Postnatal development in children with growth restriction (follow-up study)
Volochaeva M.V., Kan N.E., Tyutyunnik V.L., Leonova A.A., Soldatova E.E., Ryzhova K.O.
Objective: To investigate the characteristics of postnatal development in children with growth restriction.
Materials and methods: This retrospective cohort study included 124 pregnant women, with all newborns assessed for mass-growth parameters using INTERGROWTH-21 centile curves postnatally. The study group comprised 76 children with growth restriction, whereas the control group included 48 healthy newborns. A comprehensive assessment of their health status was conducted during hospitalization in neonatal intensive care units and after discharge.
Results: The children in both groups were comparable in terms of delivery time, Apgar score, and follow-up duration. At birth, children in the study group had significantly lower anthropometric indicators (birth weight, length, and head circumference) (p<0.001). Additionally, the study group's children were 2.9 times less likely to have harmonious development than the comparison group (p=0.031). This may indicate that the risk of disharmonious development persists despite normalization of basic birth weight and length parameters. Data have been obtained confirming the association between antenatal growth restriction and an increased risk of disorders of the central nervous system in children. Children in the study groups had a high incidence of delayed psychomotor development (p<0.05; OR= 0.27, 95% CI 0,01–0.84) and were 12 times more likely to experience impaired speech development. Additionally, children in the study group were 2.9 times less likely to exhibit harmonious development than those in the comparison group (p=0.031). This suggests that the risk of disharmonious development persists despite normalization of the basic weight and height parameters. The data confirmed an association between antenatal growth restriction and an increased risk of central nervous system disorders in children. The study group displayed a high incidence of delayed psychomotor development (p<0.05; OR=0.27, 95% CI 0.01–0.84), and was 12 times more likely to experience impaired speech development.
Conclusion: Fetal growth restriction can have long-term effects on neurocognitive development in children. This underscores the need for early monitoring and rehabilitation programs for children born with stunted growth as well as further research to clarify the role of prenatal factors in speech disorders.
Authors' contributions: Volochaeva M.V., Kan N.E., Tyutyunnik V.L., Leonova A.A., Soldatova E.E., Ryzhova K.O. – conception and design of the study, acquisition of data for analysis, review of literature, preparation and analysis of material on the topic, statistical analysis, drafting of the manuscript, editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: The study was conducted within the framework of the initiative project "Epigenetic criteria for diagnosis of fetal growth restriction from the perspective of neurogenesis dysfunction" (research project No. 19-И23 dated 8 December 2022) (registration number in the EGISU NIOKTR system (state accounting) – 123060500032-8).
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Volochaeva M.V., Kan N.E., Tyutyunnik V.L., Leonova A.A., Soldatova E.E., Ryzhova K.O.
Postnatal development in children with growth restriction (follow-up study).
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (3): 65-71 (in Russian)
https://dx.doi.org/10.18565/aig.2025.32
Keywords
The relevance of the problem concerning the relationship between growth restriction at birth and the risk of developing pathologies in adulthood is underscored by the global increase in the incidence of non-communicable diseases (NCDs), which, according to the WHO, account for 74% of deaths worldwide [1]. Epidemiological studies indicate that one in five children in developing countries is born with growth restriction or a body weight of less than 2500 g, leading to significant long-term socioeconomic consequences. The concept of Developmental Origins of Health and Disease (DOHaD) postulates that fetal growth restriction causes irreversible changes during critical periods of early development (intrauterine, neonatal, and early childhood), which results in long-term health alterations due to epigenetic mechanisms, such as modifications in DNA structure (methylation/demethylation) and changes in microRNA regulation. These processes impact the expression of genes that regulate glucose metabolism, endothelial function, and neuronal development, contributing to the onset of obesity, type 2 diabetes, hypertension, and neurodegenerative diseases in adulthood [2–4]. Accumulated data reveal that children with growth restriction have a two-fold higher risk of developing cerebral palsy, cognitive impairment, and autism spectrum disorders [5]. The risk of developing coronary heart disease increases by 40% (OR=1.4, 95% CI 1.2–1.6), and the volume of gray matter in the prefrontal cortex decreases by 8–12%, which is associated with impaired cognitive development later in life [3, 6]. It is important to note that children born with growth restriction, even those who achieve normalization of weight and height indicators ("catch-up growth"), are rarely included in neurocognitive monitoring programs. This oversight often leads to delayed diagnosis of various developmental delays and, consequently, untimely prevention and correction of these complications. The insufficient effectiveness of antenatal diagnosis of fetal growth restriction, which, according to Relph et al. [7], is only 65%, along with the lack of therapeutic options for this pregnancy complication and absence of standardized postnatal monitoring approaches for affected children, emphasizes the need for further research.
