Markers for fetal growth restriction based on the study of the polymorphism of gene regulatory regions
Gasymova Sh.R., Tyutyunnik V.L., Kan N.E., Donnikov A.E., Borisova A.G.
Objective: To study variants of gene regulatory regions and identify potential markers of fetal growth restriction.
Materials and methods: This study involved the genetic analysis of polymorphic loci of genes associated with thrombophilia, prothrombotic states, folate cycle enzyme disorders, matrix metalloproteinases and their tissue inhibitors, interleukin-1 and-10, as well as laminin and estrogen receptor genes (F2, F5, F7, F13, FGB, ITGA2, ITGB3, PAI-1, MTHFR, MTR, MTRR, MMP9, MMP2, TIMP2, TIMP3, IL10, IL1B, LAMC1, and ESR1) in 110 pregnant women with fetal growth restriction and 272 control patients (without fetal growth restriction). Polymerase chain reaction was used to genotype single nucleotide polymorphisms. The functional effects of polymorphic loci were assessed using HaploReg (for epigenetic effects) and GTEx Portal (for association with gene expression) software.
Results: In the group with fetal growth restriction, the T allele of the polymorphic locus -1296 C>T [rs5749511] of TIMP3 was found to be significantly associated (OR=3.14 (95% CI 1.08–9.13), p=0.03), as was the G allele of the polymorphic locus MTR: 2756 A>G (Asp919Gly) (OR=2.18 (95% CI 1.15–4.13), p=0.02) compared to the control group. A rare allele of the F7 polymorphic locus, 10976 G>A (Arg353Gln), was found to be protective against the development of fetal growth restriction (OR=0.46 (95% CI 0.21–1.01), p=0.05). In the group with fetal growth restriction, accumulation of the rare G allele of the MTRR polymorphic locus: 66 A>G (Ile22Met) was observed; however, the differences with the control group did not reach statistical significance. A statistically significant association was found between the 4G variant of the -675 5G>4G polymorphic locus of the SERPINE1 (PAI-1) gene and early onset fetal growth restriction.
Conclusion: The results of this study suggest that polymorphisms in the regulatory regions of TIMP3, MTR, MTRR, and PAI-1 can be considered potential markers of the risk of fetal growth restriction.
Authors' contributions: Gasymova Sh.R., Tyutyunnik V.L., Kan N.E., Donnikov A.E., Borisova A.G. – conception and design of the study, acquisition of data for analysis, collecting publications, material processing and analysis, statistical analysis, drafting and editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Gasymova Sh.R., Tyutyunnik V.L., Kan N.E., Donnikov A.E., Borisova A.G.
Markers for fetal growth restriction based on the study of the polymorphism of gene regulatory regions.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (11): 76-82 (in Russian)
https://dx.doi.org/10.18565/aig.2024.162
Keywords
Fetal growth restriction (FGR) is one of the most serious complications of pregnancy [1]. FGR is diagnosed when the estimated fetal weight (EFW) and/or abdominal circumference (AC) growth rate falls below the 10th percentile, accompanied by abnormal blood flow on Doppler ultrasound, or when the EFW and/or AC values are below the 3rd percentile [2]. In modern obstetrics, FGR is recognized as a leading cause of morbidity in newborns [3–5]. The causes of FGR are generally classified into four categories: maternal, placental, fetal, and genetic [2]. Although these causes arise from different pathophysiological mechanisms, they all result in compromised uteroplacental blood flow. Advances in diagnostic methods have enhanced our understanding of the genetic components of FGR, relating to both fetal and maternal genetics. Variations in genes, such as endothelin 1 (EDN1) and tumor necrosis factor α (TNF-α), have been identified as significant contributors to FGR development [6]. The search for genetic predictors of FGR is ongoing, underscoring the relevance of this study [7].
This study aimed to study variants of gene regulatory regions and identify potential markers of fetal growth restriction.
