Factors of energy metabolism in fetal growth restriction
Kan N.E., Soldatova E.E., Tyutyunnik V.L., Borisova A.G., Tezikov Y.V., Lipatov I.S., Sadekova A.A., Alekseev A.A., Krasnyi A.M.
Objective: This study aimed to was to investigate the blood plasma factors associated with energy metabolism in pregnant women with fetal growth restriction (FGR) and to evaluate their diagnostic performance.
Materials and methods: This cohort study involved 59 pregnant women. The study group (n=30) comprised patients diagnosed with FGR confirmed after childbirth. The control group (n=29) included women with normal pregnancies. The levels of energy metabolism factors (C-peptide, ghrelin, glucose-dependent insulinotropic polypeptide (GlP), glucagon-like peptide-1 (GLP-1), glucagon, insulin, leptin, plasminogen activator inhibitor-1 (PAI-1), resistin, and visfatin) in blood plasma were determined using a multiplex assay (10-plex Bio-Plex Pro Human Diabetes Panel test system).
Results: Analysis of maternal plasma energy metabolism factors revealed significant increases in GLP-1 and PAI-1 levels in the FGR (p=0.003 and p=0.004, respectively). Women with FGR before 37 weeks of gestation showed significant differences in leptin (p=0.05) and PAI-1 (p=0.006) levels compared with those without FGR. After 37 weeks of pregnancy, significant differences were observed in GLP-1 and glucagon levels (p=0.005 and p=0.01, respectively). This study also found that the insulin/GLP-1 ratio was significantly lower in the FGR group than in the control group (p<0.001), suggesting the development of pancreatic cell resistance to GLP-1 and a compensatory increase in its plasma levels in women with FGR. Additionally, a statistically significant direct correlation (rs=0.35, p=0.05) was observed between GLP-1 and PAI-1 (a fibrinolysis inhibitor and a pathogenetically significant factor in FGR). The combination of five factors (GLP-1, glucagon, insulin, leptin, and PAI-1) exhibited excellent diagnostic performance, with an area under the ROC curve of 0.92, a sensitivity of 96%, and a specificity of 81%.
Conclusion: The study results suggest the potential involvement of energy metabolism factors in the development of FGR and highlight prospects for further exploration. Determining the blood plasma levels of GLP-1 and PAI-1 in women with FGR could serve as new non-invasive markers for diagnosing FGR during pregnancy. Furthermore, a combination of factors (GLP-1, glucagon, insulin, leptin, and PAI-1) could identify FGR with high diagnostic accuracy.
Authors' contributions: Kan N.E., Soldatova E.E., Tyutyunnik V.L., Borisova A.G., Tezikov Yu.V., Lipatov I.S., Sadekova A.A., Alekseev A.A., Krasnyi A.M. – conception and design of the study, obtaining data for analysis, review of relevant literature, material processing and analysis, drafting and editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Kan N.E., Soldatova E.E., Tyutyunnik V.L., Borisova A.G., Tezikov Y.V., Lipatov I.S., Sadekova A.A., Alekseev A.A., Krasnyi A.M. Factors of energy metabolism in fetal growth restriction.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (5): 44-52 (in Russian)
https://dx.doi.org/10.18565/aig.2024.9
Keywords
Fetal growth restriction (FGR) is a significant issue in modern obstetrics [1]. Numerous studies have been conducted to address this issue. Although there are already established diagnostic criteria for FGR during pregnancy, the final diagnosis is typically made after the child is born [2]. Therefore, the search for new markers to diagnose this complication during pregnancy is ongoing.
Several pathogenic mechanisms and theories for the development of FGR have been proposed. Of particular interest is the theory of fetal programming, which was first proposed by David Barker [3]. According to this theory, various unfavorable factors in the intrauterine environment can lead to changes in the metabolic and immune processes. These changes ultimately shape the metabolic characteristics of the fetus and newborn, influencing their predisposition to cardiovascular, neurological, and other diseases, as well as obesity and diabetes [4].
The literature describes various factors involved in the regulation of energy metabolism [5, 6]. However, their roles in the pathogenesis of FGR, their interactions, and the contribution of each factor to the development of this obstetric syndrome remain unexplored.