This study aimed to investigate the characteristics of postnatal development in children with growth restriction.
Materials and methods
A retrospective cohort study was conducted, including 124 pregnant women with an antenatally verified diagnosis of "fetal growth restriction" (n=76) and women with physiological pregnancies (n=48), all observed and delivered at the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology, and Perinatology of the Ministry of Health of Russia. The inclusion criteria were as follows: singleton pregnancy, maternal age between 18 and 45 years, absence of severe somatic and gynecological pathology, and absence of chromosomal pathology and/or congenital malformations in the fetus. The diagnosis of "fetal growth restriction" was made according to the Delphi criteria [8] and the clinical guidelines of the Ministry of Health of Russia titled "Insufficient fetal growth requiring medical care to the mother (fetal growth restriction)" [9]. Exclusion criteria included multiple pregnancies, chromosomal pathology, congenital malformations of the fetus, severe extragenital pathology, and pregnancies resulting from assisted reproductive technologies.
Postnatally, the weight and height indicators of newborns (n=124) were assessed using INTERGROWTH-21 centile curves [10, 11], allowing for confirmation of the diagnosis of "growth restriction," identification of newborns corresponding to gestational age based on weight and height characteristics, and formation of the main group consisting of 76 children with growth restriction, along with a comparison group of 48 children without growth restriction. All children included in the study underwent follow-up observation after discharge from the Scientific and Pediatric Advisory Department. A comprehensive health assessment was conducted during hospitalization in the neonatal department and after discharge. This study was conducted in accordance with ethical standards, and approval was obtained from the local ethics committee. The women were enrolled in the study after signing an informed consent form.
Statistical analysis
The distribution of continuous variables was tested for normality using the Shapiro–Wilk test (for samples with fewer than 50 observations) or the Kolmogorov–Smirnov test (for samples with more than 50 observations). For numerical variables that were not normally distributed, the results were reported as median (Me) and interquartile range [Q1; Q3]. Categorical variables are described using counts and percentages. Comparisons of two independent groups for continuous variables with a non-normal distribution were conducted using the non-parametric Mann–Whitney U-test. To analyze categorical data in contingency tables, Pearson's chi-square test was used for expected frequencies ≥10 and Fisher's exact test was applied for expected frequencies <10. The odds ratio (OR) with 95% confidence interval (95% CI) was calculated as a quantitative measure of the effect when comparing relative indicators. In cases where zero values were present in the contingency table, the Haldane-Anscombe correction was used to calculate the odds ratio. The odds ratio (OR) with a 95% confidence interval (95% CI) was calculated as a quantitative measure of the effect of the relative indicators. In cases where zero values were present in the contingency table, Haldane–Anscombe correction was employed to calculate the odds ratio. Statistical significance was set at p<0.05.
Results
The results of the comprehensive analysis of the somatic and gynecological history and the characteristics of the course of pregnancy are presented in Table 1.
