Бедный ответ на овариальную стимуляцию (ОС) – состояние, при котором развивается/получено менее четырех фолликулов и/или ооцитов после стимуляции яичников при условии, что предполагалось получение большего количества фолликулов и ооцитов [1]. Среди женщин, проходящих протоколы экстракорпорального оплодотворения (ЭКО), бедный ответ на ОС встречается в 9–24% случаев [2]. Данное состояние часто обусловлено возрастным снижением репродуктивной функции, однако может встречаться и у молодых женщин [3]. Пациентки с бедным ответом на ОС обычно имеют более высокие базальные уровни фолликулостимулирующего гормона (ФСГ) [4], более низкие уровни антимюллерова гормона (АМГ) и меньшее количество ооцитов, полученных с использованием стандартных протоколов стимуляции яичников, по сравнению с женщинами с нормальным ответом на ОС [5]. В результате меньшего числа полученных ооцитов, у данных пациенток наблюдаются более высокая частота отмены циклов [6] и более низкие показатели частоты наступления беременности, по сравнению с пациентами с нормальным ответом на ОС [7]. Для повышения результативности лечения бесплодия у пациенток с данным диагнозом были предложены различные варианты протоколов стимуляции яичников, в том числе использование адъювантной терапии [8]. Андрогеновый прайминг был представлен в качестве одного из вариантов предварительной терапии, которая может повысить эффективность проведения ЭКО у пациенток с бедным ответом на ОС за счет увеличения внутрияичниковой концентрации андрогенов [9, 10]. В данной статье рассмотрены основные вопросы относительно эффективности и безопасности применения андрогенового прайминга у пациенток с бедным ответом на ОС в протоколах ЭКО.
Бедный ответ на овариальную стимуляцию
Гетерогенность определения бедного ответа на ОС, используемого в исследованиях, привела к необходимости принятия критериев постановки данного диагноза. Согласно Болонским критериям, группу риска бедного овариального ответа на стимуляцию в программах ВРТ составляют женщины, у которых имеются как минимум 2 из следующих 3 критериев [5]:
1) возраст 40 лет и старше или наличие факторов риска (генетические нарушения, например, синдром Тернера, операции на яичниках, эндометриоидные кисты и химиотерапия);
2) бедный ответ на стимуляцию яичников в анамнезе (<4 фолликулов при стандартном протоколе стимуляции яичников);
3) сниженный овариальный резерв (5–7 антральных фолликулов, АМГ 0,5–1,1 нг/мл).
Для улучшения результатов лечения в 2016 г. группа POSEIDON (Patient Oriented Strategies Encompassing Individualized Oocyte Number) предложила уйти от термина «бедный ответ на ОС» к концепции низкого прогноза. Кроме того, группа POSEIDON ввела две новые категории нарушений ответа при ОС [11]:
1) «субоптимальный ответ», определяемый как получение от 4 до 9 ооцитов, связанный в любом возрасте со значительно более низкой частотой родов живым плодом, по сравнению с нормальными ответчиками, т. е. с получением 10–15 ооцитов;
2) «сниженный ответ», при котором требуются более высокая доза гонадотропинов и более длительная стимуляция для получения адекватного количества ооцитов (более трех).
Кумулятивная частота родов живым плодом прямо пропорционально зависит от числа полученных ооцитов и возраста женщины [12]. Исходя из этого, количество ооцитов, необходимое для достижения максимальной эффективности ЭКО, может быть индивидуализировано с учетом возраста пациентки [13]. Эти принципы были учтены при создании критериев POSEIDON, которые включают: возраст женщины, маркеры овариального резерва (уровень АМГ), количество антральных фолликулов (КАФ) и количество полученных ооцитов в предыдущих программах ВРТ. Согласно этим критериям, пациентки с плохим прогнозом делятся на 4 группы (табл. 1) [11].

Женский возраст является критически важным критерием в классификации POSEIDON, так как возраст в наибольшей степени связан с хромосомным набором эмбрионов и, как следствие, частотой родов живым плодом. Применение критериев POSEIDON позволяет определить пациенток с низким прогнозом и стратифицировать таких пациенток в одну из четырех групп женщин с «субоптимальным или сниженным ответом яичников» на экзогенную стимуляцию гонадотропинами (табл. 1). Кроме того, группа POSEIDON представила новую меру клинического успеха ВРТ, а именно получение определенного числа ооцитов, необходимого для получения по крайней мере одной эуплоидной бластоцисты для переноса у каждого пациента. Используя критерии POSEIDON, врач может не только выявить и классифицировать пациентов с низким прогнозом в ВРТ, но и разработать индивидуальный план лечения для повышения результативности проведения ЭКО в каждой из четырех групп [13].
