Lipidome profile of follicular fluid in patients with ovarian endometrioma and its role in predicting IVF outcomes
Vardanyan V.A., Smolnikova V.Yu., Chagovets V.V., Makarova N.P., Kalinina E.A.
Relevance: Lipidome profile of follicular fluid (FF) in patients with ovarian endometrioma (OMA) offers valuable information about oocyte competence, embryo viability, and IVF outcomes.
Objective: To create a model for predicting the probability of clinical pregnancy in patients with OMA undergoing IVF based on the lipidome profile of follicular fluid.
Materials and methods: Patients baseline characteristics were collected, lipidome profile of FF and IVF outcomes in patients with OMA were analyzed (n=41). The lipidome signature of follicular fluid was analyzed using high-performance liquid chromatography coupled with mass spectrometry (HPLC-MS). Orthogonal projections to latent structures discriminant analysis (OPLS-DA) were used to create a differential model.
Results: Comparative analysis of FF samples from women who achieved pregnancy versus those with negative result revealed differences in lipidome signature within each group that correlated with IVF outcomes. Based on these findings, a predictive OPLS model was developed. The model allows to differentiate pregnant and non-pregnant women based on the levels of lipids belonging to the following classes: cholesteryl esters, phosphatidylcholines, sphingomyelins and triglycerides.
Conclusion: Lipidome profiling of follicular fluid using HPLC-MS allows for fairly accurate differentiation between women who achieve pregnancy and those who do not. These results are consistent with international data and confirm the potential for using this method to predict IVF outcomes in patients with ovarian endometriomas.
Authors' contributions: Smolnikova V.Yu., Chagovets V.V., Vardanyan V.A. – study concept and design; Vardanyan V.A., Chagovets V.V. – data collection and processing, statistical analysis, manuscript writing; Smolnikova V.Yu. – overall supervision of the clinical study and methodology, coordination of authors; Makarova N.P., Kalinina E.A. – editing.
Conflicts of interest: The authors declare no conflicts of interest.
Funding: The study had no sponsorship.
Ethical Approval: The study was approved by the local Ethics Committee of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Vardanyan V.A., Smolnikova V.Yu., Chagovets V.V., Makarova N.P., Kalinina E.A. Lipidome profile of follicular fluid in patients with ovarian endometrioma and its role in predicting IVF outcomes.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (10): 98-106 (in Russian)
https://dx.doi.org/10.18565/aig.2025.209
Keywords
External genital endometriosis (EGE) is a benign gynecological disease characterized by the growth of tissue outside the uterine cavity, similar in morphological and functional properties to the endometrium [1]. According to epidemiological studies, women with EGE have an increased risk of infertility, 2–4 times higher than the general population [2].
It is known that ovarian endometrioma (OMA) can have a negative impact on folliculogenesis, which leads to a decrease in oocyte quality and, consequently, a low fertilization rate and the production of a higher number of embryos of average and unsatisfactory quality [3]. However, the pathophysiological mechanisms that are behind this effect are poorly understood, which makes the search for minimally invasive biomarkers associated with oocyte quality relevant [4].
Follicular fluid (FF) provides the microenvironment for oocyte development and is an easily accessible biological substrate for research [5]. Changes in the composition of FF associated with pathological processes in the body disrupt the processes of oogenesis, specifying the ability of oocytes to fertilize, the subsequent development of the fertilized egg, as well as the viability of the embryo [6, 7].
The first publication appeared on the potential of metabolomics in the search for biomarkers of oocyte quality and embryo viability appeared in 2007 [8]. Since then, studies on the metabolomic profile of the fertilized fluid and blood serum of women with various gynecological diseases associated with infertility (EGE, polycystic ovary syndrome, premature ovarian failure) have been conducted [9, 10].
According to the results of a systematic review carried out by Adamyan L.V. et al., elevated levels of lactate, phospholipids and other lipids are observed in the follicular fluid of patients with OMAs [11]. It is assumed that the predominance of lipid metabolism together with OMA is a compensatory mechanism in response to mitochondrial dysfunction [12].
The aim of this study was to test a hypothesis about the influence of the lipid composition of the FF in the presence of ovarian endometrioma on the quality of oocytes, as well as to create a predictive model for the onset of pregnancy in patients with OMA based on the lipid profile of the follicular fluid.