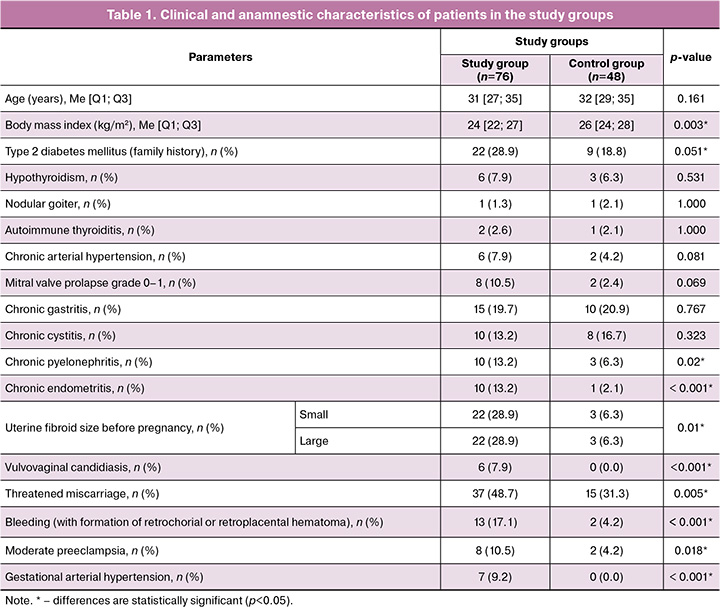
According to the analysis, both in the study group and in the control group, all children were comparable in key parameters: gestational age at delivery (in the group with fetal growth restriction, the median gestational age at delivery was 249.5 [229; 265] days, in the control group it was 245 [224; 260] days; p=0.345); the Apgar score at the 1st (p=0.822) and 5th minutes (p=0.838), as well as the duration of observation after discharge (in the group with fetal growth restriction: 14 [9; 26] months, in the control group–15 [11; 26] months; p=0.959). At birth, children with fetal growth restriction were characterized by significantly lower anthropometric indicators. In the study group, the median birth weight corresponded to the 3rd [3; 5] percentile, in the control group it was 14th [10; 15] percentile (p<0.001). The median birth length in children with growth restriction was 8th [2; 30] percentile, in the control group was 41st [17; 56] percentile (p<0.001), and the median head circumference in the study group was 30th percentile [9; 51], in the control group, 64 [51; 85] percentile (p<0.001). However, upon further observation, the differences in physical development leveled out, and the median body weight in the group with growth restriction was 9220 [7715; 11806] g, in the control group was 9815 [8000; 10300] g (p=0.872), and the median height was 78 [69; 85] cm and 82 [71; 83] cm, respectively (p=0.801). At the same time, children with growth restriction were 2.9 times less likely to have average harmonious development (p=0.031; OR=2.9, 95% CI 1.1–5.7).
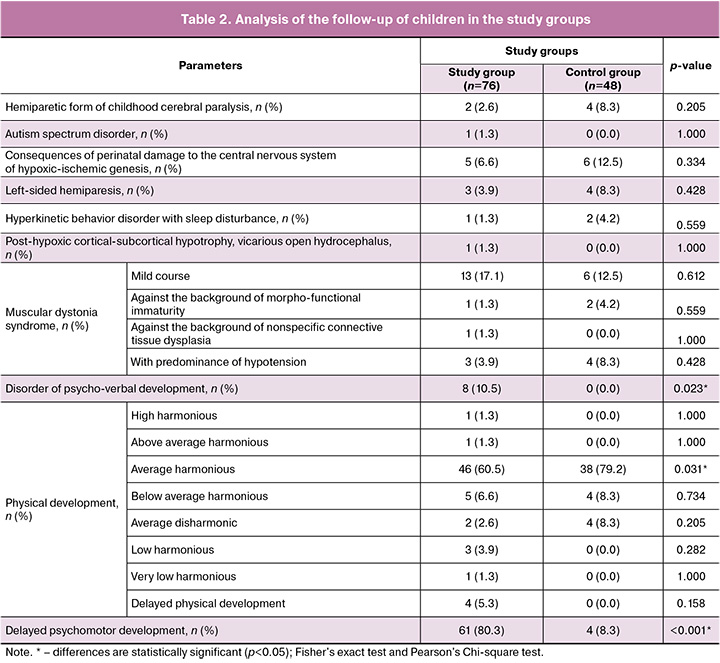
The assessment of the health status of the children in both groups was comparable. In 32.9% (25/76) of observations in the growth restriction group and 33.3% (16/48) in the control group, children were assessed by pediatricians as “healthy” (p=0.960), without significant differences in the frequency of central nervous system pathologies, neurological disorders, and eye diseases. However, the analysis showed that children from the growth restriction group had a high frequency of psychomotor developmental delay, which occurred 44.7 times more often than in the control group (p<0.001; OR=0.02, 95% CI 0.007–0.07). Also, children in the study group were 12.04 times more likely to have a psychomotor development disorder (p=0.023), but the odds ratio did not reach statistical significance (OR=0.083; 95% CI 0.005–1.474), which may be due to the limited sample size (Table 2).