Андрогеновый прайминг
Андрогеновый прайминг включает в себя применение стероидных гормонов, таких как дегидроэпиандростерон (ДГЭА) и тестостерон, перед протоколом ЭКО с целью повышения внутрияичниковой концентрации андрогенов [14, 15]. Предполагается, что снижение уровня эндогенных андрогенов может быть связано со снижением чувствительности яичников к ФСГ [16]; соответственно, прием экзогенных андрогенов перед ОС теоретически может приводить к повышению количества и качества получаемых ооцитов при ОС и улучшению клинических исходов ЭКО [17–20].
Для повышения концентрации андрогенов в «микросреде» яичников применяют стероидные гормоны, а именно:
1) тестостерон [16] – основной половой гормон, андроген, вводится трансдермально (кожный пластырь или гель), перорально или в виде подкожного имплантата. Оптимальная доза или продолжительность введения тестостерона не установлены [17];
2) ДГЭА – стероидный гормон, вырабатываемый в ретикулярной зоне надпочечников и тека-клетках яичников. Он является предшественником половых гормонов тестостерона и эстрадиола. Как правило, вводится перорально в дозе 75 мг/день [15].
Механизм действия. Согласно теории двух клеток – двух гонадотропинов (рисунок), в клетках теки андрогены (андростендион, ДГЭА и тестостерон) вырабатываются в ответ на стимуляцию лютеинизирующим гормоном (ЛГ). После диффузии в гранулезные клетки андрогены превращаются в дигидротестостерон или эстрогены (эстрадиол и эстрон) ферментом ароматаза Р450 под действием ФСГ [21, 22]. Предполагается, что введение экзогенных андрогенов до ОС может повышать экспрессию рецепторов ФСГ в клетках гранулезы, создавая больший пул фолликулов, реагирующих на экзогенную стимуляцию ФСГ [23].
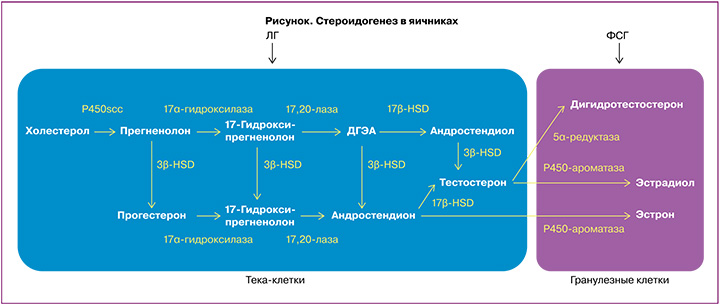
Андрогены могут влиять на фолликулогенез непосредственно через собственные рецепторы (ARs) [24] или косвенно, благодаря участию в синтезе эстрогенов. В яичниках ARs представлены в клетках гранулезы и тека-клетках, а также в строме яичников, и присутствуют на большинстве стадий развития фолликула. ARs не обнаружены в примордиальных фолликулах, но линейно коррелируют с ростом фолликула от стадии первичного к преовуляторному фолликулу [25, 26]. Обнаружено, что в первичных фолликулах ARs начинают синтезироваться раньше, чем рецепторы ФСГ, и в преантральных фолликулах концентрация ARs выше, чем концентрация рецепторов ФСГ, что указывает на роль андрогенов в развитии фолликулов на начальных стадиях [25, 27]. Так, кратковременное воздействие андрогенов в исследованиях in vivo на животных увеличивает синтез мРНК рецепторов ФСГ в гранулезных клетках малых антральных фолликулов [28] и усиливает ФСГ-индуцированное образование цАМФ, необходимое для транскрипции генов, участвующих в контроле пролиферации и дифференцировке гранулезных клеток [29]. В исследованиях in vitro на яичниках человека наблюдается значимая положительная связь между экспрессией мРНК ARs гранулезных клеток, содержанием андрогенов в фолликулярной жидкости и экспрессией мРНК рецепторов ФСГ в клетках гранулезы [24].
