Prediction of fetal growth restriction using machine learning algorithms
Kan N.E., Leonova A.A., Tyutyunnik V.L., Soldatova E.E., Ryzhova K.O., Serebriakova A.P.
Objective: To investigate the significant clinical and anamnestic predictors of fetal growth restriction (FGR) and develop effective predictive models using machine learning methods (MLM).
Materials and methods: This retrospective study included 620 pregnant women who were observed and delivered at the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. The study group comprised 300 patients with FGR, while the control group included 320 patients with healthy pregnancies. An analysis of the clinical and anamnestic data was conducted to build MLM models, including logistic regression and random forest.
Results: The logistic regression model identified the following predictors: age over 40 years, height less than 1.60 m, chronic arterial hypertension, smoking, a history of FGR, and threatened miscarriage in the first trimester with the formation of retrochorial hematoma and bleeding. This model predicts the development of FGR with a sensitivity of 73% and specificity of 80% (AUC 0.81). An alternative model constructed using random forest demonstrated an increased sensitivity of 78% and a decreased specificity of 74% (AUC 0.79). Within the random forest framework, the most significant contributors to the accuracy of the prognosis were age over 40 years, height less than 1.60 m, chronic arterial hypertension, a history of surgery resulting in a uterine scar, a history of FGR, and threatened miscarriage in the first trimester with retrochorial hematoma without bleeding.
Conclusion: Both models exhibited high predictive value for screening for FGR. Logistic regression offers interpretability, whereas random forest enhances the accuracy by accounting for nonlinear relationships. Implementing these models in clinical practice will optimize the monitoring of pregnant women at risk.
Authors’ contributions: Kan N.E., Leonova A.A., Tyutyunnik V.L., Soldatova E.E., Ryzhova K.O., Serebriakova A.P. – conception and design of the study, obtaining data for analysis, review of publications, processing and analysis of material on the topic, drafting of the manuscript, editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: The study was conducted within the framework of the initiative project «Epigenetic Criteria for Diagnosing Fetal Growth Delay from the Perspective of Neurogenesis Dysfunction» (Research Project No. 19-И23 dated December 8, 2022) (Registration number in the EGISU NIOKTR system (state accounting) – 123060500032-8).
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Kan N.E., Leonova A.A., Tyutyunnik V.L., Soldatova E.E., Ryzhova K.O., Serebriakova A.P.
Prediction of fetal growth restriction using machine learning algorithms.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (7): 40-46 (in Russian)
https://dx.doi.org/10.18565/aig.2025.135
Keywords
Fetal growth restriction remains one of the most significant challenges in modern obstetrics, associated with a high risk of perinatal complications [1–6] and long-term adverse consequences [2, 7–10]. Despite advances in prenatal diagnostics, current screening methods exhibit limited effectiveness owing to the complex, multifactorial pathogenesis of this pregnancy complication [11–14]. Therefore, it is critical to develop accurate prognostic tools. Modern machine learning technologies present new opportunities to address this issue by enabling the analysis of complex relationships between clinical, anamnestic, and instrumental parameters [15–18].
The use of artificial intelligence algorithms to predict fetal growth restriction is particularly promising because these algorithms can uncover hidden patterns in large heterogeneous datasets. Unlike traditional statistical approaches, machine learning methods such as random forest can accommodate nonlinear interactions between predictors and automatically identify the most significant combinations of risk factors [19–21].
The random forest method offers significant advantages for predictive tasks because of its resistance to overfitting, achieved through two key mechanisms: bagging (bootstrap aggregating) and random feature selection at each tree node [15, 20]. Bagging enhances the model robustness by aggregating predictions from multiple individual trees trained on different versions of the original dataset [20]. A notable advantage of this algorithm is its ability to automatically assess feature significance, facilitating the identification of key prognostic factors among numerous potential variables, without the need for additional analytical methods. Furthermore, the random forest method exhibits high stability when handling incomplete data and various types of variables, including categorical and numerical features. This significantly simplifies the data preprocessing stage compared with other machine-learning algorithms. These characteristics contribute to the widespread use of the random forest method in applied research, where model interpretability, robustness to changes in input data, and minimal pre-processing requirements are essential [19, 21].