Materials and methods
The study was conducted at the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. A total of 110 pregnant women with FGR (study group) were examined and divided into two subgroups: 1A with early onset FGR (22–316 weeks of gestation, n=34) and 1 B with late-onset FGR (32–40 weeks of gestation, n=76). The study also included 272 patients without FGR who had physiological pregnancy and served as the control group. All study participants underwent genetic analysis of polymorphic loci of genes associated with thrombophilic and prothrombotic states, disorders of folate cycle enzymes, matrix metalloproteinases, and their tissue inhibitors, as well as genes for interleukin-1 and -10, laminin, and the estrogen receptor. The study was reviewed and approved by the Research Ethics Committee of V.I. Kulakov NMRC for OG&P.
The study included pregnant women aged 18 to 45 years, with one fetus at 22–40 weeks of gestation, who provided informed consent to participate. Pregnant women with decompensated extragenital pathology, fetal malformations, or acute infectious diseases were excluded from the study.
The diagnosis of FGR was established based on the approved criteria outlined in the current clinical guidelines "Insufficient Fetal Growth Requiring Medical Care for the Mother (Fetal Growth Restriction)." The criteria included a slowing of the growth of EFW and/or AC <10th percentile in combination with pathological blood flow parameters according to ultrasound Doppler data or EFW and/or AC values below the 3rd percentile [2].
Analysis of polymorphic loci of genes F2, F5, F7, F13A1, FGB, ITGA2, ITGB3, SERPINE1 (PAI-1), MTHFR, MTR, MTRR, MMP9, MMP2, TIMP2, TIMP3, IL10, IL1B, LAMC1, and ESR1 was performed using real-time polymerase chain reaction.
Statistical analysis
Statistical analysis was performed using Attestat software (Russia), SPSS Statistics 17, and OriginPro 8.5 (USA). Continuous variables are presented as median (Me) with interquartile range (Q3–Q1). Categorical variables are reported as frequencies and percentages. Continuous variables were compared using the Mann-Whitney U-test, while categorical variables were compared using the chi-square test (when the expected cell count was greater than 10). A comparison of the genotypic frequencies of the analyzed alleles was performed to assess the association of the alleles with the phenotypic trait and to compare the distribution of genotypes to test the hypothesis of autosomal dominant and autosomal recessive inheritance of the trait. Differences were considered statistically significant at p<0.05.
Results
When analyzing the age, anthropometric parameters, family, and social status of the study participants, no statistically significant differences were found between the groups and subgroups of patients. The median age in subgroup 1A of the study group was 31 (29.0; 35.0) years, in subgroup 1B was 33.0 (29.0; 35.0) years, in the control group – 31.0 (28.0; 34.0) years in the control group. The median body mass index was 24.0 (22.0; 26.0) kg/m2 in subgroup 1A, 25.0 (23.0; 29.0) kg/m2 in subgroup 1B, and 25.0 (23.0; 26.0) kg/m2 in the control group. The majority of patients in both groups (study and control) were married (101/110 (91.8%) and 266/272 (97.8%), respectively), employed at the time of study entry (85/110 (77.3%) and 204/272 (75%), respectively), and housewives (25/110 (22.7%) and 68/272 (25%), respectively) (Table 1).
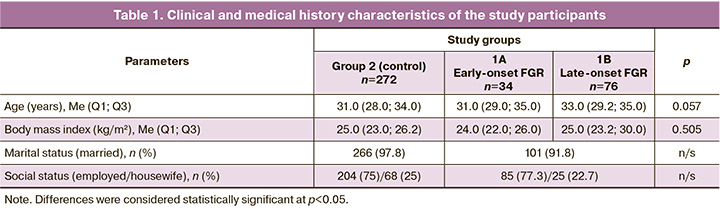
Analysis of obstetric history showed that previous pregnancies were significantly more often complicated by FGR in group 1 than in group 2 (18/110 (16.4%) and 4/272 (1.5%), respectively). There were no statistically significant differences in the incidence of other pregnancy complications (preeclampsia [6/110 (5.45%) and 4/272 (1.5%)], placental abruption [2/110 (1.8%) and 4/272 (1.5%)], and preterm labor [10/110 (9.1%) and 22/272 (8.1%)] in the groups, respectively) (Table 2).