Discussion
The leading cause of fetal growth restriction is disruption of placentation mechanisms, which results in chronic hypoxia and nutrient deficiency. This process disrupts energy metabolism in the fetal brain, leading to neuronal apoptosis in critical areas such as the hippocampus, cortex, and basal ganglia, which are essential for memory formation, motor control, and emotional regulation [12, 13]. Additionally, it affects neurogenesis and neuronal migration, resulting in structural abnormalities in the motor cortex, cerebellum, and language areas (Broca and Wernicke) [14]. Deficiencies in glucose, amino acids, and fatty acids exacerbate these issues by disrupting neurotransmitter synthesis (including dopamine and serotonin) and myelination of nerve fibers, which slows impulse transmission and causes an imbalance in the regulation of the excitation and inhibition pathways [15]. Hypoxia also increases oxidative stress, leading to the generation of free radicals that damage neuronal membranes and DNA structures [14]. Stem cells, particularly neuronal precursors, are especially vulnerable, and their depletion limits the regenerative potential of the brain tissue. Magnetic resonance imaging of the brains of children with growth restriction revealed structural changes, including a reduced volume of gray matter in the prefrontal cortex and thalamus, which correlates with cognitive deficits as well as thinning of the white matter in pathways such as the corticospinal tract and arcuate fasciculus, disrupting the integration of motor and speech functions [16, 17]. The timing of exposure to hypoxia is also critical. During the second and third trimesters, when synaptogenesis and neural network formation are active, oxygen deficiency reduces synapse density in the sensorimotor and associative areas and disorganizes connections between subcortical structures and the cortex [18, 19].
The study established that, despite "catch-up growth" restoring mass and growth indicators in the group with growth restriction after birth, functional defects in the brain persist. This explains the delayed manifestation of disorders; deficiencies become noticeable with increasing cognitive demands, such as acquisition of speech or complex motor skills. While partial compensation is possible owing to brain plasticity, the redistribution of functions can lead to an “overload” of specific areas.
Clinically, these mechanisms manifest as psychomotor and psycho-verbal delays, findings that were confirmed in the study and aligned with data from Sacchi et al. [20]. Psychomotor disorders linked to dysfunction in the motor cortex, cerebellum, and basal ganglia include delayed skill acquisition (e.g., crawling and walking), dyspraxia, and impaired coordination. Psycho-verbal disorders resulting from damage to the temporal lobes and angular gyrus are characterized by difficulties in understanding speech, limited vocabulary, and delayed formation of phrasal speech [16]. Despite the significance of these findings, the study has several methodological limitations, including the absence of standardized tools for assessing psychomotor and speech development (such as the Bayley-III or Griffiths scales), which may limit the objectivity of the identified disorders, reducing the comparability of results with international studies and the reproducibility of methods. Additionally, the short follow-up period complicates the assessment of the long-term consequences of fetal growth restriction, such as school maladjustment, specific learning difficulties, and the emergence of emotional and behavioral disorders, which often manifest at older ages. Therefore, further studies with a prospective design and extended follow-up periods (covering school age and adolescence) are essential, utilizing validated developmental scales (Bayley, Griffiths, and ADOS) for a quantitative assessment of motor, speech, and social functions.
Therefore, intrauterine hypoxia in fetal growth restriction leads to multilevel disorders that affect both the brain structure and functional networks, necessitating early diagnosis and comprehensive rehabilitation to minimize long-term consequences. We believe that the findings justify a revision of management approaches for children with growth restriction, shifting the focus from anthropometric monitoring to early diagnosis and correction of cognitive and speech deficiencies. Coordination between clinical practice, scientific research, and social policy is essential for optimizing the health trajectories of future generations.
Conclusion
The study findings confirmed that fetal growth restriction, despite compensation for weight and height indicators during follow-up, has a long-term impact on neurocognitive development. This underscores the need for early monitoring and rehabilitation programs for children born with growth restriction as well as for studies with larger samples to clarify the role of prenatal factors in the development of psychomotor disorders.