The application of modern computational technologies enables the creation of a tool that can be integrated into routine clinical practice to provide personalized risk assessments for each pregnant woman. This advancement opens avenues for timely preventive measures, optimization of pregnancy management, and consequently, improvements in perinatal outcomes.
This study aimed to investigate the significant clinical and anamnestic predictors of fetal growth restriction and develop effective predictive models using machine learning methods.
Materials and methods
A retrospective analysis of clinical and medical history characteristics was conducted on 620 pregnant women who were under observation at the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, from 2017 to 2022. The study group comprised 300 patients with an antenatally confirmed diagnosis of fetal growth restriction, as defined by the international Delphi criteria [22] and current clinical guidelines. The control group included 320 women who had a healthy pregnancy. All study participants met the following inclusion criteria: age 18–45 years, singleton pregnancy without chromosomal abnormalities or congenital malformations in the fetus, absence of severe extragenital pathology, and pregnancy resulting from assisted reproductive technologies.
To objectively verify the diagnosis in the postnatal period, all newborns underwent a comprehensive assessment of anthropometric indicators using standardized centile curves from the INTERGROWTH-21. This facilitated the final formation of the study groups: 300 children with confirmed growth restriction and 320 children whose measurements corresponded to their gestational age based on mass-height indicators [23].
Machine learning methods, specifically logistic regression and random forest, were used to analyze clinical and anamnestic data and build predictive models. The models were trained on the provided sample using the scikit-learn library in Python. Following training, their effectiveness was evaluated using standard metrics, such as sensitivity, specificity, and area under the ROC curve (AUC-ROC).
The study adhered to ethical standards, and approval was obtained from the local ethics committee. Inclusion in the study was conducted after the women provided informed voluntary consent.
Statistical analysis
Statistical analysis of the data was performed using modern methods that consider the distribution characteristics of the studied indicators. The normality of the distribution of quantitative variables was assessed using the Shapiro–Wilk test for small samples (fewer than 50 observations) and the Kolmogorov–Smirnov test for larger samples. In cases of deviation from a normal distribution, quantitative data were presented as medians with interquartile ranges, providing a more accurate description of the central tendency and value spread. Categorical variables were analyzed by indicating both absolute values and their percentage contributions to the total sample, allowing for a comprehensive understanding of the structure of the studied features.
Comparative analyses between groups were conducted using the non-parametric Mann–Whitney U test for quantitative indicators that deviated from a normal distribution, ensuring the reliability of the results when working with data of different natures. For qualitative variables, the Pearson χ² test was employed when a sufficient number of expected observations was present, whereas Fisher's exact test was used for cases with a small number of expected values, guaranteeing the correctness of statistical conclusions regardless of subgroup size. The effect size of qualitative variables was estimated by calculating the odds ratio (OR) and constructing a 95% confidence interval (CI). To process contingency tables with zero values, a special Haldane–Anscombe correction was applied to enhance the accuracy of the estimates. In all instances, differences were considered statistically significant at a level of p<0.05, which is in line with the generally accepted standards of evidence-based medicine.
Results
During the study, a comprehensive assessment of the demographic and anthropometric parameters of the pregnant women was conducted. An analysis of somatic diseases and obstetric and gynecological histories was performed, as well as an analysis of the features of the course of gestation. The table presents the clinical and anamnestic characteristics and features of the course of pregnancy in the study groups.
As shown in Table 1, women over 40 were statistically significantly more likely to be in the study group (OR=2.15; 95% CI: 1.48–3.12; p<0.001). Additionally, women in the fetal growth restriction group were significantly more likely to be short (less than 1.60 m) (OR=3.07; 95% CI: 1.90–4.96; p<0.001) and have a body weight of up to 52 kg at the time of pregnancy (OR=1.66; 95% CI: 1.12–2.47; p=0.012).