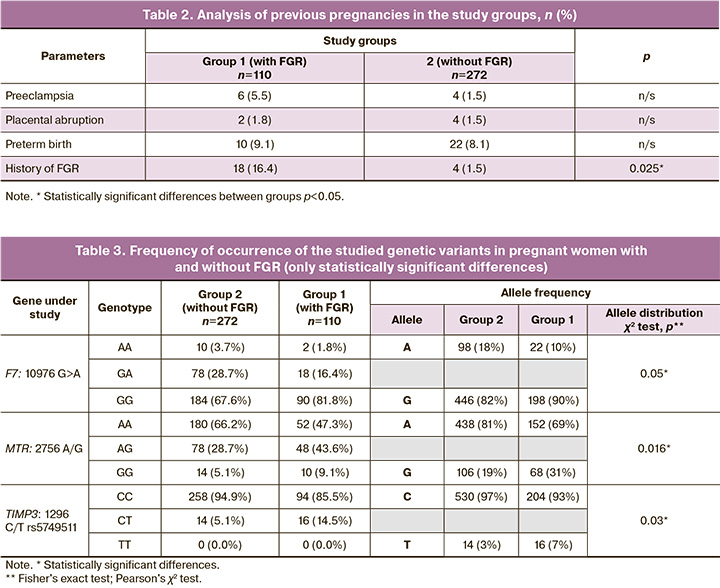
Analysis of the distribution of polymorphic genes associated with thrombophilic and prothrombotic conditions (F2, F5, F7, F13A1, FGB, ITGA2, ITGB3, SERPINE1), folate cycle enzyme disorders (MTHFR, MTR, MTRR), matrix metalloproteinase genes (MMP2, MMP9), tissue inhibitors of metalloproteinases (TIMP2 and TIMP3), and interleukin-1 (IL1B), interleukin-10 (IL10), laminin gene (LAMC1), and estrogen receptor (ESR1) genes in the pregnant women of the study groups revealed significant differences for the gene variant F7:10976 G>A, MTR: 2756 A/G, TIMP3:1296 C/T rs5749511 (Table 3). No statistically significant differences were found for variants of the other genes.
Table 4 shows the significant differences in the distribution of PAI-1 genotypes: -675 5G/4G in subgroups 1A and 1 B of the study group. In our study, among the genes responsible for hemostasis, only the distribution of alleles of the polymorphic locus of the F7 gene, 10976 G>A (Arg353Gln), showed a borderline significant association with FGR (p=0.05); however, according to the autosomal dominant model, the rare allele was a protective factor in relation to the development of this pregnancy complication (OR=0.46 (95% CI 0.21–1.01), p=0.05). The frequencies of protective genotypes in early onset FGR (subgroup 1A) and late-onset FGR (subgroup 1 B) did not differ and was 4/34 (11.8%) and of 14/76 (18.4%), respectively (p=0.7).
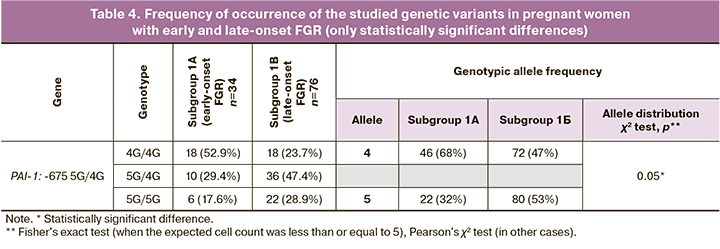
The frequency of the 4G allele of the -675 5G>4G polymorphic locus of the SERPINE1 (PAI-1) gene did not differ between patients with and without FGR (p>0.05). It should be noted that when comparing the early and late forms of this complication (subgroups 1A and 1 B), a statistically significant association between the 4G variant and early onset FGR was observed (72 (67.6%) vs. 46 (47.4%), p=0.05). According to the autosomal recessive model, OR=3.63 (95% CI 1.08–12.17), p=0.03). When studying the influence of genes associated with disorders of folate cycle enzymes, the following results were obtained. A statistically significant association with FGR was observed for the polymorphic locus MTR: 2756 A>G (Asp919Gly). The genotypic frequency of the G allele was significantly higher in pregnant women with FGR than in the control group (without FGR) (68 (30.9%) and 106 (19.5%), respectively; p=0.02). According to the autosomal dominant model, OR=2.18 (95% CI 1.15–4.13). In addition, an accumulation of a rare G allele of the MTRR polymorphic locus was observed in the FGR group: 66 A>G (Ile22Met) (136 (61.8%) and 312 (57.4%) by group, respectively), but the differences were not statistically significant (which may be due to sample size). There were also no statistically significant differences between subgroups 1A and 1 B (p=0.7).