Therefore, it is crucial to study the levels of factors that regulate energy metabolism and elucidate the underlying pathogenetic mechanisms of this pregnancy complication. These investigations are of great scientific interest and motivated this study.
The objective of this study was to investigate the blood plasma factors associated with energy metabolism in pregnant women with fetal growth restriction and to evaluate their diagnostic performance.
Materials and methods
This cohort study included 59 pregnant women and was conducted at Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, Moscow, Russia (Center).
The study group (group 1, n=30) comprised patients diagnosed with FGR confirmed after childbirth. The control group (group 2, n=29) included women with normal pregnancies without FGR according to clinical examination and functional investigation results.
The antenatal diagnosis of FGR was made according to the Delphi Consensus Document criteria for early- and late-onset FGR. Birth weight and length indicators were assessed according to the INTERGROWTH-21 percentile scale for full-term and preterm infants [7]. When the birth weight and length of the newborn were less than the 10th percentile, a diagnosis of small or low gestational weight was established.
Patients were selected based on their need to seek medical care from the Center's specialized departments. To exclude the influence of the onset of labor on the levels of the studied factors of energy metabolism, maternal blood was collected during pregnancy outside the delivery period. All patients signed an informed consent to participate in this study. This study was reviewed and approved by the Research Ethics Committee of the Center.
The criteria for inclusion in the study were gestational age 22–40 weeks, age 18–45 years, singleton pregnancy complicated by FGR (for the study group), normal pregnancy (control group), signed informed consent to participate in the study, and absence of regular labor activity.
Exclusion criteria were spontaneous labor, multiple pregnancies, preeclampsia during pregnancy, the use of a donor egg, severe non-obstetric comorbidities and antiphospholipid syndrome, acute infectious and genetic diseases of the pregnant woman, fetal malformations, and hemolytic disease of the fetus.
The levels of maternal plasma factors involved in blood energy metabolism (C-peptide, ghrelin, glucose-dependent insulinotropic polypeptide, glucagon-like peptide-1 (GLP-1), glucagon, insulin, leptin, plasminogen activator inhibitor-1 (PAI-1), resistin, and visfatin) were determined using a multiplex assay. Venous blood (5 ml) was collected in vacuum tubes containing ethylenediaminetetraacetic acid (EDTA) during pregnancy outside the delivery period. The samples were processed within 30 minutes of blood collection. To obtain plasma, venous blood samples were centrifuged twice at 500 and 5000 g for 10 min. Aliquots of 500 μL were stored at -80°C.
Plasma levels of energy metabolic factors were determined according to the manufacturer's protocol using a standard 10-plex Bio-Plex Pro Human Diabetes Panel test system (Bio-Rad, Hercules, California, USA) on a Bio-Plex 200 system (Bio-Rad, Hercules, California, USA). The results were processed using the Bio-Plex Manager 6.0 properties application (Bio-Rad, Hercules, CA, USA). The factor concentrations are presented in ng/ml.
Statistical analysis
Statistical analysis and plotting were performed using R (version 4.0.2, R Foundation for Statistical Computing, Vienna, Austria) and OriginPro 8.5 (OriginLab Corporation Software, Northampton, MA, USA). The Mann–Whitney U test was used to determine the significance of differences in continuous variables. Fisher's exact test was used to compare the categorical variables. Correlations were assessed using Spearman's rank correlation coefficient (rs). Data are presented as the median (Me) and lower and upper quartiles (Q1; Q3). The graphs in the figures are presented as percentiles (min; 25; 50; 75; max). Categorical variables were presented as counts and percentages. Logistic regression analysis was used to assess the potential diagnostic value of the studied parameters. Logistic regression was used to determine the significance of the factors studied and build a model. The probability of having FGR was calculated using the formula: P=1/(1+e-z). The quality of the resulting model was assessed by constructing an ROC curve, determining the area under the ROC curve (AUC), and calculating the sensitivity and specificity of the developed model at the cut-off point. Differences were considered statistically significant at p<0.05.
Results and discussion
Characteristics of the women included in this study are shown in Table 1. The baseline clinical and anamnestic characteristics of all patients were comparable.