Smoking (OR=4.53; 95% CI: 1.84–11.15; p<0.001), chronic arterial hypertension (OR=7.11; 95% CI: 2.96–17.07; p<0.001), the presence of intrauterine adhesions (OR=6.25; 95% CI: 1.36–28.68; p=0.01), history of uterine surgeries leading to the formation of a scar on its wall (OR=3.45; 95% CI: 2.28–5.22; p<0.001), fetal growth restriction (OR=8.12; 95% CI: 2.42–27.24; p<0.001), and preeclampsia (OR=27.78; 95% CI: 1.64–470.93; p<0.001) were also significantly more common in the study group.
Statistical analysis revealed significant differences in the prevalence of chronic inflammatory diseases between groups. The study group showed a significantly lower frequency of chronic cervicitis (OR=0.53; 95% CI: 0.38–0.73; p<0.001), salpingo-oophoritis (OR=0.47; 95% CI: 0.23–0.98; p=0.039), and tonsillitis (OR=0.65; 95% CI: 0.43–0.99; p=0.049) than the control group. The most pronounced inverse relationship was noted for chronic sinusitis (OR=0.13; 95% CI: 0.03–0.59; p=0.001).
In a comparative analysis of complications of this pregnancy, significant differences were established in mild or moderate hyperemesis gravidarum, which was significantly more common in the control group (OR=0.68; 95% CI: 0.49–0.95; p=0.028); threatened miscarriage with the formation of a retrochorial hematoma without bleeding was significantly more common in the study group (p<0.001), while threatened miscarriage with the formation of a hematoma and bleeding in the control group (OR=0.31; 95% CI: 0.19–0.50; p<0.001).
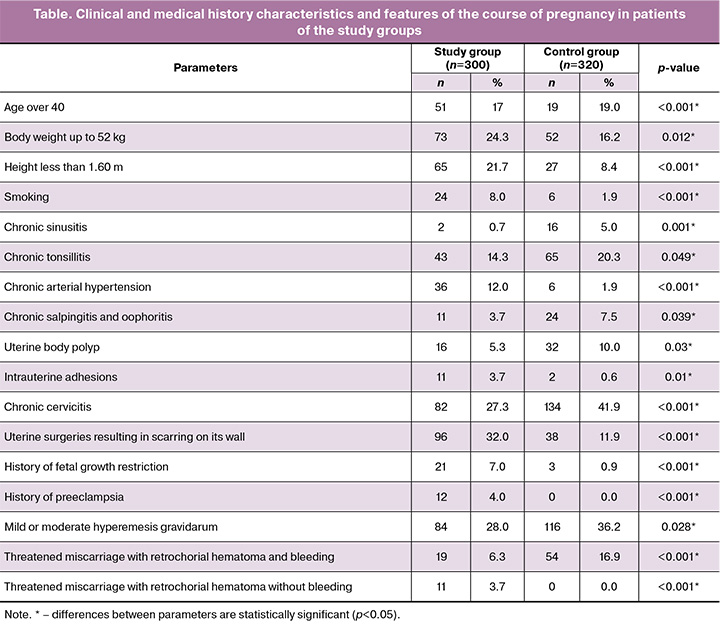
Based on a comprehensive analysis of clinical and anamnestic data using the logistic regression, six key predictors were selected: age over 40 years; height below 1.6 m; chronic arterial hypertension; smoking; fetal growth restriction in the history and threatened miscarriage with the formation of a retrochorial hematoma and bleeding. The probability of developing fetal growth restriction was determined using the following formula:
P=1/(1+e-z)
Z=-0.0002 + (0.0396×X1) + (0.0531×X2) + (0.0484×X3) + (0.0485×X4) + (0.0388×X5) + (-0.0310×X6),
where e is the base of the natural logarithm, and has a value of 2.71828182845904.
X1 – age >40 years;
X2 – height <1.60 m;
X3 – chronic arterial hypertension;
X4 – smoking;
X5 – history of fetal growth restriction;
X6 – threatened miscarriage with the presence of a retrochorial hematoma and bleeding.
The resulting prognostic model for the development of fetal growth restriction demonstrated good discriminatory ability (AUC=0.81; 95% CI: 0.75–0.87) (Fig. 1). At an optimal threshold value of 0.5, the model showed balanced diagnostic performance: sensitivity – 73%; specificity – 80%. A narrow CI confirmed the reliability of the results.