Materials and methods
The study was conducted at the Department of Assisted Technologies in Infertility Treatment named after prof. B.V. Leonov, as well as in the Metabolomics and Bioinformatics Laboratory of the Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology of the Ministry of Health of Russia.
Patients were included in the study after obtaining written informed consent. The inclusion criteria: age 20–38 years; body mass index (BMI) ≤25 kg/m2; primary unilateral OMA of up to 3 cm verified on ultrasound scan.
The exclusion criteria included general contraindications according to the Order of the Ministry of Health of Russia No. 803n dated July 31, 2020 "On the procedure for using assisted reproductive technologies (ART), contraindications and restrictions to their use", as well as the presence of an ovarian endometrioma larger than 3 cm, suspicion of pelvic malignancy according to ultrasound examination, tumor markers CA 125, CA 19-9 levels above reference values; polycystic ovary syndrome; premature ovarian failure; endometrial pathology; interstitial and/or subserous uterine fibroids over 4 cm, submucous fibroids deforming the uterine cavity; genital malformations; ART program with donor oocytes; male or female karyotype abnormalities; marked male factor and a testicular biopsy to obtain sperm; BMI >30 kg/m2.
Ovarian stimulation was started in the early follicular phase with gonadotropins (recombinant or highly purified urinary preparations) administered daily. The starting gonadotropin dose was determined individually based on age, ovarian reserve, BMI, and medical history (if there was a history of ovarian stimulation). To prevent premature ovulation, a gonadotropin-releasing hormone antagonist was administered when the leading follicle reached a diameter of 13–14 mm. When the dominant follicle was 17–18 mm, a trigger for final oocyte maturation was administered (human chorionic gonadotropin (hCG) at a standard dose of 10,000 IU intramuscularly 35 hours before transvaginal ovarian puncture). From the day of puncture, therapy was prescribed to ensure adequate secretory transformation of the endometrium (dydrogesterone 60 mg/day orally or micronized progesterone 600 mcg/day vaginally). In all cases, a day 5 blastocyst embryo was transferred. Pregnancy was detected 12–14 days after embryo transfer based on the level of the β-hCG in the blood. Clinical pregnancy was diagnosed by visualizing the gestational sac in the uterine cavity 21 days after embryo transfer.
The groups’ allocation was carried out according to the criterion of "onset/absence of pregnancy." Group I (n=16) included patients with pregnancy, group II (n=25) – with a negative result.
Laboratory stage
Lipids were extracted from the follicular fluid on the day of transvaginal ovarian puncture using a modified Folch method [13]. The molecular composition of the samples was verified by high-performance liquid chromatography with mass spectrometric detection (HPLC-MS) on a Dionex UltiMate 3000 liquid chromatograph (Thermo Scientific, Germany) coupled to a Maxis Impact qTOF mass analyzer (Bruker Daltonics, Germany). Lipids were identified by precise mass and characteristic fragments.
Statistical analysis
Data collection, its subsequent correction, systematization of initial information, and visualization of the obtained results were carried out in Microsoft Office Excel (2016). Statistical processing of the results was performed using the Python programming language (v. 3.12, Python Software Foundation).
Quantitative indicators were assessed for normal distribution using the Shapiro–Wilk test. Testing for normality revealed that the study data were not normally distributed (with the exception of patient age). Therefore, further calculations were performed using nonparametric statistical methods.
In case of describing quantitative indicators with a distribution different from normal, the median was defined as the center of the distribution, and quartiles (Me [Q1; Q3]) were used as variation indicators. The Mann–Whitney U-test was used to compare two unrelated samples.
In case of describing quantitative indicators with a normal distribution, arithmetic means (M) and standard deviations (SD) were calculated. When comparing the means of two unrelated samples, Student's t-test was performed.
Qualitative results were expressed as absolute numbers with percentages (%). Categorial data in groups were compared using the Pearson χ2 test. In cases where the number of expected observations in any cell of the four-field table was less than 10, Fisher's exact test was used to assess the significance level of differences. Differences were considered statistically significant at p≤0.05, using a two-tailed p-value.
Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA) was conducted to classify patients according to pregnancy/non-pregnancy criteria [14]. Identified lipids were considered as independent variables in the models. Pregnancy/non-pregnancy served as the dependent variable. The quality of the developed models was assessed by constructing a receiver operating characteristic (ROC) curve, determining its area, sensitivity, and specificity. In addition, OPLS-DA was used to identify the lipids most significant for classification. This was accomplished through variable influence on projection (VIP) analysis. Lipids with VIP >1 were considered potential markers for pregnancy [15].
Results
All patients included in the study suffered minimal influence of external factors that could have affected the results. The medical history of the patients with ovarian cancer at the time of the IVF/ICSI program are presented in the table.

Given the data indicating changes in the lipid profile of the FF in patients of advanced reproductive age and with exogenous-constitutional obesity, the study included patients under 38 years with a BMI≤25 kg/m2. No significant differences were found in the age and BMI in both patient groups. The average age was 32.2 (2.1) years in patients who had become pregnant and 31.8 (3.9) years in patients with a negative pregnancy result.
Menstrual cycle parameters, age at menarche, age at first sexual intercourse, general and gynecological medical history, as well as serum hormone levels did not differ significantly between the study groups. According to the obstetric history, Group I included an equal number of patients with both primary and secondary infertility. Group II was dominated by women with primary infertility. The duration of infertility did not differ significantly between the groups. No significant differences were found in endometrioma size; on average, it was 16.5 mm in Group I and 19.0 mm in Group II.
Different ovarian stimulation protocols may affect the FF metabolomic profile, which could potentially significantly influence the results of the study. That is why all patients underwent ovarian stimulation with gonadotropin-releasing hormone antagonist [16]. When assessing the ovarian stimulation parameters, the average total dose of gonadotropins, the duration of ovarian stimulation, and the ovarian sensitivity index (the average number of obtained cumulus-oocyte complexes (COC) to the total dose of gonadotropins ratio × 1.000) did not differ significantly [17].
When analyzing the embryological stage of patients with pregnancy and patients with a negative treatment result in Group I, no significant differences were found in the number of COC obtained, mature (MII), “immature” and degenerative oocytes, as well as in the number of morulae and blastocysts. However, among patients who achieved pregnancy, the number of 2PN zygotes, fertilization rate, and total blastocyst count were statistically higher than in non-pregnant patients. The fertilization rate averaged 83.0% in Group I and 67.2% in Group II with in vitro fertilization (IVF) (p=0.001). When using intracytoplasmic sperm injection (ICSI) for fertilization, this indicator was 95.2% and 79.9%, respectively (p<0.001). In the ovarian stimulation cycle, clinical pregnancy at the end of the IVF treatment cycle occurred in 16/41 (39.02%) women with OMAs; in 25/41 (60.8%) patients, the treatment result was negative.
A total of 181 FF samples from 41 patients were selected for the study. The lipid fraction was extracted from the FF samples, and its composition was analyzed using HPLC-MS. The analysis identified 156 lipids. The obtained data on the lipid composition of the FF were subjected to orthogonal discriminant analysis using the partial least squares method (Orthogonal Partial Least Squares Discriminant Analysis, OPLS-DA) to identify differences between the study groups and assess the possibility of classifying samples according to the onset of pregnancy in these patients or their failure to conceive.

When constructing the OPLS model, FF lipid levels served as independent variables, and the dependent variable was the outcome of the ART program. Figure 1 shows the OPLS model scores constructed from the analysis of the FF lipidome in patients with primary OMA at the time of the IVF/ICSI program. Green dots correspond to FF samples from patients who achieved pregnancy, and red dots mean FF samples from patients with a negative pregnancy result. The resulting OPLS-DA model demonstrates a clear trend of separation between the FF lipid profile of pregnant and non-pregnant patients.

The results of the ROC analysis of the OPLS-DA model, constructed by analyzing the FF lipid levels in patients with OMA, are presented in Figure 2. The horizontal axis (X-axis) corresponds to the test specificity, and the vertical axis (Y-axis) to the test sensitivity. The area under the ROC curve (AUC) of this OPLS-DA reached 1, and the sensitivity and specificity of the model, calculated using the lipid profiles of the studied samples, were equal to 1, which is in line with the complete separation of the clusters corresponding to the study groups (Fig. 1).