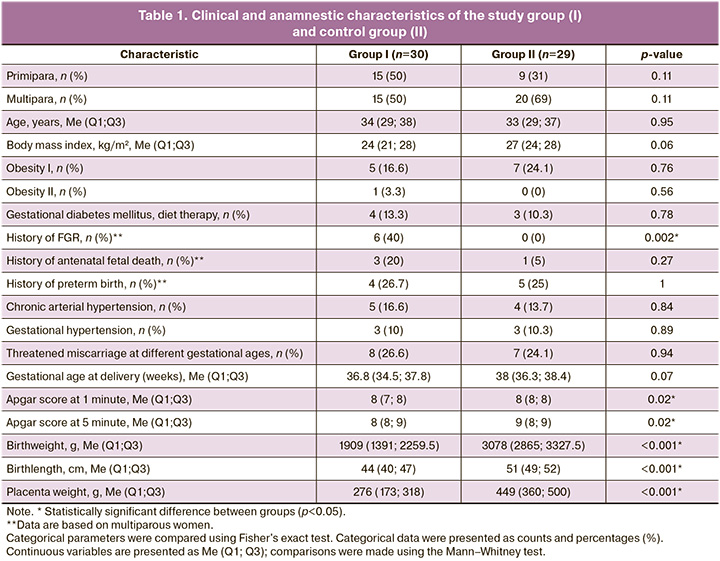
The age of the pregnant women was 34 (29–38) years in the study group and 33 (29–37) years in the control group (p=0.95). There were no statistically significant differences in the weight and height indicators (p>0.05). Analysis of the initial somatic health of the patients did not show any statistically significant differences. Analysis of obstetric history showed that, among multiparous women, a history of FGR was significantly more common in the study group (6/15, 40%, p=0.002).
The Apgar scores of newborns ranged from 5 to 9 points. Birth weight and length of newborns according to the INTERGROWTH-21 percentile scales were significantly different between the study and control groups. The birth weights of the children in the study and control groups were 1909 (1391; 2259.5) g and 3078 (2865; 3327.5) g, respectively (p<0.001). The percentile of birth weight was 2.0 (1.0; 3.0) and 59 (46; 85), respectively, by group with p<0.001.
The identification of the most significant factors involved in the regulation of energy metabolism during FGR was carried out outside the delivery period to exclude the influence of the onset of labor on the level of the studied factors. The levels of energy metabolism factors (ng/ml) in maternal blood plasma are presented in Table 2.
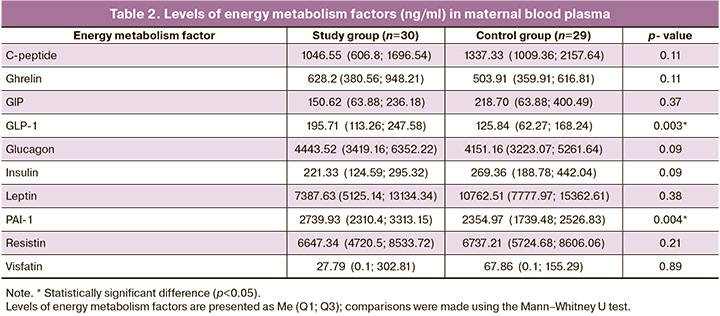
According to the data presented, the study of GLP-1 and PAI-1 is of great interest because when comparing the blood plasma of pregnant women in the studied groups, statistically significant differences were obtained.
The level of GLP-1 in the study group was statistically significantly higher than in the control group (p=0.003) and amounted to 195.71 (113.26; 247.58) ng/ml, and in women with a normal pregnancy – 125.84 (62 .27; 168.24) ng/ml.
A similar trend was observed for PAI-1 levels. Its levels were also significantly higher in patients with pregnancies complicated by FGR (p=0.004). The level of PAI-1 was 2739.93 (2310.4; 3313.15) ng/ml in the study group and 2354.97 (1739.48; 2526.83) ng/ml in the control group.
When studying the factors of energy metabolism during pregnancy at 26-40 weeks, a sharp change in the level of the studied factors was found after 37 weeks of pregnancy. Taking into account the identified trend, the level of factors was studied in both groups before 37 weeks of pregnancy and after 37 weeks of pregnancy; therefore, all patients were divided into two subgroups according to gestational age. The results of the studied factors of energy metabolism in the blood plasma of pregnant women with FGR and women with normal pregnancies, depending on the duration of pregnancy, are presented in Figure 1.