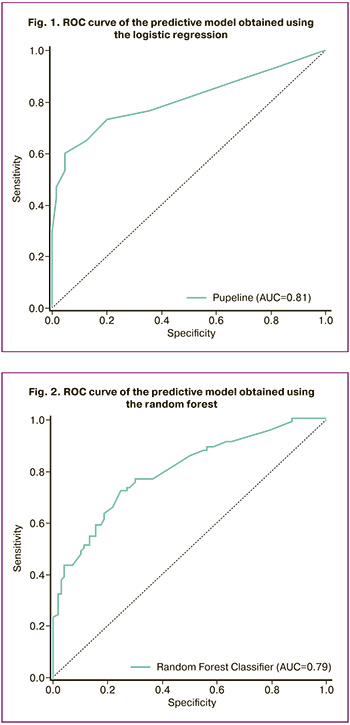
To verify the model and consider complex nonlinear relationships between variables, the random forest method was applied. The resulting machine learning model showed a comparable prognostic value (AUC=0.79), while maintaining a clinically significant sensitivity of 78% and specificity of 74% at a threshold value of 0.5 (Fig. 2). However, the identified risk factors for the development of fetal growth restriction differed from those identified using the logistic regression method. The most significant predictors of the development of fetal growth restriction when using the random forest method: age over 40 years; height below 1.60 m; chronic arterial hypertension; uterine surgeries leading to the formation of a scar on its wall; fetal growth restriction in the history and threatened miscarriage with the formation of a retrochorial hematoma without bleeding.
Discussion
The study findings revealed significant differences in the clinical and anamnestic characteristics of the pregnant women across the study groups. These data demonstrate that fetal growth restriction is associated with a complex of maternal factors, including age-related features, anthropometric data, and concomitant somatic diseases [24–27]. Women over 40 years of age were significantly more prevalent in the ctudy group (OR=3.25; 95% CI: 1.85–5.69). These findings align with contemporary concepts regarding the age-associated risk of placental insufficiency [24]. Additionally, the identified anthropometric features – short stature (<1.60 m) and underweight (<52 kg) – may indicate constitutional risk factors for the development of placental dysfunction [27].
An analysis of the prevalence of somatic diseases revealed a significant correlation between chronic arterial hypertension and the development of fetal growth restriction, underscoring the critical role of vascular disorders in the pathogenesis of this condition [25].
The study results indicate that threatened miscarriage with retrochorial hematoma formation, regardless of the presence of bleeding, is a significant predictor of fetal growth restriction. Retrochorial hematoma involves impaired placentation and damage to the chorionic vessels, leading to decreased placental perfusion and the development of chronic fetal hypoxia, ultimately resulting in fetal growth restriction [28]. The absence of external signs of bleeding in retrochorial hematoma may suggest subclinical placental insufficiency, explaining its detection primarily through methods that account for nonlinear relationships, such as the random forest method.
The machine learning techniques employed in this study (logistic regression and random forest methods) demonstrated comparable predictive values (AUC 0.81 and 0.79, respectively) with different sets of significant predictors. Both methods confirmed the importance of factors such as age > 40 years, short stature (<1.60 m), chronic arterial hypertension, and a history of fetal growth restriction. However, the random forest method also emphasized additional indicators, including a history of uterine surgery resulting in a scar on the uterine wall and complications during the first trimester of pregnancy, specifically threatened miscarriage with retrochorial hematoma without bleeding. These results highlight the value of combining both methods to develop a more comprehensive predictive model, where logistic regression offers clear clinical interpretation, whereas the random forest method identifies less obvious yet potentially significant predictors.
Conclusion
The increase in sensitivity (78% versus 73%) accompanied by a decrease in specificity (74% versus 80%) in the random forest model compared to the logistic regression model reflects fundamental differences in the training strategies of these algorithms. From a clinical perspective, this balance of indicators may be preferable for screening tasks where minimizing the number of missed cases of pathology is critical, even at the expense of increasing false-positive results.
A comprehensive analysis of clinical and anamnestic factors, combined with modern machine learning methods, can significantly enhance the accuracy of predicting fetal growth restriction and optimize the management of pregnant women at risk. Further studies will not only validate the presented models but will also help develop strategies for preventing this complication of pregnancy.
