Effectiveness of donor-recipient ovarian stimulation programs in oocyte donors at different phases of the menstrual cycle
Objective: To compare the effectiveness of donor-recipient programs for ovarian stimulation of oocyte donors during the follicular and luteal phases of the menstrual cycle.Lapina V.S., Martazanova B.A., Durinyan E.R., Amyan T.S., Korolkova A.I., Gavisova A.A.
Materials and methods: The study included 114 women:30 oocyte donors who underwent ovarian stimulation in both the follicular and luteal phases of the menstrual cycle and 84 recipients who underwent embryo transfer in a cryopreserved cycle. Donor oocytes were retrieved in the follicular and luteal phases in 36 and 48 of these recipients, respectively. The embryological parameters and clinical outcomes of the donor-recipient programs in both groups were evaluated.
Results: An analysis of the embryological parameters of the donor-recipient programs showed no difference in the fertilization rates of the donor oocytes received at different phases of the menstrual cycle, nor in the blastulation rates, number of high-quality blastocysts, and number of cryopreserved embryos. The pregnancy rate in recipients in cryopreserved cycles for embryo transfer, clinical pregnancy rate, reproductive loss rate up to 12 weeks, implantation rate, and live birth rate also did not differ significantly when donor oocytes obtained in the follicular and luteal phases of the cycle were used (p>0.05).
Conclusion: Ovarian stimulation of oocyte donors in the luteal phase of the menstrual cycle does not adversely affect embryological parameters and clinical outcomes of donor-recipient programs. In this context, the implementation of ovarian stimulation in the luteal phase of the cycle is important for different groups of patients to optimize the timing and increase the effectiveness of treatment.
Authors' contributions: Lapina V.S., Martazanova B.A., Durinyan E.R., Amyan T.S., Korolkova A.I., Gavisova A.A. – conception and design of the study, review of relevant literature, collection of material, statistical analysis, drafting of the manuscript, final approval for submission.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Lapina V.S., Martazanova B.A., Durinyan E.R., Amyan T.S., Korolkova A.I., Gavisova A.A. Effectiveness of donor-recipient ovarian stimulation programs in
oocyte donors at different phases of the menstrual cycle.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (9): 107-114 (in Russian)
https://dx.doi.org/10.18565/aig.2023.153
Keywords
The study of folliculogenesis patterns in the normal menstrual cycle has shown the feasibility of recruiting a cohort of antral follicles not only in the early follicular phase, but also throughout the menstrual cycle [1–3]. This provides the rationale for performing ovarian stimulation on any day of the menstrual cycle. The literature provides data on successful ovarian stimulation in an in vitro fertilization (IVF) program at different phases of the menstrual cycle. The first experience of ovarian stimulation with the start of the program at any phase of the menstrual cycle (“random-start” protocols) appeared because of the need to preserve genetic material in cancer patients before conducting gametotoxic therapy (chemotherapy and/or radiation therapy) [4, 5]. Research results indicate satisfactory quantity and quality of oocytes and embryos in assisted reproductive technology (ART) programs, regardless of the menstrual cycle phase [6, 7].
There are also data in the literature on the high clinical efficacy of ART programs (IVF/ICSI) with ovarian stimulation in the luteal phase of the menstrual cycle in patients with a "poor" response. In 2014, Kuang Y. et al. [8] successfully introduced stimulation in the follicular and luteal phases of the cycle (Shanghai Protocol), followed by cryopreservation of the resulting embryos and transfer in a cryopreserved cycle. In 242 cycles, this approach was shown to be highly effective based on oocyte/embryo quality assessment, resulting in a clinical pregnancy rate of 48.9% [8].
However, stimulation regimens, choice of gonadotropins, and methods to prevent the spontaneous surge of luteinizing hormone (LH) and luteinization of follicles when ovarian stimulation is initiated during the luteal phase have not yet been fully determined. The search continues for markers of ovarian reserve that are most informative for the subsequent selection of the optimal day to begin ovarian stimulation.