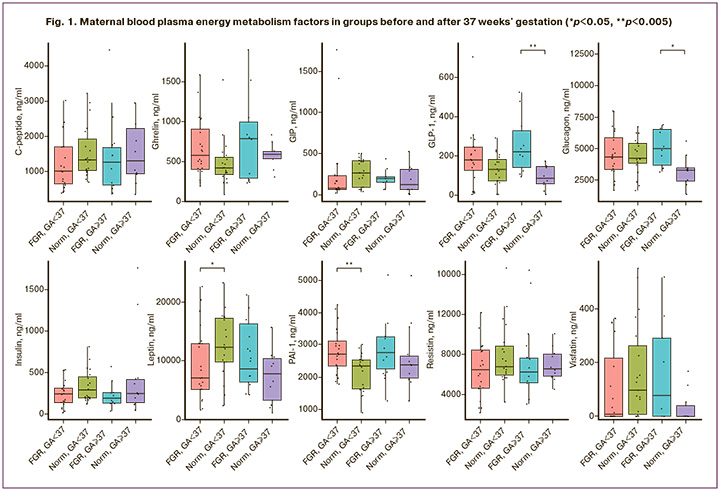
Statistically significant differences were observed in the levels of leptin (p=0.05) and PAI-1 (p=0.006) in women with and without FGR up to 37 weeks of pregnancy.
The level of PAI-1was was significantly increased in patients with pregnancies complicated by FGR before 37 weeks of gestation (p=0.006). Thus, the level of PAI-1 in the study group was 2718.11 (2329.29; 3117.11) ng/ml, and 2344.68 (1636.21; 2532.66) ng/ml in the control group.
The level of leptin in the study group was statistically significantly lower than in the control group (p=0.05) and amounted to 7048.32 (5125.95; 12904.13) ng/ml, and 12276.41 (9747 .23; 17202.28) ng/ml in women with a normal pregnancy.
Dysregulation of leptin is associated with abnormal intrauterine fetal growth and subsequent low birth weight [8]. Normal fetal growth depends on complex interactions between the maternal, placental, and intrauterine environments. Leptin is a peptide hormone best known for its role in energy homeostasis and secretion from adipose tissue but is primarily secreted by the placenta during pregnancy [9]. Thus, leptin, produced by placental tissues and adipocytes of the mother and fetus, plays an integral role in fetal growth during normal pregnancy, is critical for the development and functioning of the placenta, regulates blastocyst formation, and plays an important role in implantation and placentation [10–12]. According to the literature, pregnancy is a state of maternal hyperleptinemia that is closely associated with maternal insulin resistance and mobilization of nutrients from maternal fat stores to meet the energy needs of the growing fetus [13]. Leptin levels gradually increase with the progression of pregnancy and reach a peak in the third trimester of pregnancy. Owing to the molecular weight of leptin, maternal leptin cannot freely cross the placental barrier and thus does not affect leptin levels in the placenta, amniotic fluid, or umbilical cord blood [13].
Studies have reported both lower [14] and higher [15, 16] maternal leptin levels in patients with FGR than in gestational age-matched neonates. Other studies have found no correlation between maternal leptin levels and fetal weight. In our study, the level of leptin was significantly reduced in women with pregnancies complicated by FGR, which indicates the possible role of leptin in the development of this pregnancy complication; however, this requires further study with a correlation analysis of maternal, fetal, and placental leptin to clarify the mechanisms of FGR formation.
After 37 weeks of pregnancy, statistically significant differences were observed in GLP-1 and glucagon levels in the blood plasma of pregnant women in both the groups (p=0.005 and p=0.01, respectively).
The level of GLP-1 in pregnant women with FGR after 37 weeks was statistically significantly higher than in the control group (p=0.005) and amounted to 219.76 (140.17; 326.18) ng/ml, and in women with a normal pregnancy – 83.63 (1.3; 144.56) ng/ml.