References
- Lei T.Y., Li D.Z. Perinatal outcome of late-onset fetal growth restriction: etiology matters. Ultrasound Obstet. Gynecol. 2022; 60(5): 707-8. https://dx.doi.org/10.1002/uog.26090
- Bahia M.L.R., Velarde G.C., Silva F.C.D., Araujo Júnior E., Sá R.A.M. Adverse perinatal outcomes in fetuses with severe late-onset fetal growth restriction. J. Matern. Fetal Neonatal Med. 2022; 35(25): 8666-72. https:/dx.doi.org/10.1080/14767058.2021.1995858
- Wu B.A., Chand K.K., Bell A., Miller S.L., Colditz P.B., Malhotra A. et al. Effects of fetal growth restriction on the perinatal neurovascular unit and possible treatment targets. Pediatr. Res. 2024; 95(1): 59-69. https:/dx.doi.org/10.1038/s41390-023-02805-w
- Muniz C.S., Dias B.F., Motoyama P.V.P., Almeida C.T.C., Feitosa F.E.L., Araujo Júnior E. et al. Doppler abnormalities and perinatal outcomes in pregnant women with early-onset fetal growth restriction. J. Matern. Fetal Neonatal Med. 2022; 35(25): 7276-79. https:/dx.doi.org/10.1080/14767058.2021.1946786
- Dall'Asta A., Stampalija T., Mecacci F., Zegarra R.R., Sorrentino S., Minopoli M. et al. Incidence, clinical features and perinatal outcome in anomalous fetuses with late-onset growth restriction: cohort study. Ultrasound Obstet. Gynecol. 2022; 60(5): 632-39. https:/dx.doi.org/10.1002/uog.24961
- Rizzo G., Mappa I., Bitsadze V., Słodki M., Khizroeva J., Makatsariya A. et al. Role of Doppler ultrasound at time of diagnosis of late-onset fetal growth restriction in predicting adverse perinatal outcome: prospective cohort study. Ultrasound Obstet. Gynecol. 2020; 55(6): 793-8. https:/dx.doi.org/10.1002/uog.20406
- Misgina K.H., Levine L., Boezen H.M., Bezabih A.M., van der Beek E.M., Groen H. Influence of perinatal distress on adverse birth outcomes: a prospective study in the Tigray region, Northern Ethiopia. PLOS One. 2023; 18(7): e0287686. https:/dx.doi.org/10.1371/journal.pone.0287686
- Sacchi C., Marino C., Nosarti C., Vieno A., Visentin S., Simonelli A. Association of intrauterine growth restriction and small for gestational age status with childhood cognitive outcomes: a systematic review and meta-analysis. JAMA Pediatr. 2020; 174(8): 772-81. https:/dx.doi.org/10.1001/jamapediatrics.2020.1097
- Sacchi C., O'Muircheartaigh J., Batalle D., Counsell S.J., Simonelli A., Cesano M. et al. Neurodevelopmental outcomes following intrauterine growth restriction and very preterm birth. J. Pediatr. 2021; 238: 135-44. https:/dx.doi.org/10.1016/j.jpeds.2021.07.002
- Fernandez-Rodriguez B., Alba C. de, Galindo A., Recio D., Villalain C., Pallas C.R. et al. Obstetric and pediatric growth charts for the detection of late-onset fetal growth restriction and neonatal adverse outcomes. J. Perinat. Med. 2020; 49(2): 216-24. https:/dx.doi.org/10.1515/jpm-2020-0210
- Kingdom J., Ashwal E., Lausman A., Liauw J., Soliman N., Figueiro-Filho E. et al. Guideline No. 442: Fetal growth restriction: screening, diagnosis, and management in singleton pregnancies. J. Obstet. Gynaecol. Can. 2023; 45(10): 102154. https:/dx.doi.org/10.1016/j.jogc.2023.05.022
- Melamed N., Baschat A., Yinon Y., Athanasiadis A., Mecacci F., Figueras F. et al. FIGO (international Federation of Gynecology and obstetrics) initiative on fetal growth: best practice advice for screening, diagnosis, and management of fetal growth restriction. Int. J. Gynaecol. Obstet. 2021; 152 Suppl. 1(Suppl. 1): 3-57. https:/dx.doi.org/10.1002/ijgo.13522