Oocyte donors are an ideal model for assessing the impact of the initiation of superovulation stimulation on oocyte and embryo quality, as they represent a cohort of healthy, potentially or proven fertile women who voluntarily undergo an ovarian stimulation program [9].
In this context, it seems relevant and promising to study the characteristics of ovarian stimulation in different phases of the menstrual cycle in donor-recipient ART programs. Studies [8] conducted in this direction have shown the absence of an adverse effect of high concentrations of progesterone on the day of the start of ovarian stimulation in the luteal phase of the cycle on the response of the ovaries and the number and quality of oocytes obtained.
The main phase of the study was a comparative evaluation of embryologic parameters and clinical outcomes of donor-recipient programs using donor oocytes obtained at different phases of the menstrual cycle.
This study aimed to compare the effectiveness of donor-recipient programs for the ovarian stimulation of oocyte donors during the follicular and luteal phases of the menstrual cycle.
Materials and methods
This prospective observational study was conducted at the Department of Preservation and Restoration of the Reproductive Function of Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation.
The study included 114 women: 30 oocyte donors who underwent ovarian stimulation in both the follicular and luteal phases of the menstrual cycle, and 84 recipients who underwent embryo transfer in a cryopreserved cycle. Group 1 (n=36) included recipients who used donor oocytes obtained in the follicular phase and Group 2 (n=48) included recipients who used donor oocytes obtained in the luteal phase. Embryological parameters and clinical outcomes of donor-recipient programs were assessed in both groups.
Inclusion criteria for oocyte donors were as follows: age from 18 to 35 years; basal FSH concentration<10 mIU/ml; the presence of more than 10 antral follicles on day 2 of the menstrual cycle; body mass index 18–25 kg/m2; and informed consent to participate in the study.
The inclusion criteria for recipients were age 25–49 years; failure to obtain their own oocytes in IVF programs: premature depletion of ovarian function, confirmed clinically and by laboratory tests, genetic diseases in the patient, congenital anomalies or surgical removal of the ovaries, a sharp decrease in ovarian reserve in women of late age, a history of unsuccessful ART cycles (IVF/ICSI) when receiving oocytes of low quality or embryos with developmental disorders at the stages of early embryogenesis; and informed consent to participate in the study.
Exclusion criteria were pronounced male factor, large interstitial or subserous uterine fibroids, stage III–IV external and internal endometriosis, submucosal uterine fibroids, and uterine malformations.
Oocyte donors entered the IVF program twice and were divided into 2 groups according to the phase of the cycle in which ovarian stimulation was performed. The same donor was stimulated twice: the first time in the follicular phase and again three months later in the luteal phase.
Protocols for the stimulation of oocyte donors during the follicular and luteal phases of the cycle were presented in detail in a previous study.
Fertilization of donor oocytes was performed only by ICSI using the sperm of the recipient's partner. The embryos were cultured in single droplets. Embryo quality was evaluated according to the Gardner blastocyst grading system (1999) [10]. Cryopreservation was performed for embryos with excellent and good quality.
As part of the program for the transfer of cryopreserved embryos, female recipients underwent preparation of the endometrium against the background of hormone replacement therapy (from day 4 of the menstrual cycle, 6 mg/day of estradiol valerate, with an M-echo size of 8–10 mm and a clear three-layer structure of the endometrium, 600 mg/day of micronized progesterone). On day 6 of taking micronized progesterone, one thawed embryo was transferred. Pregnancy was diagnosed by a positive serum β-subunit of human chorionic gonadotropin (>100 IU/L) on day 14 after embryo transfer, followed by visualization of the gestational sac using vaginal ultrasound on day 21 after transfer.
The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P of the Ministry of Health of the Russian Federation.
Statistical analysis
The research results were processed on a personal computer using SPSSV22.0 and a statistical analysis package for Microsoft Office Excel 2007.