Активация ARs также увеличивает экспрессию инсулиноподобного фактора роста 1 (ИФР-1) и его рецепторного гена в гранулезных клетках, клетках теки растущих фолликулов и в ооцитах первичных фолликулов, тем самым облегчая влияние ИФР-1 как при рекрутировании фолликулов, так и при последующем фолликулярном развитии [30, 31]. Андрогены, вырабатываемые растущими фолликулами, могут способствовать переходу от примордиальных к первичным фолликулам и от первичных к вторичным фолликулам посредством усиления действия ИФР-1, а также могут усиливать действие ФСГ на стимулирование последующего роста из преантральных в антральные фолликулы [24, 31, 32].
Таким образом, применение андрогенового прайминга может приводить к увеличению количества растущих преантральных и антральных фолликулов и увеличивать пролиферацию гранулезных клеток [33]. В совокупности со «спасением» рекрутированных фолликулов от атрезии при экзогенном воздействии ФСГ при ОС [34] предполагается, что лечение андрогенами женщин с бедным ответом на ОС может улучшить реакцию яичников на ОС [35].
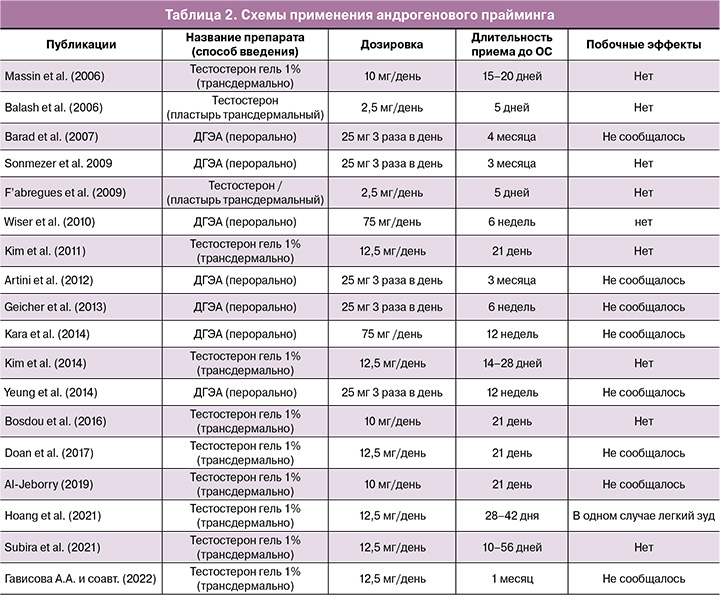
Длительность приема и дозировки. На сегодняшний момент не существует общепринятых дозировок и длительности использования андрогенов в качестве адъювантной терапии. Как правило, тестостерон используется в виде трансдермального 1% геля в дозировке 10–12,5 мг/день или пластыря (2,5 мг/день) длительностью от 5 до 56 дней до ОС. Тогда как пероральные формы ДГЭА в дозировке 75 мг в день – от 6 недель до 4 месяцев до проведения ЭКО. Основные схемы приема стероидных гормонов при андрогеновом прайминге представлены в таблице 2.
В данном обзоре были рассмотрены данные опубликованных метаанализов [6, 17, 18, 35–40] относительно влияния предварительной терапии андрогенами на основные показатели эффективности проведения ЭКО, такие как число полученных ооцитов, частота наступления клинической беременности (ЧНКБ) и частота родов живым плодом.
Количество полученных ооцит-кумулюсных комплексов
Систематический обзор и метаанализ Bosdou J.K. et al. [18], Noventa M. et al. [38], метаанализ Luo S. et al. [6] показали, что количество полученных ооцит-кумулюсных комплексов (ОКК) было выше у женщин, которые предварительно принимали препараты тестостерона, по сравнению с женщинами, не получавшими лечения. Также авторы более поздних систематических обзоров и метаанализов 2022 г. [39, 40] пришли к выводу, что у женщин с бедным ответом на ОС, перенесших ЭКО, прием тестостерона ассоциирован со значительным увеличением количества полученных ОКК.
Однако, по данным метаанализа Sunkara S.K. et al. (2011) [35], не было обнаружено различий в количестве полученных ооцитов между теми, кто получал андрогенные добавки, по сравнению с теми, кто их не получал. Систематический обзор и метаанализ Gonza´lez-Comadran M. et al. (2012) [37] также не показал различий в количестве и качестве полученных ооцитов. Аналогично, по данным метаанализа 2020 г., прием андрогенов у женщин до ОС не увеличивает количества полученных ОКК в сравнении с женщинами, которые не получали андрогены до ОС [36]. Авторы систематического обзора и метаанализа Neves A.R. et al. (2022) сообщили об отсутствии различий в количестве полученных ооцитов при проведении прайминга ДГЭА в сравнении с плацебо или отсутствием лечения [40].