Matrix metalloproteinases (MMPs) are thought to be enzymes involved in normal trophoblast invasion and remodeling of the spiral arteries [7]. This is crucial for the normal course of pregnancy and fetal growth according to gestational age. Therefore, we investigated the association of matrix metalloproteinase genes (MMP2, MMP3, and MMP9) and tissue inhibitors of metalloproteinases (TIMP2 and TIMP3) with the development of FGR and its forms. A statistically significant association between the T allele of the TIMP3 polymorphic locus: -1296 C>T [rs5749511] and the development of FGR was found. The genotypic frequency of the T allele was 16 (7.3%) in the group with FGR and 14 (2.6%) in the group without FGR (p=0.03). According to the autosomal dominant inheritance model, OR=3.14 (95% CI 1.08–9.13), p=0.03. There were no statistically significant differences between the early- and late-onset FGR groups (p=0.7).
Discussion
According to the literature, more than 7 million women globally develop placenta-associated pregnancy complications including preeclampsia, FGR, and gestational hypertension [9]. Physiological hypercoagulation occurs during pregnancy and, when compounded by hereditary hemostatic defects, can lead to intravascular thrombogenesis and adverse outcomes for both the mother and fetus [10]. In this study, with the exception of the F7 gene, no statistically significant differences were found in genes associated with thrombophilia and prothrombotic states between pregnant women with and without FGR. Coagulation factor VII (F7, pro-convertin) plays a key role in the blood coagulation cascade. In cases where arginine is replaced by glutamine at position 353 in the factor VII activation area, gene expression and activity decrease by 50% in the homozygous state [11]. While this reduces the risk of coronary disorders, it increases bleeding tendencies. Literature indicates that the G/A and A/A genotypes of the F7 gene significantly reduce gene expression, providing a protective effect against thrombosis and myocardial infarction [12]. Our results support the protective role of the A allele (353Gln) of the F7 gene in FGR, consistent with other studies on the role of thrombophilia in FGR pathogenesis [13]. During pregnancy, physiological hypercoagulation can lead to adverse outcomes if even minor fibrinolytic system changes occur, causing hyper- or hypofibrinolysis [14].
Current research presents conflicting findings regarding the role of PAI-1 in obstetric pathologies. PAI-1’s main function is to limit fibrinolytic activity, and the polymorphic 4G variant increases gene expression, increases PAI-1 levels, and decreases thrombolytic system activity [15]. Phenotypic differences in PAI-1 genotypes are due to the fact that both activators and repressors can bind to the 5G promoter, while only activators bind to the 4G promoter [16]. The PAI activity increases throughout pregnancy and returns to normal levels after birth. In our study, we found an association between the 4G variant, which elevates PAI levels, and early-onset FGR, in which the genetically determined PAI increase is relatively pronounced.
Our study revealed a statistically significant association between the polymorphic locus MTR: 2756 A>G (Asp919Gly) and FGR. Vitamin B12-dependent methionine synthetase, encoded by the MTR gene, reverses homocysteine to methionine conversion. Methionine synthetase activity requires reductive methylation by methionine synthetase reductase. Both the MTR 2756G and MTRR 66G alleles are linked to folate cycle disruptions [17]. Other studies have shown that in cases of maternal vitamin B12 deficiency, the 2756G variant of the MTR gene may contribute to fetoplacental insufficiency and FGR [18]. Folate supplementation, as clinically recommended, reduces the negative effects of folate cycle gene polymorphisms. All women in this study consumed folic acid, which may explain the minimal effect of folate cycle gene polymorphisms. During early pregnancy, physiological vascular transformation of spiral arteries in the placenta is critical for fetal development, and abnormal MMP activity is associated with impaired placental development [19]. MMPs, a family of 28 endopeptidases, play a central role in tissue remodeling by degrading extracellular matrix components. MMP activity is regulated by tissue inhibitors of metalloproteinases (TIMPs: TIMP1, TIMP2, TIMP3, and TIMP4) [20, 21]. Therefore, the regulation of the MMP-TIMP system is vital for normal pregnancy progression.
