyperstimulation, a daily dose of 5 mg of letrozole is recommended, whereas a dose of 2.5 mg may suffice for correcting estradiol concentrations in cases of reduced ovarian reserve.
References
- Zhao J., Liu J., Chen K., Li S., Wang Y., Yang Y. et al. What lies behind chemotherapy-induced amenorrhea for breast cancer patients: a meta-analysis. Breast Cancer Res. Treat. 2014; 145(1): 113-28. https://dx.doi.org/10.1007/s10549-014-2914-x.
- Anderson R.A., Themmen A.P.N., Al-Qahtani A., Groome N.P., Cameron D.A. The effects of chemotherapy and long-term gonadotrophin suppression on the ovarian reserve in premenopausal women with breast cancer. Hum. Reprod. 2006; 21(10): 2583-92. https://dx.doi.org/10.1093/humrep/del201.
- Del Castillo L.M., Buigues A., Rossi V., Soriano M.J., Martinez J., De Felici M. et al. The cyto-protective effects of LH on ovarian reserve and female fertility during exposure to gonadotoxic alkylating agents in an adult mouse model. Hum. Reprod. 2021; 36(9): 2514-28. https://dx.doi.org/10.1093/humrep/deab165.
- Raphael J., Trudeau M.E., Chan K. Outcome of patients with pregnancy during or after breast cancer: a review of the recent literature. Curr. Oncol. 2015; 22(Suppl. 1): S8-18. https://dx.doi.org/10.3747/co.22.2338.
- Kontzoglou K., Stamatakos M., Tsaknaki S., Goga H., Kostakis A., Safioleas M. Successful pregnancy after breast cancer therapy: dream or reality? Int. Semin. Surg. Oncol. 2009; 6: 7. https://dx.doi.org/10.1186/1477-7800-6-7.
- Azim H.A., Santoro L., Pavlidis N., Gelber S., Kroman N., Azim H. et al. Safety of pregnancy following breast cancer diagnosis: a meta-analysis of 14 studies. Eur. J. Cancer. 2011; 47(1): 74-83. https://dx.doi.org/10.1016/j.ejca.2010.09.007.
- Kroman N., Jensen M.B., Melbye M., Wohlfahrt J., Mouridsen H.T. Should women be advised against pregnancy after breast-cancer treatment? Lancet. 1997; 350(9074): 319-22. https://dx.doi.org/10.1016/S0140-6736(97)03052-3.
- Blakely L.J., Buzdar A.U., Lozada J.A., Shullaih S.A., Hoy E., Smith T.L. et al. Effects of pregnancy after treatment for breast carcinoma on survival and risk of recurrence. Cancer. 2004; 100(3): 465-9. https://dx.doi.org/10.1002/cncr.11929.
- Azim H.A., Kroman N., Paesmans M., Gelber S., Rotmensz N., Ameye L. et al. Prognostic impact of pregnancy after breast cancer according to estrogen receptor status: a multicenter retrospective study. J. Clin. Oncol. 2013; 31(1): 73-9. https://dx.doi.org/10.1200/JCO.2012.44.2285.
- Oktay K., Harvey B.E., Partridge A.H., Quinn G.P., Reinecke J., Taylor H.S. et al. Fertility Preservation in Patients With Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2018; 36(19): 1994-2001. https://dx.doi.org/10.1200/JCO.2018.78.1914.
- Nye L., Rademaker A., Gradishar W.J. Breast cancer outcomes after diagnosis of hormone-positive breast cancer and subsequent pregnancy in the tamoxifen era. Clin. Breast Cancer. 2017; 17(4): e185-9. https://dx.doi.org/10.1016/j.clbc.2016.12.014.
- Lambertini M., Peccatori F.A., Demeestere I., Amant F., Wyns C., Stukenborg J.B. et al.; ESMO Guidelines Committee. Fertility preservation and post-treatment pregnancies in post-pubertal cancer patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2020; 31(12): 1664-78. https://dx.doi.org/10.1016/j.annonc.2020.09.006.