Таким образом, имеющиеся данные свидетельствуют о том, что применение ДГЭА до ОС не увеличивает количество полученных ооцитов в программах ЭКО у женщин с бедным ответом на ОС, а прием тестостерона имеет противоречивые данные.
Частота наступления клинической беременности
Систематические обзоры и метаанализы Gonza´lez-Comadran M. et al. [37], Bosdoue J.K. et al. [18], Luo S. et al. [6], Noventa M. et al. [38], Neves A.R. et al. [40], Katsika E.T. et al. [39] показали, что женщины, получавшие тестостерон до ОС, имели статистически значимо более высокую ЧНКБ по сравнению с женщинами, не получавшими данную терапию. В Кокрейновском метаанализе 2015 г., оценивающем влияние лечения тестостероном и ДГЭА перед ОС у женщин с бедным ответом в сравнении с плацебо, авторами были сделаны выводы об увеличении ЧНКБ (ОШ 1,81; 95% ДИ 1,25–2,62) [17]. Применение ДГЭА у пациенток с бедным ответом на стимуляцию было оценено в метаанализе 2020 г. [36], по результатам которого ЧНКБ была выше у пациенток, принимавших данный препарат, по сравнению с пациентками, которым лечение не проводилось.
Напротив, по данным метаанализа 2011 г., оценивающего эффективность приема андрогенов у пациенток с бедным ответом, ЧНКБ не отличалась между пациентами, применявшими андрогеновый прайминг и не применявшими его [35]. Более того, по данным систематического обзора и метаанализа 2020 г., применение тестостерона у женщин с бедным ответом на ОС не показало существенной разницы в данном показателе, по сравнению с контрольной группой, не получавшей лечения [36]. Применение прайминга ДГЭА в сравнении с женщинами, которым не применялся прайминг или использовалось плацебо, не влияло на увеличение ЧНКБ по результатам систематического обзора и метаанализа 2022 г. [40].
Таким образом, существуют неоднозначные данные о влиянии предварительного приема тестостерона и ДГЭА на ЧНКБ у женщин с бедным ответом на ОС.
Частота родов живым плодом
Систематические обзоры и метаанализы Gonza´lez-Comadran M. et al. (2012) [37], Bosdoue J.K. et al. [18], Luo S. et al. (2014) [6], Noventa M. et al. (2019) [38], Katsika E.T. et al. (2022) [39], Neves A.R. et al. (2022) [40], обобщившие данные по применению тестостерона перед ОС в сравнении со стандартной стимуляцией яичников у пациенток с бедным ответом, пришли к выводу, что женщины, получавшие тестостерон перед ОС, имели статистически значимо более высокую частоту родов живым плодом. Также авторами Кокрейновского метаанализа 2015 г. были получены данные о повышении частоты родов живым плодом при предварительном лечении тестостероном (4 РКИ; ОШ 2,60; 95% ДИ 1,30–5,20) [17]. Одновременно с этими данными, систематический обзор и метаанализ Sunkara S.K. et al. (2011) [35] резюмировал, что андрогеновый прайминг у пациентов с бедным ответом на ОС в программах ЭКО не повышает частоту родов живым плодом. Систематический обзор и метаанализ Neves A.R. et al. (2022) [40] не обнаружил статистически значимой разницы в частоте родов живым плодом у пациенток, получавших ДГЭА до и во время ОС, в сравнении с пациентками, не получавшими лечения данным препаратом.