References
- United Nations Children’s Fund (UNICEF), World Health Organization (WHO). UNICEF-WHO Low birthweight estimates: Levels and trends 2000–2015. Geneva: World Health Organization; 2019 Licence: CC BY-NC-SA 3.0 IGO.
- Lapehn S., Paquette A.G. The rlacental epigenome as a molecular link between prenatal exposures and fetal health outcomes through the DOHaD Hypothesis. Curr. Environ. Health Reports. 2022; 9(3): 490-501. https://dx.doi.org/10.1007/s40572-022-00354-8.
- Кан Н.Е., Леонова А.А., Тютюнник В.Л., Хачатрян З.В. Особенности нейрогенеза при задержке роста плода. Акушерство и гинекология. 2022; 11: 24-30. [Kan N.E., Leonova A.A., Tyutyunnik V.L., Khachatryan Z.V. Features of neurogenesis in fetal growth restriction. Obstetrics and Gynecology. 2022; (11): 24-30. (in Russian)]. https://dx.doi.org/10.18565/aig.11.24-30.
- Солдатова Е.Е., Кан Н.Е., Тютюнник В.Л., Волочаева М.В. Задержка роста плода с позиции фетального программирования. Акушерство и гинекология. 2022; 8: 5-10. [Soldatova E.E, Kan N.E, Tyutyunnik V.L, Volochaeva M.V. Fetal growth retardation from the perspective of fetal programming. Obstetrics and Gynecology. 2022; (8): 5-10. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.8.5-10.
- Sacchi C., Marino C., Nosarti C., Vieno A., Visentin S., Simonelli A. Association of intrauterine growth restriction and small for gestational age status with childhood cognitive outcomes. JAMA Pediatr. 2020; 174(8): 772. https://dx.doi.org/10.1001/jamapediatrics.2020.1097.
- Crispi F., Miranda J., Gratacós E. Long-term cardiovascular consequences of fetal growth restriction: biology, clinical implications, and opportunities for prevention of adult disease. Am. J. Obstet. Gynecol. 2018; 218(2S): 869-79. https://dx.doi.org/10.016/j.ajog.2017.12.012.
- Relph S., Vieira M.C., Copas A., Alagna A., Page L., Winsloe C. et al.; DESiGN Trial Team and DESiGN Collaborative Group. Characteristics associated with antenatally unidentified small-for-gestational-age fetuses: prospective cohort study nested within DESiGN randomized controlled trial. Ultrasound Obstet. Gynecol. 2023; 61(3): 356-66. https://dx.doi.org/10.1002/uog.26091.
- Gordijn S.J., Beune I.M., Thilaganathan B., Papageorghiou A., Baschat A.A., Baker P.N. et al. Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet. Gynecol. 2016; 48(3): 333-9. https://dx.doi.org/10.1002/uog.15884.
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Недостаточный рост плода, требующий предоставления медицинской помощи матери (задержка роста плода). М.; 2022. 71 с. [Ministry of Health of the Russian Federation. Clinical guidelines. Insufficient growth of the fetus, requiring the provision of medical care to the mother (fetal growth retardation). Moscow; 2022. 71 p. (in Russian)].
- Leite D.F.B., de Melo E.F J.r., Souza R.T., Kenny L.C., Cecatti J.G. Fetal and neonatal growth restriction: new criteria, renew challenges. J. Pediatr. 2018; 203: 462-3. https://dx.doi.org/10.1016/j.jpeds.2018.07.094.
- Рюмина И.И., Байбарина Е.Н., Нароган М.В., Маркелова М.М., Орловская И.В., Зубков В.В., Дегтярев Д.Н. Использование международных стандартов роста для оценки физического развития новорожденных и недоношенных детей. Неонатология: новости, мнения, обучение. 2023; 11(2): 48-52. [Ryumina I.I., Baibarina E.N., Narogan M.V., Markelova M.M., Orlovskaya I.V., Zubkov V.V., Degtyarev D.N. Using international growth standards to assess the physical development of newborns and premature infants. Neonatology: News, Opinions, Training. 2023; 11(2): 48-52. (in Russian)].
- Gilchrist C., Cumberland A., Walker D., Tolcos M. Intrauterine growth restriction and development of the hippocampus: implications for learning and memory in children and adolescents. Lancet Child Adolesc. Health. 2018; 2(10): 7764. https://dx.doi.org/10.1016/S2352-4642(18)30245-1.