A similar trend was observed when glucagon was studied. Its level was also statistically significantly increased in the study group (p=0.01) and amounted to 5023.19 (3705.38; 6539.45) ng/ml, and in the control group – 3296.34 (1382.64; 3479. 17) ng/ml.
GLP-1 binds to its receptors in the pancreas and causes insulin secretion [17, 18]. To evaluate the effect of GLP-1 on insulin secretion during FGR after 37 weeks, we examined the insulin/GLP-1 ratio in the study and control groups. It was found that in the FGR group, the insulin/GLP-1 ratio was significantly reduced (p<0.001), amounts to 1.03 (0.63; 2.06), in contrast to the control group, where the value was 2.49 (1.58; 7.08) (Fig. 2).
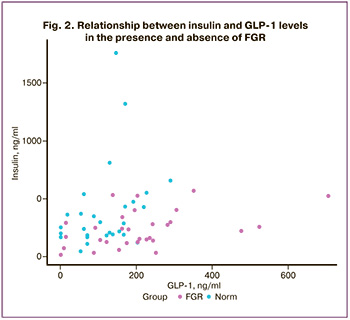
Based on the above, it can be assumed that women with pregnancies complicated by FGR exhibit resistance to GLP-1 at the pancreatic cell receptor level, leading to increased secretion of GLP-1 in the blood plasma during pregnancy [19].
Increased levels of GLP-1 in the FGR may influence various processes in the mother's body [20]. Upon binding to GLP-1R, cyclic adenosine monophosphate (cAMP) is synthesized, which in turn activates cAMP-dependent protein kinase (PKA), which positively regulates the expression of PAI-1 [21]. This was confirmed by a statistically significant direct correlation between GLP-1 and PAI-1 levels in the study group (Spearman correlation coefficient, rs=0.35, p=0.05). No statistically significant correlation was observed in the control group (rs=-0.22, p=0.25).
Increased levels of PAI-1 (fibrinolysis inhibitor) in the blood of pregnant women can directly lead to FGR due to the increased production and accumulation of fibrin in the endothelium of the spiral uterine arteries [22]. During pregnancy, major changes occur in the hemostatic system, affecting uteroplacental blood flow. Endovascular invasion of trophoblast cells causes physiological adaptation of the spiral uterine arteries necessary to provide increased blood flow to the intervillous space as pregnancy prolongs [23]. Most of the vascular endothelium and underlying smooth muscle are replaced by trophoblasts, and fibrin or fibrinoid forms the morphological substrate of the walls of spiral arteries [24]. Compared with endothelial cells, trophoblasts have a reduced ability to lyse fibrin. Previous studies have shown that this change is associated with increased levels of PAI-1 and PAI-2. During pregnancy complicated by FGR, there is limited physiological adaptation of the spiral uterine arteries, which leads to various vascular lesions, including the development of placental insufficiency due to higher fibrin deposition. This is explained by the high concentration of PAI-1 in maternal blood and in the placenta, and a decrease in the activity of plasminogen activator, compared with trophoblast cells during normal pregnancy [22, 24]. Early local elevation of PAI-1 may play an important role in limiting trophoblast invasion, increasing fibrin deposition, and subsequently reducing uteroplacental blood flow, which may lead to the development of FGR [23].
In our study, the level of PAI-1 was significantly higher in the blood plasma of pregnant women with FGR, which confirms the literature data and indicates the significant role of PAI-1 in insufficient placentation in early pregnancy and subsequently in the disturbance of uteroplacental blood flow in this group of patients.
To assess the diagnostic value using logistic regression, from the 10 studied factors of energy metabolism, 5 factors were selected by the conditional method: GLP-1, glucagon, insulin, leptin and PAI-1. The logistic regression formula for the selected factors was as follows:
P=1/(1+e-z),
Z=4.79+[GLP-1]*(-0.014)+[Glucagon]*(-0.0014)+[Insulin]*0.005+ +[Leptin]* 0.0005+[PAI-1]*( -0.0012),
where P is the probability of having FGR, e is the base of the natural logarithm and has a value of 2.718; and [GLP-1], [Glucagon], [Insulin], [Leptin], and [PAI-1] are the values of the levels of energy metabolism factors (ng/ml) in maternal blood plasma, presented in Table 2.