- Miranda J., Rodriguez-Lopez M., Triunfo S., Sairanen M., Kouru H., Parra-Saavedra M. et al. Prediction of fetal growth restriction using estimated fetal weight vs a combined screening model in the third trimester. Ultrasound Obstet. Gynecol. 2017; 50(5): 603-11. https:/dx.doi.org/10.1002/uog.17393
- Волочаева М.В., Тимофеева А.В., Федоров И.С., Кан Н.Е., Тютюнник В.Л., Рыжова К.О., Гасымова Ш.Р. Модель диагностики задержки роста плода с использованием функциональных методов исследования. Акушерство и гинекология. 2025; 2: 31-9. [Volochaeva M.V., Timofeeva A.V., Fedorov I.S., Kan N.E., Tyutyunnik V.L., Ryzhova K.O., Gasymova Sh.R. A model for diagnosing fetal growth restriction using functional diagnostic methods. Obstetrics and Gynecology. 2025; (2): 31-9 (in Russian)]. https://dx.doi.org/10.18565/aig.2025.15
- Zimmerman R.M., Hernandez E.J., Yandell M., Tristani-Firouzi M., Silver R.M., Grobman W. et al. AI-based analysis of fetal growth restriction in a prospective obstetric cohort quantifies compound risks for perinatal morbidity and mortality and identifies previously unrecognized high risk clinical scenarios. BMC Pregnancy Childbirth. 2025; 25(1): 80. https:/dx.doi.org/10.1186/s12884-024-07095-6
- Ulusoy C.O., Kurt A., Seyhanli Z., Hizli B., Bucak M., Agaoglu R.T. et al. Role of inflammatory markers and doppler parameters in late-onset fetal growth restriction: a machine-learning approach. Am. J. Reprod. Immunol. 2024; 92(4): e70004. https:/dx.doi.org/10.1111/aji.70004
- Rescinito R., Ratti M., Payedimarri A.B., Panella M. Prediction models for intrauterine growth restriction using artificial intelligence and machine learning: a systematic review and meta-analysis. Healthcare (Basel). 2023; 11(11): 1617. https:/dx.doi.org/10.3390/healthcare11111617
- Pierucci U.M., Tonni G., Pelizzo G., Paraboschi I., Werner H., Ruano R. Artificial intelligence in fetal growth restriction management: a narrative review. J. Clin. Ultrasound. 2025; 53(4): 825-31. https:/dx.doi.org/10.1002/jcu.23918
- Lee S.U., Choi S.K., Jo Y.S., Wie J.H., Shin J.E., Kim Y.H. et al. Prediction model of late fetal growth restriction with machine learning algorithms. Life (Basel). 2024; 14(11): 1521. https:/dx.doi.org/10.3390/life14111521
- Ornaghi S., Caricati A., Di Martino D.D., Mossa M., Di Nicola S., Invernizzi F. et al. Non-invasive maternal hemodynamic assessment to classify high-risk pregnancies complicated by fetal growth restriction. Front. Clin. Diabetes Healthc. 2022; 3: 851971. https:/dx.doi.org/10.3389/fcdhc.2022.851971
- Huo J., Li G., Li Ch., Li X., Liu G., Chen Q. et al. Screening for late-onset fetal growth restriction in antepartum fetal monitoring using Deep Forest and SHAP. In: Cao B.Y., Wang S.F., Nasseri H., Zhong Y.B., eds. Intelligent Systems and Computing. ICFIE 2022. Lecture Notes on Data Engineering and Communications Technologies, vol. 207. Springer, Singapore; 2024. https:/dx.doi.org/10.1007/978-981-97-2891-6_29
- Gordijn S.J., Beune I.M., Thilaganathan B., Papageorghiou A., Baschat A.A., Baker P.N. et al. Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet. Gynecol. 2016; 48(3): 333-9. https:/dx.doi.org/10.1002/uog.15884