- Sacchi C., De Carli P., Mento G., Farroni T., Visentin S., Simonelli A. Socio-Emotional and Cognitive Development in Intrauterine Growth Restricted (IUGR) and Typical Development Infants: Early Interactive Patterns and Underlying Neural Correlates. Rationale and Methods of the Study. Front. Behav. Neurosci. 2018; 12: 315. https://dx.doi.org/10.3389/fnbeh.2018.00315.
- Murray D.M. Biomarkers in neonatal hypoxic-ischemic encephalopathy-Review of the literature to date and future directions for research. Handb. Clin. Neurol. 2019; 162: 281-93. https://dx.doi.org/10.1016/B978-0-444-64029-1.00013-8.
- Oke S.L., Hardy D.B. The role of cellular stress in intrauterine growth restriction and postnatal dysmetabolism. Int. J. Mol. Sci. 2021; 22(13): 6986. https://dx.doi.org/10.3390/ijms22136986.
- Miller S.L., Huppi P.S., Mallard C. The consequences of fetal growth restriction on brain structure and neurodevelopmental outcome. J. Physiol. 2016; 594(4): 807-23. https://dx.doi.org/10.1113/JP271402.
- Shah D.K., Ponnusamy V., Evanson J., Kapellou O., Ekitzidou G., Gupta N. et al. Raised plasma neurofilament light protein levels are associated with abnormal MRI outcomes in newborns undergoing therapeutic hypothermia. Front. Neurol. 2018; 9: 86. https://dx.doi.org/10.3389/fneur.2018.00086.
- Zhang Y.J. Recent research on the influence of intrauterine growth restriction on the structure and function of the nervous system. Zhongguo Dang Dai Er Ke Za Zhi. 2021; 23(11): 1184-9. https://dx.doi.org/10.7499/j.issn.1008-8830.2108044.
- Dudink I., Hüppi P.S., Sizonenko S.V., Castillo-Melendez M., Sutherland A.E., Allison B.J., Miller S.L. Altered trajectory of neurodevelopment associated with fetal growth restriction. Exp. Neurol. 2022; 347: 113885. https://dx.doi.org/10.1016/j.expneurol.2021.113885.
- Sacchi C., O'Muircheartaigh J., Batalle D., Counsell S.J., Simonelli A., Cesano M. et al. Neurodevelopmental outcomes following intrauterine growth restriction and wery preterm birth. J. Pediatr. 2021; 238: 135-144.e10. https://dx.doi.org/10.1016/j.jpeds.2021.07.002.
Received 13.02.2025
Accepted 03.03.2025
About the Authors
Maria V. Volochaeva, PhD, Senior Researcher at the Institute of Obstetrics, Physician at 1 Maternity Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, +7(919)968-72-98, volochaeva.m@yandex.ru,https://orcid.org/0000-0001-8953-7952
Natalia E. Kan, Professor, Dr. Med. Sci., Deputy Director for Research – Director of the Institute of Obstetrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, kan-med@mail.ru, Researcher ID: B-2370-2015,
SPIN: 5378-8437, Authors ID: 624900, Scopus Author ID: 57008835600, https://orcid.org/0000-0001-5087-5946
Victor L. Tyutyunnik, Professor, Dr. Med. Sci., Leading Researcher at the Center for Scientific and Clinical Research, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, tioutiounnik@mail.ru,
Researcher ID: B-2364-2015, SPIN: 1963-1359, Authors ID: 213217, Scopus Author ID: 56190621500, https://orcid.org/0000-0002-5830-5099
Anastasia A. Leonova, PhD student, Physician at Оbstetric Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, nastena27-03@mail.ru, https://orcid.org/0000-0001-6707-3464
Ekaterina E. Soldatova, PhD, Physician at Оbstetric Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, katerina.soldatova95@bk.ru, https://orcid.org/0000-0001-6463-3403
Kristina O. Ryzhova, resident, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia,
117997, Russia, Moscow, Ac. Oparin str., 4, cr.yanina@gmail.com, https://orcid.org/0009-0007-8318-435X
Corresponding author: Maria V. Volochaeva, volochaeva.m@yandex.ru