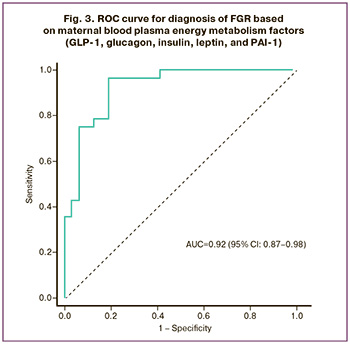
Receiver operating characteristic (ROC) analysis was performed to assess the diagnostic significance of the selected factors (Fig. 3). The area under the ROC curve for a combination of 5 factors was found to be 0.92 (95% CI: 0.87–0.98) with an optimal threshold value of 0.36, sensitivity of 96%, specificity of 81%, positive predictive value of 81% (95% CI: 70.7–89.7) and a negative predictive value of 79.3% (95% CI: 62.2–93). Women should be considered at risk for developing FGR at an optimal cutoff value of less than 0.36. According to the expert scale, the study of energy metabolism factors, such as GLP-1, glucagon, insulin, leptin, and PAI-1, has “excellent” diagnostic value, and their combination can be considered as potential markers for the diagnosis of FGR in the antenatal stage.
Conclusion
The study identified significant differences in energy metabolism factors between the groups of patients with FGR and those with normal pregnancies. These findings suggest that energy metabolism factors may play a role in the development of FGR, and warrant further investigation. In particular, the study observed a statistically significant increase in GLP-1 and PAI-1 levels in the blood plasma of women with FGR, suggesting their potential as noninvasive markers for the diagnosis of this pregnancy complication.
Despite the availability of diagnostic panels and criteria, diagnosis of FGR remains challenging. However, by considering a combination of energy metabolism factors, such as GLP-1, glucagon, insulin, leptin, and PAI-1, FGR can be accurately identified, thus improving patient management during the antenatal period.
Furthermore, it is important to emphasize the need for further research with a larger sample size along with a correlation analysis of the concentration of energy metabolism factors in both maternal and umbilical cord blood. This will help elucidate the underlying mechanisms of FGR.
References
- Nardozza L.M., Caetano A.C., Zamarian A.C., Mazzola J.B., Silva C.P., Marçal V.M. et al. Fetal growth restriction: current knowledge. Arch. Gynecol. Obstet. 2017; 295(5): 1061-77. https://dx.doi.org/10.1007/s00404-017-4341-9.
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Недостаточный рост плода, требующий предоставления медицинской помощи матери (задержка роста плода). М.; 2022. 76с. [Ministry of Health of the Russian Federation. Clinical guidelines. Insufficient fetal growth requiring maternal medical care (fetal growth restriction). Moscow; 2022. 76p. (in Russian)].
- Hales C.N., Barker D.J. The thrifty phenotype hypothesis. Br. Med. Bull. 2001; 60: 5-20. https://dx.doi.org/10.1093/bmb/60.1.5.
- Oke S.L., Hardy D.B. The role of cellular stress in intrauterine growth restriction and postnatal dysmetabolism. Int. J. Mol. Sci. 2021; 22(13): 6986. https://dx.doi.org/10.3390/ijms22136986.
- Солдатова Е.Е., Кан Н.Е., Тютюнник В.Л., Волочаева М.В. Задержка роста плода с позиции фетального программирования. Акушерство и гинекология. 2022; 8: 5-10. [Soldatova E.E., Kan N.E., Tyutyunnik V.L., Volochaeva M.V. Fetal growth retardation from the perspective of fetal programming. Obstetrics and Gynecology. 2022; (8): 5-10. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.8.5-10.
- Кан Н.Е., Солдатова Е.Е., Тютюнник В.Л., Волочаева М.В., Садекова А.А., Красный А.М. Диагностическая значимость определения экспрессии генов энергетического метаболизма при задержке роста плода. Акушерство и гинекология. 2023; 8: 48-55. [Kan N.E., Soldatova E.E., Tyutyunnik V.L., Volochaeva M.V., Sadekova A.A., Krasnyi A.M. Diagnostic significance of determining the expression of energy metabolism genes in fetal growth retardation. Obstetrics and Gynecology. 2023; (8): 48-55. (in Russian)]. https://dx.doi.org/10.18565/aig.2023.93.