In the study by Song G. et al. [22], the polymorphic locus -1296 C>T [rs5749511] of TIMP3 was analyzed in association with unexplained recurrent spontaneous abortions in a Chinese population. While no statistically significant associations were found, the T allele frequency was 1.6 times higher in the abortion group than in the control group. Another study showed reduced TIMP-3 expression in the endometrium of women experiencing spontaneous miscarriages, unexplained infertility, and IVF implantation failures compared to control groups [23, 24]. Various polymorphisms in TIMP1 and TIMP3 genes have been linked to placentation disorders and complications, such as preeclampsia and FGR [25]. Our data confirmed the association of the MMP-TIMP system, specifically the polymorphic locus -1296 C>T [rs5749511] in TIMP3, with FGR development.
Conclusion
The study of regulatory region variants in F2, F5, F7, F13, FGB, ITGA2, ITGB3, PAI-1, MTHFR, MTR, MTRR, MMP9, MMP2, TIMP2, TIMP3, IL10, IL1B, LAMC1, and ESR1 in 110 pregnant women with FGR and 272 without FGR revealed that FGR was significantly associated with the polymorphic loci -66 A>G (Ile22Met) in MTRR, 2756 A>G (Asp919Gly) in MTR, and -1296 C>T [rs5749511] in TIMP3. In addition, the 10976 G>A (Arg353Gln) allele of the F7 gene appeared to be protective against FGR. Differences between early- and late-onset FGR were noted in the 4G variant of the -675 5G>4G locus in SERPINE1 (PAI-1). The influence of other genes on FGR was not supported.
When applied to clinical practice, our findings may help improve FGR prediction and management strategies for high-risk pregnancies, thereby enhancing perinatal outcomes. Identifying genetic markers of FGR remains a priority, necessitating further research with advanced technologies, such as next-generation sequencing (NGS).
References
- Alfirevic Z., Stampalija T., Dowswell T. Fetal and umbilical Doppler ultrasound in high-risk pregnancies. Cochrane Database Syst. Rev. 2017; 6(6): CD007529. https://dx.doi.org/10.1002/14651858.CD007529.pub4.
- Министерство здравоохранения Российской Федерации. Недостаточный рост плода, требующий предоставления медицинской помощи матери (задержка роста плода). Клинические рекомендации (протокол лечения). М.; 2022. 71с. [Ministry of Health of the Russian Federation. Insufficient growth of the fetus, requiring the provision of medical care to the mother (fetal growth retardation). Clinical guidelines (treatment protocol). Moscow; 2022. 71p. (in Russian)].
- Pels A., Beune I.M., van Wassenaer-Leemhuis A.G., Limpens J., Ganzevoort W. Early-onset fetal growth restriction: a systematic review on mortality and morbidity. Acta Obstet. Gynecol. Scand. 2020; 99(2): 153-66. https://dx.doi.org/10.1111/aogs.13702.
- Crispi F., Miranda J., Gratacos E. Long‐term cardiovascular consequences of fetal growth restriction: biology, clinical implications, and opportunities for prevention of adult disease. Am. J. Obstet. Gynecol. 2018; 218(2S): S869-S879. https://dx.doi.org/10.1016/j.ajog.2017.12.012.
- Солдатова Е.Е., Кан Н.Е., Тютюнник В.Л., Волочаева М.В. Задержка роста плода с позиции фетального программирования. Акушерство и гинекология. 2022; 8: 5-10. [Soldatova E.E., Kan N.E., Tyutyunnik V.L., Volochaeva M.V. Fetal growth retardation from the perspective of fetal programming. Obstetrics and Gynecology. 2022; (8): 5-10. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.8.5-10.
- Nowakowska B.A., Pankiewicz K., Nowacka U., Niemiec M., Kozłowski S., Issat T. Genetic background of fetal growth restriction. Int. J. Mol. Sci. 2021; 23(1): 36. https://dx.doi.org/10.3390/ijms23010036.