Эти результаты свидетельствуют о том, что применение ДГЭА до ОС не связано с увеличением частоты родов живым плодом в программах ЭКО у женщин с бедным ответом на ОС, и, соответственно, этот препарат, вероятно, не рекомендуется перед ОС. Существуют неоднозначные данные о влиянии приема тестостерона на частоту родов живым плодом у пациенток с бедным ответом на ОС. Хотя метаанализ Katsika E.T. et al. (2022) является самым крупным на сегодняшний день, и его результаты показали, что вероятность беременности и родов живым плодом увеличивается у женщин с бедным ответом на ОС, предварительно получавших трансдермальный тестостерон, обзор характеризуется определенными ограничениями, которые следует учитывать при интерпретации его результатов [39]. Вершиной доказательной медицины является гайдлайн ESHRE по ОС. По его данным, на сегодняшний день не рекомендуется назначение тестостерона и ДГЭА перед стимуляцией яичников у женщин с бедным ответом на ОС, так как имеются противоречивые данные относительного того, что предварительная адъювантная терапия андрогенами перед ОС улучшает ответ яичников у женщин с бедным ответом. Также недостаточно данных по дозировке, продолжительности применения и безопасности использования андрогенового прайминга [41].
Безопасность
Немалую роль при использовании андрогенов перед протоколами ЭКО играют длительность приема, дозировка и безопасность относительно краткосрочных и отдаленных влияний на здоровье женщины и плода. Примечательно, что у женщин, применяющих ДГЭА и тестостерон, могут быть побочные эффекты, связанные с приемом андрогенов. Экзогенное введение тестостерона может влиять на либидо, минеральную плотность костной ткани, мышечную массу, жировую массу, распределение тканей, настроение, энергию и психологическое благополучие [42]. ДГЭА в сравнении с плацебо/без лечения может приводить к усилению угревой сыпи [43, 44]. Yeung T.W.Y. et al. в исследовании 2013 г. сообщили, что до 22% участников в группе ДГЭА жаловались на увеличение акне, по сравнению с 8,3% в группе плацебо [45]; в другом исследовании отмечалось, что у некоторых женщин наблюдалась повышенная выработка кожного сала, а у некоторых развился переходный гирсутизм [46]. Помимо перечисленных побочных эффектов, характерных для приема андрогенов, описан один случай жалоб женщины на головокружение [44], но не было указано, была ли она в группе вмешательства или в контрольной группе. Все другие исследования не отметили никаких побочных эффектов ни у одного из своих участников. В систематическом обзоре и метаанализе Katsika E.T. et al. (2022) [39] указано, что ни в одном из исследований тестостерона не сообщалось о каких-либо побочных эффектах. Таким образом, введение трансдермальных форм тестостерона и использование ДГЭА в дозах 75 мг представляется безопасным [47–49], поскольку в проанализированных исследованиях не сообщалось о побочных эффектах, за исключением зуда в месте нанесения после трансдермального введения тестостерона в одном случае [50] (табл. 2). Однако долгосрочные эффекты предварительного лечения тестостероном в настоящее время не изучены. Точно так же в настоящее время нет данных о детях, рожденных после предварительного лечения тестостероном [39].
В большинстве приведенных здесь исследований авторы использовали короткие курсы лечения андрогенами. Поскольку рецепторы андрогенов синтезируются в гранулезных клетках на ранних стадиях созревания фолликулов, логично предположить, что продление приема тестостерона в течение более длительного периода может увеличить пул фолликулов, чувствительных к гонадотропинам, и, следовательно, увеличить количество ооцитов, доступных для извлечения. Это предположение все еще нуждается в проверке с помощью должным образом спланированных испытаний, но потенциальная возможность повысить результативность лечения бесплодия при помощи «андрогенизации» должна учитывать возможные, связанные с данной терапией побочные эффекты [18].
Заключение
В опубликованных систематических обзорах и метаанализах в настоящее время не может быть сделано однозначных выводов относительно эффективности применения андрогенов у женщин с бедным ответом на стимуляцию яичников. Необходимо учитывать ограничения в рандомизированных исследованиях, на основании которых проводились метаанализы. Кроме того, количество исследований, включенных в метаанализ, все еще относительно невелико. Это приводит к тому, что оценки лечения подвержены некоторой неточности, что снижает достоверность результатов. Также следует учитывать, что определение бедного ответа яичников варьировалось в разных исследованиях. Присутствовала значительная неоднородность в отношении типа, дозы и продолжительности предварительного лечения тестостероном и ДГЭА. Требуется проведение дальнейших релевантных РКИ для подтверждения пользы и безопасности терапии андрогенами до ОС у женщин с бедным ответом в программах ЭКО. В настоящее время существуют противоречивые данные о положительном влиянии приема тестостерона и ДГЭА перед ОС на клинические исходы ЭКО, и, вероятно, эти препараты не рекомендованы для использования у женщин с бедным ответом на ОС.