- Рюмина И.И., Байбарина Е.Н., Нароган М.В., Маркелова М.М., Орловская И.В., Зубков В.В., Дегтярев Д.Н. Использование международных стандартов роста для оценки физического развития новорожденных и недоношенных детей. Неонатология: новости, мнения, обучение. 2023; 11(2): 48-52. [Ryumina I.I., Baibarina E.N., Narogan M.V., Markelova M.M., Orlovskaya I.V., Zubkov V.V., Degtyarev D.N. The usage of the international growth standards to assess the physical development of newborn and premature children. Neonatology: News, Opinions, Training. 2023; 11(2): 48-52. (in Russian)]. https://dx.doi.org/10.33029/2308-2402-2023-11-2-48-52.
- de Knegt V.E., Hedley P.L., Kanters J.K., Thagaard I.N., Krebs L., Christiansen M. et al. The role of leptin in fetal growth during pre-eclampsia. Int. J. Mol. Sci. 2021; 22(9): 4569. https://dx.doi.org/10.3390/ijms22094569.
- Masuzaki H., Ogawa Y., Sagawa N., Hosoda K., Matsumoto T., Mise H. et al. Nonadipose tissue production of leptin: leptin as a novel placenta-derived hormone in humans. Nat. Med. 1997; 3(9): 1029-33. https://dx.doi.org/10.1038/nm0997-1029.
- Schanton M., Maymó J.L., Pérez-Pérez A., Sánchez-Margalet V., Varone C.L. Involvement of leptin in the molecular physiology of the placenta. Reproduction. 2018; 155(1): R1-R12. https://dx.doi.org/10.1530/REP-17-0512.
- D'Ippolito S., Tersigni C., Scambia G., Di Simone N. Adipokines, an adipose tissue and placental product with biological functions during pregnancy. Biofactors. 2012; 38(1): 14-23. https://dx.doi.org/10.1002/biof.201.
- Pérez-Pérez A., Toro A., Vilariño-García T., Maymó J., Guadix P., Dueñas J.L. et al. Leptin action in normal and pathological pregnancies. J. Cell. Mol. Med. 2018; 22(2): 716-27. https://dx.doi.org/10.1111/jcmm.13369.
- Khant Aung Z., Grattan D.R., Ladyman S.R. Pregnancy-induced adaptation of central sensitivity to leptin and insulin. Mol. Cell. Endocrinol. 2020; 516: 110933. https://dx.doi.org/10.1016/j.mce.2020.110933.
- Yildiz L., Avci B., Ingeç M. Umbilical cord and maternal blood leptin concentrations in intrauterine growth retardation. Clin. Chem. Lab. Med. 2002; 40(11): 1114-7. https://dx.doi.org/10.1515/CCLM.2002.195.
- Mise H., Yura S., Itoh H., Nuamah M.A., Takemura M., Sagawa N. et al. The relationship between maternal plasma leptin levels and fetal growth restriction. Endocr. J. 2007; 54(6): 945-51. https://dx.doi.org/10.1507/endocrj.k06-225.
- Savvidou M.D., Sotiriadis A., Kaihura C., Nicolaides K.H., Sattar N. Circulating levels of adiponectin and leptin at 23-25 weeks of pregnancy in women with impaired placentation and in those with established fetal growth restriction. Clin. Sci. (Lond). 2008; 115(7): 219-24. https://dx.doi.org/10.1042/CS20070409.
- Müller T.D., Finan B., Bloom S.R., D'Alessio D., Drucker D.J., Flatt P.R. et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019; 30: 72-130. https://dx.doi.org/10.1016/j.molmet.2019.09.010.
- Drucker D.J., Habener J.F., Holst J.J. Discovery, characterization, and clinical development of the glucagon-like peptides. J. Clin. Invest. 2017; 127(12): 4217-27. https://dx.doi.org/10.1172/JCI97233.
- Hefetz L., Ben-Haroush Schyr R., Bergel M., Arad Y., Kleiman D., Israeli H. et al. Maternal antagonism of Glp1 reverses the adverse outcomes of sleeve gastrectomy on mouse offspring. JCI Insight. 2022; 7(7): e156424. https://dx.doi.org/10.1172/jci.insight.156424.