Statistical analysis was performed using generally accepted methods of variation statistics. Quantitative variables showing normal distribution are expressed as mean (M) and standard deviation (SD) and presented as M (SD). Categorical variables were reported as frequencies and percentages.
The distribution of continuous variables was tested for normality using the Kolmogorov-Smirnov test and graphical data analysis. Variables that did not meet normality assumptions were compared using a nonparametric Mann-Whitney test. Differences were considered statistically significant at p-level of less than 0.05.
Categorical variables were compared using the chi-square test (χ2).
Sample size
No sample size calculation was performed because of the lack of similar studies at the time of the data collection.
The primary outcome was the number of mature oocytes obtained from oocyte donors and the number of embryos obtained from recipients. The secondary outcome was the recipient pregnancy rate.
Results
Clinical and medical history data of the donors
The age of the oocyte donors was 27.7 (4.2) years, the mean body mass index was 21.1 (3.1) kg/m2.
Most oocyte donors had a history of childhood infections. None of the donors had somatic comorbidities, as determined by specialist conclusions. Three donors had a history of laparoscopic appendectomy.
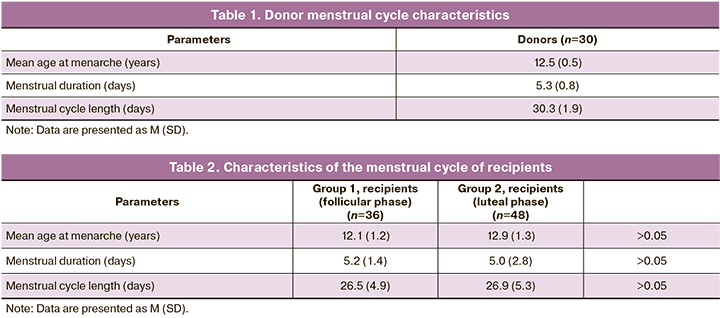
The menstrual cycle characteristics of donors are presented in Table 1. All the donors had regular menstrual cycles. The mean age at menarche was 12.5 (0.5) years.
Clinical and anamnestic data of recipients
The mean age of recipients in group 1 was 38.54 (6.5) years (follicular phase) and 39.53 (5.5) years in group 2 (luteal phase). The body mass index was 24.3 (4.1), and 23.9 (3.8) kg/m2, respectively.
The characteristics of the recipients’ menstrual cycles are presented in Table 2. The mean age at menarche in group 1(follicular phase) was 12.1 (1.2) years, and 12.9 (1.3) years in group 2 (luteal phase).
No statistically significant differences were found between the recipients in either group.
The duration of infertility also did not differ significantly between the groups, amounting to 6.3 (3.4) years in group 1 and 5.7 (4.1) in group 2 (p>0.05).
The history of gynecologic diseases is presented in Table 3.