- Рюмина И.И., Байбарина Е.Н., Нароган М.В., Маркелова М.М., Орловская И.В., Зубков В.В., Дегтярев Д.Н. Использование международных стандартов роста для оценки физического развития новорожденных и недоношенных детей. Неонатология: новости, мнения, обучение. 2023; 11(2): 48-52. [Ryumina I.I., Baibarina E.N., Narogan M.V., Markelova M.M., Orlovskaya I.V., Zubkov V.V., Degtyarev D.N. Using international growth standards to assess the physical development of newborns and premature infants. Neonatology: News, Opinions, Training. 2023; 11(2): 48-52 (in Russian)]. https:/dx.doi.org/10.33029/2308-2402-2023-11-2-48-52
- Zhang P., Haymar T., Al-Sayyed F., Dygulski S., Dygulska B., Devi A. et al. Placental pathology associated with maternal age and maternal obesity in singleton pregnancy. J. Matern. Fetal Neonatal Med. 2022; 35(25): 9517-26. https:/dx.doi.org/10.1080/14767058.2022.2044777
- Vasapollo B., Novelli G.P., Farsetti D., Valensise H. Maternal peripheral vascular resistance at mid gestation in chronic hypertension as a predictor of fetal growth restriction. J. Matern. Fetal Neonatal Med. 2022; 35(25): 9834-36. https:/dx.doi.org/10.1080/14767058.2022.2056443
- Dall'Asta A., Minopoli M., Zegarra R.R., Di Pasquo E., Ghi T. An update on maternal cardiac hemodynamics in fetal growth restriction and pre-eclampsia. J. Clin. Ultrasound. 2023; 51(2): 265-72. https:/dx.doi.org/10.1002/jcu.23392
- Yang L., Feng L., Huang L., Li X., Qiu W., Yang K. et al. Maternal factors for intrauterine growth retardation: systematic review and meta-analysis of observational studies. Reprod. Sci. 2023; 30(6): 1737-45. https:/dx.doi.org/10.1007/s43032-021-00756-3
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Недостаточный рост плода, требующий предоставления медицинской помощи матери (задержка роста плода). М.; 2022. 71 с. [Ministry of health of the Russian Federation. Clinical guidelines. Insufficient growth of the fetus, requiring the provision of medical care to the mother (fetal growth retardation). Moscow; 2022. 71 p. (in Russian)].
Received 19.05.2025
Accepted 17.06.2025
About the Authors
Natalia E. Kan, Professor, Dr. Med. Sci., Honored Scientist of the Russian Federation, Deputy Director for Research – Director of the Institute of Obstetrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4,kan-med@mail.ru. Researcher ID: B-2370-2015, SPIN: 5378-8437, Authors ID: 624900, Scopus Author ID: 57008835600, https://orcid.org/0000-0001-5087-5946
Anastasia A. Leonova, PhD student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, +7(937)453-54-27, nastena27-03@mail.ru, https://orcid.org/0000-0001-6707-3464
Victor L. Tyutyunnik, Professor, Dr. Med. Sci., Leading Researcher at the Center for Scientific and Clinical Research, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, tioutiounnik@mail.ru,
Researcher ID: B-2364-2015, SPIN: 1963-1359, Authors ID: 213217, Scopus Author ID: 56190621500, https://orcid.org/0000-0002-5830-5099
Ekaterina E. Soldatova, Researcher at the Obstetric Department of the Institute of Obstetrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, katerina.soldatova95@bk.ru, https://orcid.org/0000-0001-6463-3403
Kristina O. Ryzhova, Resident, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia,
117997, Russia, Moscow, Ac. Oparina str., 4, cr.yanina@gmail.com, https://orcid.org/0009-0007-8318-435X
Anna P. Serebriakova, obstetrician-gynecologist at the Day Hospital Department, Primorsky Regional Perinatal Center, 690042, Russia, Vladivostok, Mozhayskaya st., 1B, serebriakovanna@gmail.com, https://orcid.org/0000-0001-7014-2627
Corresponding author: Anastasia A. Leonova, nastena27-03@mail.ru