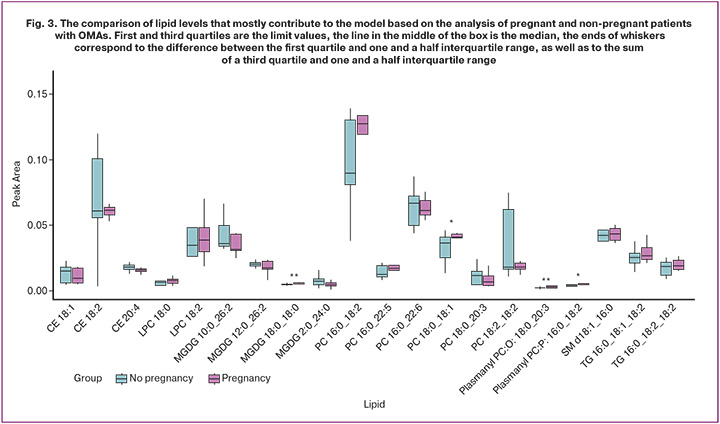
The lipid molecules that contributed most to the construction of these models were identified: cholesterol esters, which include oleic, linoleic and arachidonic fatty acids (CE 18:1, CE 18:2, CE 20:4), lysophosphatidylcholines (LPC 18:0, LPC 18:2), phosphatidylcholine, sphingomyelins, triglycerides and plasmalogens (Fig. 3).
Discussion
The study included patients with good ovarian reserve and a normal ovarian response to gonadotropin stimulation. Importantly, among patients who achieved pregnancy, there was a statistically significant increase in the number of normally fertilized oocytes and blastocysts produced. Therefore, higher fertilization and blastulation rates in this group, along with the FF lipid profile, may serve as clinically significant prognostic markers for pregnancy.
A previous study conducted at the Department of Assisted Technologies in Infertility Treatment named after prof. B.V. Leonov of Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology of the Ministry of Health of Russia, confirmed the hypothesis about the prognostic significance of the FF lipid profile and blood serum in the onset of pregnancy in patients with tubal-peritoneal factor of infertility [18]. The aim of our study was to develop a model that would predict the outcome of ART programs in patients with tubal-peritoneal factor of infertility.
Multivariate analysis revealed that the ratio of certain lipid levels significantly differed in patients who achieved pregnancy compared to those who did not. The study proposed a panel of metabolites that could be used to predict the outcome of ART programs in patients with OMAs at the time of IVF/ICSI. The most significant metabolites determining outcome in the study groups included the following lipids: cholesterol esters, phosphatidylcholines, sphingomyelins, and triglycerides.
A number of studies have shown that the FF metabolomic profile reflects the quality of the oocyte and its functional capabilities (competence), as well as the viability of the embryo developed as a result of fertilization and its ability to implant, which makes it possible to use this method to predict the outcomes of ART programs [7, 19].
It is known that the follicular fluid of patients with OMAs is characterized by a high content of glycerol, ketone bodies, as well as sphingolipids and phosphatidylcholines [20, 21].
Lipids maintain intrafollicular homeostasis and provide the oocyte with the energy necessary for subsequent meiotic divisions and embryonic cleavage. Furthermore, cholesterol and its esters are precursors of steroid hormones, which also play an important role in oogenesis [22].
According to the results of our study, TG 16:0_16:1_18:2_18:1 triglycerides and PC 16:0_20:3 phospholipids are of greatest importance for the onset of pregnancy in women with endometriomas, which is consistent with the results of other studies and confirms the important role of these lipids in the maturation of oocytes and ensuring adequate embryonic development at an early stage. The results of a study conducted by Zarezadeh R. et al. showed that phospholipids containing saturated fatty acids had a negative impact on oocyte maturation, while increased oleic acid level in triglycerides was associated with a higher number of mature oocytes. A positive effect of arachidonic acid in phospholipids on blastocyst formation rate was also found [23]. Sturmey R.G. et al. proposed the predictive role of phospholipids in blastocyst formation frequency. High polyunsaturated fatty acid in FF triglycerides positively correlated with the number of excellent and good quality blastocysts, while low polyunsaturated fatty acid content tended to reduce blastocyst formation frequency rate. Activation of beta-oxidation of fatty acids occurs at the stage of blastocyst formation, and a decrease in the efficiency of beta-oxidation of fatty acids leads to the arrest of embryo development [24].