- Zhai J., Li Z., Zhou Y., Yang X. The role of plasminogen activator inhibitor-1 in gynecological and obstetrical diseases: an update review. J. Reprod. Immunol. 2022; 150: 103490. https://dx.doi.org/10.1016/j.jri.2022.103490.
- Григорьева К.Н., Бицадзе В.О., Хизроева Д.Х., Третьякова М.В., Блинов Д.В., Макацария Н.А., Цибизова В.И., Накаидзе И.А., Гашимова Н.Р., Грандоне Э., Макацария А.Д. Металлопротеиназы как биохимические маркеры патологии беременности. Акушерство, гинекология и репродукция. 2022; 16(1): 38-47. [Grigor’eva K.N., Bitsadze V.O., Khizroeva D.Kh., Tret’yakova M.V., Blinov D.V., Makatsariya N.A., Tsibizova V.I., Nakaidze I.A., Gashimova N.R., Grandone E., Makatsariya A.D. Metalloproteinases as biochemical markers of pregnancy pathology. Obstetrics, Gynecology and Reproduction. 2022; 16(1): 38-47. (in Russian)]. https://dx.doi.org/10.17749/2313-7347/ob.gyn.rep.2022.275.
- Donmez H.G., Beksac M.S. Association of single nucleotide polymorphisms (4G/5G) of plasminogen activator inhibitor-1 and the risk factors for placenta-related obstetric complications. Blood Coagul. Fibrinolysis. 2023; 34(6): 396-402. https://dx.doi.org/10.1097/MBC.0000000000001242.
- Шостак Д.П., Пашов А.И., Патрушева В.Е., Стуров В.Г., Горбунов А.П. Исследование генов системы гемостаза у беременных в европейской популяции. Сибирское медицинское обозрение. 2018; 2: 5-12. [Shostak D.P., Pashov A.I., Patrusheva V.E., Sturov V.G., Gorbunov A.P. Study of genes of the hemostasis system in pregnant women in the European population. Siberian Medical Review. 2018; (2): 5-12. (in Russian)]. https://dx.doi.org/10.20333/2500136-2018-2-5-12.
- Bernardi F., Castaman G., Pinotti M., Ferraresi P., Di Iasio M.G., Lunghi B. et al. Mutation pattern in clinically asymptomatic coagulation factor VII deficiency. Hum. Mutat. 1996; 8(2): 108-15. https://dx.doi.org/ 10.1002/(SICI)1098-1004(1996)8:2<108::AID-HUMU2>3.0.CO;2-7.
- Schreiber K., Sciascia S., de Groot P.G., Devreese K., Jacobsen S., Ruiz-Irastorza G. et al. Antiphospholipid syndrome. Nat. Rev. Dis. Primers. 2018; 4: 17103. https://dx.doi.org/0.1038/nrdp.2017.103.
- Mihai B.M., Salmen T., Cioca A.M., Bohîlțea R.E. The proper diagnosis of thrombophilic status in preventing fetal growth restriction. Diagnostics (Basel). 2023; 13(3): 512. https://dx.doi.org/10.3390/diagnostics13030512.
- Seferovic M.D., Gupta M.B. Increased umbilical cord PAI-1 levels in placental insufficiency are associated with fetal hypoxia and angiogenesis. Dis. Markers. 2016; 2016: 7124186. https://dx.doi.org/10.1155/2016/7124186.
- Wiklund P.G., Nilsson L., Ardnor S.N., Eriksson P., Johansson L., Stegmayr B. et al. Plasminogen activator inhibitor-1 4G/5G polymorphism and risk of stroke: replicated findings in two nested case-control studies based on independent cohorts. Stroke. 2005; 36(8): 1661-5. https://dx.doi.org/10.1161/01.STR.0000174485.10277.24.
- Kohler H.P., Grant P.J. Plasminogen-activator inhibitor type 1 and coronary artery disease. N. Engl. J. Med. 2000; 342(24): 1792-801. https://dx.doi.org/10.1056/NEJM200006153422406.
- Ma Z., Paek D., Oh C.K. Plasminogen activator inhibitor-1 and asthma: role in the pathogenesis and molecular regulation. Clin. Exp. Allergy. 2009; 39(8): 1136-44. https://dx.doi.org/10.1111/j.1365-2222.2009.03272.x.