- Peccatori F.A., Pup L. Del, Salvagno F., Guido M., Sarno M.A., Revelli A. et al. Fertility preservation methods in breast cancer. Breast Care. 2012; 7(3): 197-202. https://dx.doi.org/10.1159/000339671.
- Назаренко Т.А., Бурдули А.Г., Мартиросян Я.О., Джанашвили Л.Г. Криоконсервация репродуктивного материала у онкологических больных. Акушерство и гинекология. 2019; 9: 40-9. [Nazarenko T.A., Burduli A.G., Martirosyan Ya.O., Dzhanashvili L.G. Cryopreservation of reproductive material in cancer patients. Obstetrics and Gynecology. 2019; (9): 40-9. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.9.40-49.
- Bedoschi G.M., de Albuquerque F.O., Ferriani R.A., Navarro P.A. Ovarian stimulation during the luteal phase for fertility preservation of cancer patients: case reports and review of the literature. J. Assist. Reprod. Genet. 2010; 27(8): 491-4. https://dx.doi.org/10.1007/s10815-010-9429-0.
- Назаренко Т.А., Мартиросян Я.О., Бирюкова А.М., Джанашвили Л.Г., Иванец Т.Ю., Сухова Ю.В. Опыт стимуляции яичников в режиме random-start протоколов для сохранения репродуктивного материала онкологических больных. Акушерство и гинекология. 2020; 4: 52-8. [Nazarenko T.A., Martirosyan Ya.O., Biryukova A.M., Dzhanashvili L.G., Ivanets T.Yu., Sukhova Yu.V. Experience in random-start ovarian stimulation for preserving reproductive material of cancer patients. Obstetrics and Gynecology. 2020; (4): 52-8. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.4.52-58.
- Корнеева И.Е., Мартиросян Я.О., Ковальчук А.И., Назаренко Т.А., Бирюкова А.М., Веюкова М.А., Иванец Т.Ю. Особенности фолликуло-стероидо-оогенеза при стимуляции яичников в лютеиновую фазу менструального цикла. Акушерство и гинекология. 2021; 7: 107-12. [Korneeva I.E., Martirosyan Ya.O., Koval’chuk A.I., Nazarenko T.A., Biryukova A.M., Veyukova M.A., Ivanets T.Yu. Characteristics of folliculogenesis, steroidogenesis, and oogenesis during ovarian stimulation in the luteal phase of the menstrual cycle. Obstetrics and Gynecology. 2021; (7): 107-12 (in Russian)]. https://dx.doi.org/10.18565/aig.2021.7.107-112.
- Cakmak H., Rosen M.P. Ovarian stimulation in cancer patients. Fertil. Steril. 2013; 99(6): 1476-84. https://dx.doi.org/10.1016/j.fertnstert.2013.03.029.
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in premenopausal women with oestrogen receptor-positive early-stage breast cancer treated with ovarian suppression: a patient-level meta-analysis of 7030 women from four randomised trials. Lancet Oncol. 2022; 23(3): 382-92. https://dx.doi.org/10.1016/S1470-2045(21)00758-0.
Received 19.02.2025
Accepted 24.03.2025
About the Authors
Almina M. Biryukova, PhD, Clinical Supervisor at the F. Paulsen Research and Educational Center for ART with the Clinical Department, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, a_birukova@oparina4.ruTatiana A. Nazarenko, Dr. Med. Sci., Professor, Head of the Institute of Reproductive Medicine, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
117997, Russia, Moscow, Ac. Oparin str., 4, t_nazarenko@oparina4.ru, https://orcid.org/0000-0002-5823-1667
Yana O. Martirosyan, PhD, Junior Researcher at the F. Paulsen Research and Educational Center for ART with the Clinical Department, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, ya_martirosyan@oparina4.ru, https://orcid.org/0000-0002-9304-4410
Elena V. Oshkina, Oncologist at the Breast Pathology Department, V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, E_oshkina@oparina4.ru