It is important to note that studying the FF lipid profile is necessary not only to predict pregnancy in ART programs but also to improve the effectiveness of infertility treatment using IVF/ICSI. One of the challenges in this area is the creation of culture media enriched with essential energy substrates and cofactors, which will optimize embryo development in vitro.
In this study, the content of various phosphatidylcholines in the follicular fluid in patients with OMAs correlated with the outcome of the ART cycle: patients who achieved pregnancy had an increased content of PC 16:0_18:2 phosphatidylcholines, while patients with a negative pregnancy result had higher levels of PC 16:0_22:6. Dabaja M. et al. reported a negative impact of two molecules of phosphatidic acid, which is part of PA (35:6) and PA (37:7), on the outcomes of ART programs in women with endometriosis [9, 25].
Another class of lipids that correlated with ART cycle outcome in our study are plasmalogens. According to Zhang M. et al., plasmalogen levels in follicular fluid positively correlate with oocyte quality and their fertilization potential. Plasmalogens are known to be involved in a number of pathophysiological processes observed in OMAs, including hypoxia, inflammation, oxidative stress, and ferroptosis. Plasmalogens have been shown to be a substrate for lipid peroxidation, a process underlying granulosa cell ferroptosis in OMAs. It is believed that decreased plasmalogen levels in the FF reflect granulosa cell apoptosis, which confirms the importance of this class of lipids for assessing oocyte quality [26, 27].
The study results demonstrated the high significance of sphingomyelins in predicting pregnancy. Sphingomyelins act as signaling molecules, participate in the regulation of folliculogenesis and oogenesis, and facilitate intercellular communication in the cumulus-oocyte complex. Furthermore, sphingomyelins play a protective role under conditions of oxidative stress, which is necessary for maintaining oocyte/embryo viability [28].
Currently, in clinical practice, preimplantation embryo quality assessment is based on morphological and morphokinetic parameters, which have limited predictive value for pregnancy. The development of the FF metabolites panel will allow for additional assessment of embryo viability.
Understanding molecular mechanisms that underlie unsatisfactory ART outcomes in patients with OMAs will enable a personalized approach to the medical care of patients with OMAs up to 3 cm and repeated IVF failures (embryo arrest and the production of a large number of unsatisfactory quality embryos). Further research in this area may support the rationale for surgical treatment of OMAs in such women patients.
Conclusion
HPLC-MS analysis of the FF lipid profile revealed significant differences in its composition in patients with and without pregnancy. These results indicate that lipids play an important role in the follicular microenvironment, providing an optimal environment for oocyte maturation. Studying the lipid profile of follicular fluid will help understand the mechanisms underlying impaired oocyte competence in ovarian endometriomas.
Validation of the proposed lipid panel on a larger cohort of patients will enable to use this method in the future to select the most viable embryo in patients with OMAs.
References
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Эндометриоз. 2024. [Ministry of Health of the Russian Federation. Clinical guidelines. Endometriosis. 2024. (in Russian)].
- Leone U., Chiappa V., Ceccaroni M., Roviglione G., Savelli L., Ferrero S. et al. Epidemiology of infertility in women with endometriosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2024; 92: 102454. https://dx.doi.org/10.1016/j.bpobgyn.2023.102454
- Casalechi M., Di Stefano G., Fornelli G., Somigliana E., Viganò P. Impact of endometriosis on the ovarian follicles. Best Pract. Res. Clin. Obstet. Gynaecol. 2024; 92: 102430. https://dx.doi.org/10.1016/j.bpobgyn.2023.102430