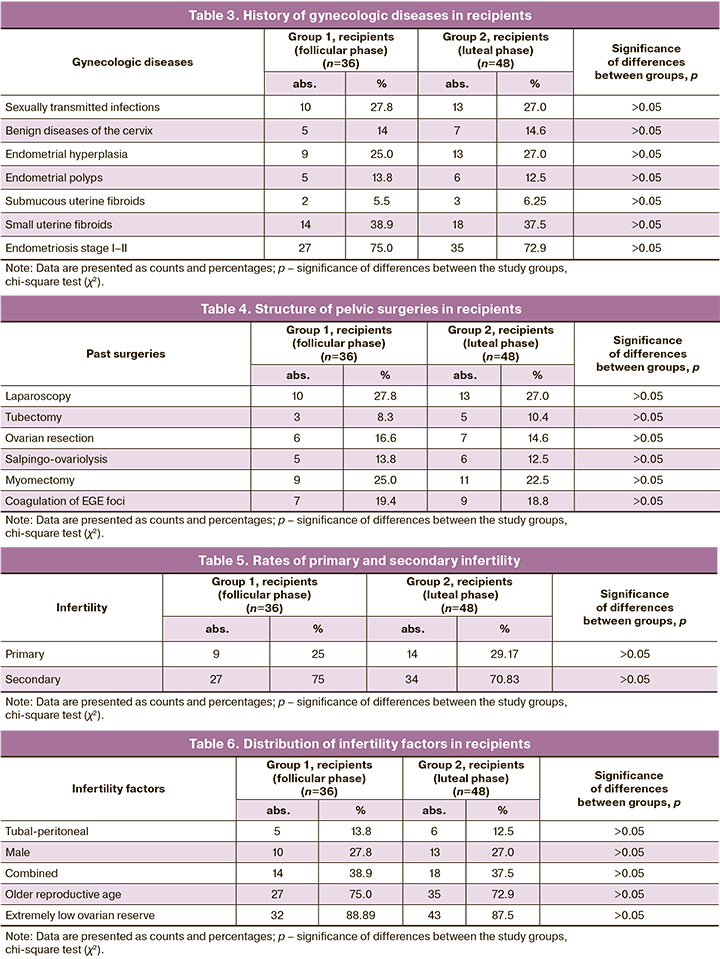
Almost one-third of the recipients had a history of pelvic surgery (Table 4). The most common pelvic surgeries were laparoscopy, myomectomy, and coagulation of foci of extragenital endometriosis.
When analyzing the data regarding the type of infertility (primary/secondary) in the study groups, no statistically significant differences were found (p>0.05) (Table 5).
The mean number of unsuccessful ART programs in recipients with a history was 3.6 (1.7) in group 1, 2.9 (2.3) in group 2 (p>0.05).
Among the factors of infertility in recipients, the most common were a decrease in the ovarian reserve and advanced reproductive age. The data are presented in Table 6.
Characteristics of the embryological stage of the studied groups
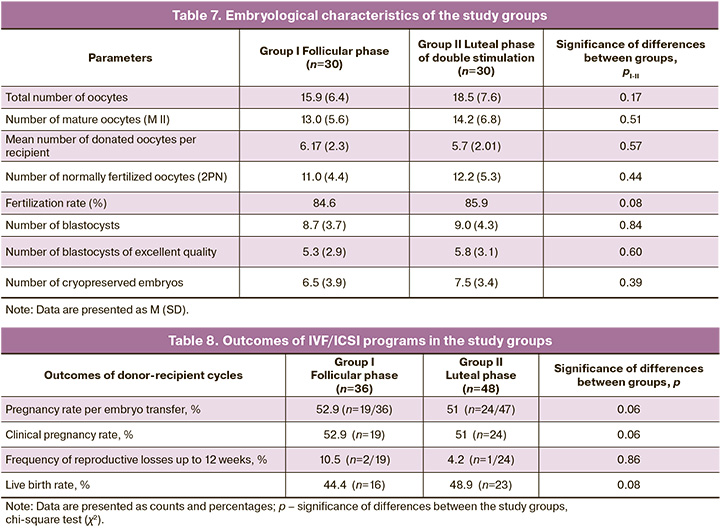
When analyzing the embryological stage of ART programs (IVF/ICSI) of the study groups, no statistically significant differences were found in the number of obtained and mature oocytes, number of donated oocytes, number of normally fertilized oocytes, and number of embryos obtained, including those of high quality, as shown in Table 7.
Comparative characteristics of the outcomes of ART programs (IVF/ICSI) in donor-recipient cycles
As part of the preparation of the endometrium for the transfer of thawed embryos, recipients in both groups received identical therapy (see section “Materials and Methods”). The transfer of one thawed embryo was performed under ultrasound guidance under aseptic conditions using a disposable flexible catheter. The endometrial thickness (M-echo) on the day of embryo transfer was 8–11 mm in both groups (p>0.05).