- Mehdi S.F., Pusapati S., Anwar M.S., Lohana D., Kumar P., Nandula S.A. et al. Glucagon-like peptide-1: a multi-faceted anti-inflammatory agent. Front. Immunol. 2023; 14: 1148209. https://dx.doi.org/10.3389/fimmu.2023.1148209.
- Ma Z., Paek D., Oh C.K. The cAMP/PKA pathway positively regulates PAI-1 expression in human mast cells. J. Allergy Clin. Immunol. 2009; 123(2): S214. https://dx.doi.org/10.1016/j.jaci.2008.12.818.
- Sheppard B.L., Bonnar J. Uteroplacental hemostasis in intrauterine fetal growth retardation. Semin. Thromb. Hemost. 1999; 25(5): 443-6. https://dx.doi.org/10.1055/s-2007-994947.
- Kaufmann P., Black S., Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol. Reprod. 2003; 69(1): 1-7. https://dx.doi.org/10.1095/biolreprod.102.014977.
- Kam E.P., Gardner L., Loke Y.W., King A. The role of trophoblast in the physiological change in decidual spiral arteries. Hum. Reprod. 1999; 14(8): 2131-8. https://dx.doi.org/10.1093/humrep/14.8.2131.
Received 17.01.2024
Accepted 24.04.2024
About the Authors
Natalia E. Kan, Professor, Dr. Med. Sci., Deputy Director of Science, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, +7(926)220-86-55, kan-med@mail.ru, Researcher ID: B-2370-2015,SPIN-code: 5378-8437, Authors ID: 624900, Scopus Author ID: 57008835600, https://orcid.org/0000-0001-5087-5946
Ekaterina E. Soldatova, PhD student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, +7(906)110-51-13, katerina.soldatova95@bk.ru, https://orcid.org/0000-0001-6463-3403
Victor L. Tyutyunnik, Professor, Dr. Med. Sci., Leading Researcher at the Center of Scientific and Clinical Research, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, +7(903)969-50-41, tioutiounnik@mail.ru,
Researcher ID: B-2364-2015, SPIN-code: 1963-1359, Authors ID: 213217, Scopus Author ID: 56190621500, https://orcid.org/0000-0002-5830-5099
Anastasia G. Borisova, PhD student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology,
Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, +7(968)735-40-81, vvv92@list.ru
Yurii V. Tezikov, Professor, Dr. Med. Sci., Head of the Department of Obstetrics and Gynecology of the Institute of Clinical Medicine, Samara State Medical University,
Ministry of Health of Russia, 443099, Russia, Samara, Chapaevskaya str., 89, +7(846)958-24-18, yra.75@inbox.ru, Researcher ID: С-6187-2018, SPIN-code: 2896-6986,
Author ID: 161372, Scopus Author ID: 6603787595, https://orcid.org/0000-0002-8946-501X
Igor S. Lipatov, Professor, Dr. Med. Sci., Professor at the Department of Obstetrics and Gynecology of the Institute of Clinical Medicine, Samara State Medical University, Ministry of Health of Russia, 443099, Russia, Samara, Chapaevskaya str., 89, +7(846)958-24-18, i.lipatoff2012@yandex.ru, Researcher ID: С-5060-2018,
SPIN-code: 9625-2947, Author ID: 161371, Scopus Author ID: 6603787595, https://orcid.org/0000-0001-7277-7431
Alsu A. Sadekova, PhD (Bio), Researcher at the Cytology Laboratory, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, +7(495)438-22-72, a_sadekova@oparina4.ru, https://orcid.org/0000-0003-4726-7477
Aleksey A. Alekseev, Junior Researcher at the Cytology Laboratory, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, +7(926)134-40-56, a_alekseev@oparina4.ru, https://orcid.org/0000-0002-5347-6884
Aleksey M. Krasnyi, PhD (Bio), Head of the Cytology Laboratory, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, +7(495)438-22-72, alexred@list.ru, https://orcid.org/0000-0001-7883-2702
Corresponding author: Ekaterina E. Soldatova, katerina.soldatova95@bk.ru