- Giacomini E., Sanchez A., Sarais V., Beitawi S., Candiani M., Viganò P. Characteristics of follicular fluid in ovaries with endometriomas. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017; 209: 34-8. https://dx.doi.org/10.1016/j.ejogrb.2016.01.032
- Brinca A.T., Peiró A.M., Evangelio P.M., Eleno I., Oliani A.H., Silva V. et al. Follicular fluid and blood monitorization of infertility biomarkers in women with endometriosis. Int. J. Mol. Sci. 2024; 25(13): 7177. https://dx.doi.org/10.3390/ijms25137177
- Fiscus J., Fraison É., Renault L., Salle B., Panthu B., Labrune E. Metabolic signature of follicular fluid to understand infertility-related diseases: a narrative review. Reprod. Biomed. Online. 2024; 48(6): 103762. https://dx.doi.org/10.1016/j.rbmo.2023.103762
- Wallace M., Cottell E., Gibney M.J., McAuliffe F.M., Wingfield M., Brennan L. An investigation into the relationship between the metabolic profile of follicular fluid, oocyte developmental potential, and implantation outcome. Fertil. Steril. 2012; 97(5): 1078-84.e1-8. https://dx.doi.org/10.1016/j.fertnstert.2012.01.122
- Singh R., Sinclair K.D. Metabolomics: approaches to assessing oocyte and embryo quality. Theriogenology. 2007; 68 Suppl. 1: S56-62. https://dx.doi.org/10.1016/j.theriogenology.2007.04.007
- Dabaja M.Z., Santos A.A., Christofolini D.M., Barbosa C.P., de Oliveira D.N., de Oliveira A.N. et al. Comparative metabolomic profiling of women undergoing in vitro fertilization procedures reveals potential infertility-related biomarkers in follicular fluid. Sci. Rep. 2022; 12(1): 20531. https://dx.doi.org/10.1038/s41598-022-24775-5
- Zhang Y., He C., He Y., Zhu Z. Follicular fluid metabolomics: tool for predicting IVF outcomes of different infertility causes. Reprod. Sci. 2025; 32(4): 921-34. https://dx.doi.org/10.1007/s43032-024-01664-y
- Adamyan L., Pivazyan L., Zarova E., Avetisyan J., Laevskaya A., Sarkisova A. et al. Metabolomic biomarkers of endometriosis: a systematic review. Journal of Endometriosis and Uterine Disorders. 2024; 7: 100077. https://dx.doi.org/10.1016/j.jeud.2024.100077
- Kobayashi H., Imanaka S. Recent progress in metabolomics for analyzing common infertility conditions that affect ovarian function. Reprod. Med. Biol. 2024; 23(1): e12609. https://dx.doi.org/10.1002/rmb2.12609
- Folch J., Lees M., Sloane Stanley G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957; 226(1): 497-509.
- Trygg J., Wold S. Orthogonal projections to latent structures (O-PLS). J. Chemom. 2002; 16: 119-28. https://dx.doi.org/10.1002/cem.695
- Wold S., Sjöström M., Eriksson L. PLS-regression: a basic tool of chemometrics. Chemometrics and Intelligent Laboratory Systems. 2001; 58(2): 109-30. https://dx.doi.org/10.1016/S0169-7439(01)00155-1
- Guo H., Zhu Q., Gao H., Lyu Q., Chai W., Wu L. et al. Metabolomics analysis of follicular fluid in ovarian endometriosis women receiving progestin-primed ovary stimulation protocol for in vitro fertilization. Sci. Rep. 2023; 13(1): 5747. https://dx.doi.org/10.1038/s41598-023-32797-w
- Revelli A., Gennarelli G., Biasoni V., Chiadò A., Carosso A., Evangelista F. et al. The ovarian sensitivity index (OSI) significantly correlates with ovarian reserve biomarkers, is more predictive of clinical pregnancy than the total number of oocytes, and is consistent in consecutive IVF cycles. J. Clin. Med. 2020; 9(6): 1914. https://dx.doi.org/10.3390/jcm9061914
- Фортыгина Ю.А., Макарова Н.П., Драпкина Ю.С., Новоселова А.В., Чаговец В.В., Франкевич В.Е., Калинина Е.А. Сравнительный анализ липидного профиля крови и фолликулярной жидкости женщин, проходящих лечение бесплодия методами вспомогательных репродуктивных технологий. Акушерство и гинекология. 2024; 4: 93-102. [Fortygina Yu.A., Makarova N.P., Drapkina Yu.S., Novoselova A.V., Gamisonia A.M., Chagovets V.V., Frankevich V.E., Kalinina E.A. Comparative analysis of blood and follicular fluid lipid profiles in women undergoing infertility treatment with assisted reproductive technologies. Obstetrics and Gynecology. 2024; (4): 93-102 (in Russian)]. https://dx.doi.org/10.18565/aig.2024.62