When analyzing the outcomes of IVF/ICSI programs in women in the study groups, no statistically significant differences were found in pregnancy, progressive pregnancy, and live birth rates (Table 8).
Discussion
Some studies [8, 11] have reported a peculiarity in the hormonal profile during ovarian stimulation in the luteal phase of the cycle, characterized by high progesterone levels on the day of ovarian stimulation. However, this did not have a negative impact on the number and quality of donor oocytes obtained. Thus, the number of obtained donor oocytes at stage MII was 13.0 (5.6) when stimulated in the follicular phase, and 14.2 (6.8) when stimulated in the luteal phase. This observation is most likely explained by the absence of progesterone receptors in oocyte-cumulus complexes. Accordingly, the indicators of early embryogenesis in recipients receiving oocytes also turned out to be comparable regardless of the phase of the menstrual cycle, which was confirmed by a similar number of normally fertilized oocytes, high-quality blastocysts, cryopreserved embryos, and the rates of clinical pregnancy and live births.
Research evidence also did not reveal differences in the number and competence of oocytes obtained during an IVF program in the luteal phase of the menstrual cycle compared to conventional ovarian stimulation [12, 13]. Moreover, some authors have demonstrated an increase in the number of aspirated oocytes during ovarian stimulation during the luteal phase [14, 15]. There are reports of comparable numbers of euploid blastocysts and good-quality oocytes and embryos in a dual stimulation program after stimulation in the luteal phase of the menstrual cycle in patients with decreased ovarian reserve, resulting in higher pregnancy rates [14]. In addition, current data did not reveal differences in the number of clinical pregnancies, birth weight, body length of newborns, or developmental anomalies between children born as a result of stimulation in the luteal phase of the cycle and those born as a result of stimulation in the follicular phase [16, 17]. However, data on embryological outcomes and treatment effectiveness in women with normal ovarian reserves who undergo IVF in the luteal phase of the cycle are very limited. As described earlier, given that oocyte donors are an ideal model for studying the influence of the period of onset of superovulation stimulation on the quality of oocytes and embryos, as they are represented by a cohort of healthy, potentially or proven fertile women [9], we chose this model.
The feasibility of performing ovarian stimulation at any phase of the menstrual cycle is relevant in various groups of patients. Thus, given the importance of an emergency IVF program for cancer patients due to the lack of time to wait for optimal conditions to start the program, the possibility of ovarian stimulation of the ovaries in the luteal phase of the menstrual cycle for many of them may be the last chance to preserve genetic material before the start of cancer treatment. In addition, the possibility of conducting an IVF program at the beginning of stimulation, regardless of the phase of the cycle, and the use of double stimulation allow women with a decrease in ovarian reserve to receive higher rates of success in the IVF program. Emergency preservation of genetic material for patients when planning surgical interventions for pelvic organs is also relevant. Another important aspect is the possibility of carrying out ovarian stimulation of the ovaries in oocyte donors on any day of the menstrual cycle, which allows optimizing donor preparation time and accelerating the period of pregnancy in recipients.
Conclusion
Ovarian stimulation of oocyte donors in the luteal phase of the menstrual cycle does not adversely affect embryological parameters and clinical outcomes of donor-recipient programs. In this context, implementation of ovarian stimulation in the luteal phase of the cycle is important for different groups of patients to optimize the timing and increase the effectiveness of treatment.
References
- McNatty K.P., Hillier S.G., van den Boogaard A.M., Trimbos-Kemper T.C., Reichert L.E., van Hall E.V. Follicular development during the luteal phase of the human menstrual cycle. J. Clin. Endocrinol. Metab. 1983; 56(5): 1022-31. https://dx.doi.org/10.1210/jcem-56-5-1022.
- Baerwald A.R., Adams G.P., Pierson R.A. Ovarian antral folliculogenesis during the human menstrual cycle: a review. Hum. Reprod. Update. 2012; 18(1): 73-91. https://dx.doi.org/10.1093/humupd/dmr039.