- Laraqui A., Allami A., Carrié A., Coiffard A.S., Benkouka F., Benjouad A. et al. Influence of methionine synthase (A2756G) and methionine synthase reductase (A66G) polymorphisms on plasma homocysteine levels and relation to risk of coronary artery disease. Acta Cardiol. 2006; 61(1): 51-61. https://dx.doi.org/10.2143/AC.61.1.2005140.
- Furness D.L., Fenech M.F., Khong Y.T., Romero R., Dekker G.A. One-carbon metabolism enzyme polymorphisms and uteroplacental insufficiency. Am. J. Obstet. Gynecol. 2008; 199(3): 276.e1-8. https://dx.doi.org/10.1016/j.ajog.2008.06.020.
- Burton G.J., Jauniaux E. Pathophysiology of placental-derived fetal growth restriction. Am. J. Obstet. Gynecol. 2018; 218(2S): S745-S761. https://dx.doi.org/10.1016/j.ajog.2017.11.577.
- Nikolov A., Popovski N. Role of gelatinases MMP-2 and MMP-9 in healthy and complicated pregnancy and their future potential as preeclampsia biomarkers. Diagnostics (Basel). 2021; 11(3): 480. https://dx.doi.org/10.3390/diagnostics11030480.
- Song G., Yan J., Zhang Q., Li G., Chen Z.J. Association of tissue inhibitor of metalloproteinase gene polymorphisms and unexplained recurrent spontaneous abortions in Han Chinese couples. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014; 181: 84-8. https://dx.doi.org/10.1016/j.ejogrb.2014.07.013.
- Konac E., Alp E., Onen H.I., Korucuoglu U., Biri A.A., Menevse S. Endometrial mRNA expression of matrix metalloproteinases, their tissue inhibitors and cell adhesion molecules in unexplained infertility and implantation failure patients. Reprod. Biomed. Online. 2009; 19(3): 391-7. https://dx.doi.org/10.1016/s1472-6483(10)60174-5.
- Jokimaa V., Oksjoki S., Kujari H., Vuorio E., Anttila L. Altered expression of genes involved in the production and degradation of endometrial extracellular matrix in patients with unexplained infertility and recurrent miscarriages. Mol. Hum. Reprod. 2002; 8(12): 1111-6. https://dx.doi.org/10.1093/molehr/8.12.1111.
- Cruz J.O., Conceição I.M.C.A., Sandrim V.C., Luizon M.R. Comprehensive analyses of DNA methylation of the TIMP3 promoter in placentas from early-onset and late-onset preeclampsia. Placenta. 2022; 117: 118-21. https://dx.doi.org/10.1016/j.placenta.2021.12.003.
Received 10.07.2024
Accepted 18.10.2024
About the Authors
Shagane R. Gasymova, Junior Researcher at the Department of Fetal Medicine, Institute of Obstetrics; Diagnostic Medical Sonographer at the Department of Ultrasound and Functional Diagnostics, Obstetrician-Gynecologist, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology,Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, +7(916)542-22-99, shagane2501@mail.ru, https://orcid.org/0009-0001-2626-6670
Victor L. Tyutyunnik, Professor, Dr. Med. Sci., Leading Researcher at the Center for Scientific and Clinical Research, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, tioutiounnik@mail.ru,
Researcher ID: B-2364-2015, SPIN-code: 1963-1359, Authors ID: 213217, Scopus Author ID: 56190621500, https://orcid.org/0000-0002-5830-5099
Natalia E. Kan, Professor, Dr. Med. Sci., Deputy Director for Research, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, kan-med@mail.ru, Researcher ID: B-2370-2015, SPIN-code: 5378-8437,
Authors ID: 624900, Scopus Author ID: 57008835600, https://orcid.org/0000-0001-5087-5946
Andrey E. Donnikov, PhD, Head of the Laboratory of Molecular Genetic Methods, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, donnikov@dna-technology.ru, https://orcid.org/0000-0003-504-2406
Anastasia G. Borisova, PhD student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, vvv92@list.ru, https://orcid.org/0009-0004-5234-1584
Corresponding author: Shagane R. Gasymova, shagane2501@mail.ru