- Jiang Y.C., Che Q., Lu X., Liu M., Ye Y., Cao X. et al. Follicular fluid and plasma lipidome profiling and associations towards embryonic development outcomes during ART treatment. Front. Endocrinol. (Lausanne). 2024; 15: 1464171. https://dx.doi.org/10.3389/fendo.2024.1464171
- Vouk K., Hevir N., Ribič-Pucelj M., Haarpaintner G., Scherb H., Osredkar J. et al. Discovery of phosphatidylcholines and sphingomyelins as biomarkers for ovarian endometriosis. Hum. Reprod. 2012; 27(10): 2955-65. https://dx.doi.org/10.1093/humrep/des152
- Pocate-Cheriet K., Santulli P., Kateb F., Bourdon M., Maignien C., Batteux F. et al. The follicular fluid metabolome differs according to the endometriosis phenotype. Reprod. Biomed. Online. 2020; 41(6): 1023-37. https://dx.doi.org/10.1016/j.rbmo.2020.09.002
- Liu T., Qu J., Tian M., Yang R., Song X., Li R. et al. Lipid metabolic process involved in oocyte maturation during folliculogenesis. Front. Cell. Dev. Biol. 2022; 10: 806890. https://dx.doi.org/10.3389/fcell.2022.806890
- Zarezadeh R., Nouri M., Hamdi K., Shaaker M., Mehdizadeh A., Darabi M. Fatty acids of follicular fluid phospholipids and triglycerides display distinct association with IVF outcomes. Reprod. Biomed. Online. 2021; 42(2): 301-9. https://dx.doi.org/10.1016/j.rbmo.2020.09.024
- Sturmey R.G., Reis A., Leese H.J., McEvoy T.G. Role of fatty acids in energy provision during oocyte maturation and early embryo development. Reprod. Domest. Anim. 2009; 44 Suppl. 3: 50-8. https://dx.doi.org/10.1111/j.1439-0531.2009.01402.x
- Turkoglu O., Zeb A., Graham S., Szyperski T., Szender J.B., Odunsi K. et al. Metabolomics of biomarker discovery in ovarian cancer: a systematic review of the current literature. Metabolomics. 2016; 12(4): 60. https://dx.doi.org/10.1007/s11306-016-0990-0
- Zhang M., Wang Y., Di J., Zhang X., Liu Y., Zhang Y. et al. High coverage of targeted lipidomics revealed lipid changes in the follicular fluid of patients with insulin-resistant polycystic ovary syndrome and a positive correlation between plasmalogens and oocyte quality. Front. Endocrinol. (Lausanne). 2024; 15: 1414289. https://dx.doi.org/10.3389/fendo.2024.1414289
- Zhang Y., Liu X., Deng M., Xu C., Zhang Y., Wu D. et al. Ferroptosis induced by iron overload promotes fibrosis in ovarian endometriosis and is related to subpopulations of endometrial stromal cells. Front. Pharmacol. 2022; 13: 930614. https://dx.doi.org/10.3389/fphar.2022.930614
- Turathum B., Gao E.-M., Grataitong K., Liu Y.-B., Wang L., Dai X. et al. Dysregulated sphingolipid metabolism and autophagy in granulosa cells of women with endometriosis. Front. Endocrinol. (Lausanne). 2022; 13: 906570. https://dx.doi.org/10.3389/fendo.2022.906570
Received 06.08.2025
Accepted 13.10.2025
About the Authors
Victoria A. Vardanyan, PhD student, Department of IVF named after Prof. B.V. Leonov, Academician V.I. Kulakov National Medical Research Center for Obstertrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, +7(903)299-60-77, vvictoria5@yandex.ru,https://orcid.org/0000-0001-9057-1736
Veronika Yu. Smolnikova, Dr. Med. Sci., Leading Researcher, Department of IVF named after Prof. B.V. Leonov, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, v_smolnikova@oparina4.ru,
https://orcid.org/0000-0002-8025-4849
Vitaly V. Chagovets, PhD (in Physics and Mathematics), Head of the Laboratory of Metabolomics and Bioinformatics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, vvchagovets@gmail.com,
https://orcid.org/0000-0002-5120-376X
Natalya P. Makarova, Dr. Med. Sci., Leading Researcher, Department of IVF named after Prof. B.V. Leonov, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4., makarova@oparina4.ru,
https://orcid.org/0000-0003-1396-7272
Elena A. Kalinina, Dr. Med. Sci., Professor, Head of the IVF Department named after Prof. B.V. Leonov, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, e_kalinina@oparina4.ru,
https://orcid.org/0000-0002-8922-2878