- Kirillova A., Martazanova B., Mishieva N., Semenova M. Follicular waves in ontogenesis and female fertility. Biosystems. 2021; 210: 104558.https://dx.doi.org/10.1016/j.biosystems.2021.104558.
- Bedoschi G.M., de Albuquerque F.O., Ferriani R.A., Navarro P.A. Ovarian stimulation during the luteal phase for fertility preservation of cancer patients: case reports and review of the literature. J. Assist. Reprod. Genet. 2010; 27(8): 491-4. https://dx.doi.org/10.1007/s10815-010-9429-0.
- Sönmezer M., Türkçüoğlu I., Coşkun U., Oktay K. Random-start controlled ovarian hyperstimulation for emergency fertility preservation in letrozole cycles. Fertil. Steril. 2011; 95(6): 2125.e9-11. https://dx.doi.org/10.1016/j.fertnstert.2011.01.030.
- Cakmak H., Katz A., Cedars M.I., Rosen M.P. Effective method for emergency fertility preservation: random-start controlled ovarian stimulation. Fertil. Steril. 2013; 100(6): 1673-80. https://dx.doi.org/10.1016/j.fertnstert.2013.07.1992.
- Mishieva N., Martazanova B., Bogatyreva K., Korolkova A., Kirillova A., Veyukova M. et al. Cumulus cell gene expression in luteal-phase-derived oocytes after double stimulation in one menstrual cycle. Reprod. Biomed. Online. 2020; 41(3): 518-26. https://dx.doi.org/10.1016/j.rbmo.2020.05.002.
- Kuang Y., Chen Q., Hong Q., Lyu Q., Ai A., Fu Y. et al. Double stimulations during the follicular and luteal phases of poor responders in IVF/ICSI programmes (Shanghai protocol). Reprod. Biomed. Online. 2014; 29(6): 684-91.https://dx.doi.org/10.1016/j.rbmo.2014.08.009.
- Martinez F., Racca A., Rodríguez I., Polyzos N.P. Ovarian stimulation for oocyte donation: a systematic review and meta-analysis. Hum. Reprod. Update. 2021; 27(4): 673-96. https://dx.doi.org/10.1093/humupd/dmab008.
- Gardner D.K., Schoolcraft W.B. Culture and transfer of human blastocysts. Curr. Opin. Obstet. Gynecol. 1999; 11(3): 307-11. https://dx.doi.org/10.1097/00001703-199906000-00013.
- Богатырева Х.А., Мишиева Н.Г., Мартазанова Б.А., Лапина В.С., Абубакиров А.Н. Эффективность протоколов стимуляции функции яичников в различные фазы менструального цикла у пациенток со сниженным овариальным резервом. Акушерство и гинекология. 2017; 11: 78-83. [Bogatyreva Kh.A., Mishieva N.G., Martazanova B.A., Lapina V.S., Abubakirov A.N. Efficiency of ovarian stimulation protocols in different phases of menstrual cycle in patients with diminished ovarian reserve. Obstetrics and Gynecology. 2017; (11): 78-83. (in Russian)]. https://dx.doi.org/10.18565/aig.2017.11.78-83.
- Jochum F., Sananès N., Teletin M., Lichtblau I., Rongières C., Pirrello O. Luteal phase stimulation, the future of fertility preservation? Retrospective cohort study of luteal phase versus follicular phase stimulation. J. Gynecol. Obstet. Hum. Reprod. 2019; 48(2): 91-4. https://dx.doi.org/10.1016/j.jogoh.2018.11.003.
- Королькова А.И., Мишиева Н.Г., Мартазанова Б.А., Бурменская О.В., Веюкова М.А., Екимов А.Н., Трофимов Д.Ю., Абубакиров А.Н. Значимость копийности митохондриальной ДНК в клетках кумулуса пациенток позднего репродуктивного возраста. Акушерство и гинекология. 2019;10: 108-14. [Korolkova A.I., Mishieva N.G., Martazanova B.A., Burmenskaya O.V., Veyukova M.A., Ekimov A.N., Trofimov D.Yu., Abubakirov A.N. Implications of mitochondrial DNA copy number in cumulus cells in late reproductive-age women. Obstetrics and Gynecology. 2019; (10): 108-14. (in Russian)].https://dx.doi.org/10.18565/aig.2019.10.108-114.
- Kalra S.K., Ratcliffe S., Gracia C.R., Martino L., Coutifaris C., Barnhart K.T. Randomized controlled pilot trial of luteal phase recombinant FSH stimulation in poor responders. Reprod. Biomed. Online. 2008; 17(6): 745-50.https://dx.doi.org/10.1016/s1472-6483(10)60400-2.
- Королькова А.И., Мишиева Н.Г., Мартазанова Б.А., БурменскаяО.В., Екимов А.Н., Трофимов Д.Ю., Веюкова М.А., Кириллова А.О., Абубакиров А.Н. Повышение эффективности программ ЭКО на основании определения копийности митохондриальной ДНК в трофэктодерме эмбрионов. Акушерство и гинекология. 2019; 3: 98-104. [Korolkova A.I., Mishieva N.G., Martazanova B.A., Bourmenskaya O.V., Ekimov A.N., Trofimov D.Yu., Veyukova M.A., Kirillova A.O., Abubakirov A.N. Increasing the effectiveness of IVF programs by determining mitochondrial DNA copy number in embryonic trophectoderm. Obstetrics and Gynecology. 2019; (3): 98-104. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.3.98-104,
- Vaiarelli A., Cimadomo D., Alviggi E., Sansone A., Trabucco E., Dusi L. et al. The euploid blastocysts obtained after luteal phase stimulation show the same clinical, obstetric and perinatal outcomes as follicular phase stimulation-derived ones: a multicenter study. Hum. Reprod. 2020; 35(11): 2598-608.https://dx.doi.org/10.1093/humrep/deaa203.
- Королькова А.И., Мишиева Н.Г., Бурменская О.В., Екимов А.Н., Абубакиров А.Н., Богатырева Х.А. Современные методы селекции эмбрионов при проведении программ вспомогательных репродуктивных технологий. Акушерство и гинекология. 2018; 2: 13-8. [Korolkova A.I., Mishieva N.G., Burmenskaya O.V., Ekimov A.N., Abubakirov A.N., Bogatyreva Kh.A. Current embryo selection techniques in the implementation of assisted reproductive technology programs. Obstetrics and Gynecology. 2018; (2): 13-8. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.2.13-18.
Received 15.06.2023
Accepted 23.08.2023
About the Authors
Vera S. Lapina, Obstetrician-Gynecologist at the 1st Gynecology Department, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(909)920-23-05, v_lapina@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.Bella A. Martazanova, Ph.D., Senior Researcher at the 1st Gynecology Department, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
+7(967)123-88-24, dr.bella.ivf@gmail.com, 117997, Russia, Moscow, Ac. Oparina str., 4.
Evelina R. Durinyan, Ph.D., Senior Researcher at the 1st Gynecology Department, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
+7(916)612-99-30, evelina_durinyan@mail.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Tatiana S. Amyan, Ph.D., Junior Researcher of the 1st Gynecology Department, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(926)163-28-33, t_amyan@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Anna I. Korolkova, Ph.D., Researcher of the 1st Gynecology Department, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(915)322-08-79, korolkovaai@icloud.com, 117997, Russia, Moscow, Ac. Oparina str., 4.
Alla A. Gavisova, Ph.D., Head of the 1st Gynecology Department, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, +7(916)829-05-90,
a_gavisova@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Corresponding author: Vera S. Lapina, v_lapina@oparina4.ru



