The role of pathological hemostasis in formation of perinatal complications of the novel coronavirus infection
Matusevich E.M., Yuryev S.Yu., Nikolaeva M.G., Frankevich V.E., Frankevich N.A., Popova I.S., Nemtseva T.N.
Objective: To investigate relationship between maternal and fetal complications after novel coronavirus (nCoV) infection (COVID-19 infection) in the presence of polymorphism of gene encoding proteins of the hemostasis system.
Materials and methods: A comparative study of 270 cases of pregnancy and delivery was carried out. Among them, 170 women were infected with novel coronavirus, and 100 women had no COVID-19 with a proven absence of antibodies to SARS-CoV-2 in the blood. Real-time PCR was used for detection of gene polymorphism of F2, F5, F7, F13, FGB, ITGA2, ITGB3, SERPINE1 in the peripheral blood samples. The enzyme-linked immunosorbent assay (ELISA) was used to quantify specific proteins – soluble fms-like tyrosine kinase 1; placental growth factor; tissue plasminogen activator (t-PA); plasminogen activator inhibitor-type 1 (PAI-1).
Results: Gene polymorphism of the hemostasis system influences the course of novel coronavirus infection, the concentration of the major regulatory proteins and determines formation of perinatal complications. Maternal carriage of the plasminogen activator inhibitor-type 1 (675 4G/4G) allele during pregnancy is associated with mild course of COVID-19 infection. At the same time, homozygous carriage of PAI-1(675) 4G/4G determines the development of severe preeclampsia. Carriage of F13(103) T allele is associated with increased risk of infection and clinical significance of the disease with circulation of the most aggressive SARS-CoV-2 Beta variant and increased concentration of t-PA and PlGF in the blood. Moreover, the novel coronavirus infection leads additionally to increased concentration of these proteins at delivery. Homozygous carriage of F13(103) T/T increases the risk of premature placental abruption.
Conclusion: The mechanisms of gestational complications after nCoV infection are mediated by accumulation of supraphysiological concentrations of proangiogenic factors and fibrinolysis activators at delivery, and their action is modulated by the presence of genetic risk factors associated with hereditary thrombophilia.
Authors' contributions: Matusevich E.M., Yuryev S.Yu. – the concept and design of the study; Matusevich E.M., Popova I.S., Nemtseva T.N. – material collection and processing; Frankevich V.E., Frankevich N.A., Matusevich E.M. – statistical data processing; Matusevich E.M., Yuryev S.Yu., Frankevich V.E., Frankevich N.A., Nikolaeva M.G. – writing the text of the article; Yuruev S.Yu., Nikolaeva M.G., Frankevich V.E., Frankevich N.A. – text editing.
Conflicts of interest: The authors confirm that they have no conflicts of interest to declare.
Funding: The study was carried out at the expense of the grant of the Russian Science Foundation No. 24-64-00006, http://rscf.ru/project/24-64-00006/.
Ethical Approval: The study was approved by the local Bioethics Committee of the Siberian State Medical University the Ministry of Health of Russia (Approval document No. № 8993 of December 21, 2022).
Patient Consent for Publication: The patients have signed informed consent for publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Matusevich E.M., Yuryev S.Yu., Nikolaeva M.G., Frankevich V.E., Frankevich N.A.,
Popova I.S., Nemtseva T.N. The role of pathological hemostasis in formation
of perinatal complications of the novel coronavirus infection.
Akusherstvo i Gynecologia/Obstetrics and Gynecology. 2024; 6: 46-55 (in Russian)
https://dx.doi.org/10.18565/aig.2024.5
Keywords
In April 2023, the Lancet published the results of analysis of the effectiveness of organizational measures aimed at reducing infection and mortality from COVID-19. By the middle of 2022, the main successful policies were vaccination, mask-wearing, mobility-restricting and shifting to remote work and learning [1]. Despite this, by September 2023, the WHO registered more than 760 million cases of novel coronavirus (2019-nCoV) infection caused by the coronavirus SARS-CoV-2, and about 6.9 million deaths caused by COVID-19. In 2022, more complete analysis of the characteristics of 2019-nCoV infection in the “obstetric” population reported higher incidence of the disease, severity of infection and mortality among pregnant and puerperant women. The initial data on the absence of the specific impact of COVID-19 on the course of pregnancy and the fetus in contrast to influenza virus H1N1, have proved untenable. A significant negative effect of comorbid conditions, such as obesity and hypertension, was reported. No direct relationship between the severity of the course of 2019-nCoV infection and damage to the placenta and fetus was found [2].
Management strategy during the pandemic is complicated by the necessity to monitor carefully monitor the maternal and fetal condition for a severity of the infectious process, on the one hand, and to minimize outpatient visits and hospitalizations, on the other hand. In the time of pandemic, high workload of healthcare personnel in medical facilities makes it impossible to increase the volume of outpatient or inpatient care. There is a reasonable possibility that a risk-based approach, formation of a high risk group from COVID-19 among pregnant women and pathogenetically based prevention of complications, along with total vaccination, can reduce the number of maternal and perinatal losses [3, 4].
The studies of main pathogenetic mechanisms of 2019-nCoV infection enable to propose the quantitative characteristics of proteins-markers of placental dysfunction and the fibrinolytic system as predictors of the development of complications. Immunothrombosis of the placental microvasculature, which is specific for 2019-nCoV infection due to increased concentration of pro-inflammatory molecules, even in the absence of clinical and laboratory presentation of cytokine storm will lead to imbalance between pro-angiogenic and anti-angiogenic factors. It has been proven that any systemic inflammation at the initial stage is accompanied by hypofibrinolysis [5]. This sign is doubly pathognomonic for infectious inflammation, since the infectious agent often uses plasmin to increase virulence, thereby leading to plasminogen depletion [6].
Despite the lack of clinically proven methods for regulation of fibrinolysis, especially in the obstetric practice, establishing clear criteria for the risk group for perinatal complications due to nCoV infection, which are associated with placental dysfunction and impairment of the fibrinolytic system, can become the basis for the development of innovative methods for treatment of viral infections in pregnant women and algorithms for subsequent management and delivery.
The purpose of the study was to investigate relationship between formation of maternal and perinatal complications of COVID-19 infection during pregnancy in the presence of polymorphism of genes encoding proteins of the hemostasis system.
Material and methods
The analysis of documentation related to the course of pregnancy and 2019-nCoV infection (in the main group of patients with COVID-19) was done. The characteristics of gestation, delivery and perinatal complications in novel coronavirus disease (COVID-19) depending on the severity of infection, SARS-CoV-2 strains (Alpha, Beta, Gamma, Delta, and Omicron) were studied. The associations between gestational age at which the patient had SARS-CoV-2 infection, pregnancy complications and perinatal outcomes were defined.
In the comparative case-control study, 270 women in continuous sampling gave birth from January to December 2021. Depending on having or nor having nCoV infection, the patients were divided into 2 groups: the main group consisted of 170 women who had coronavirus infection at different gestational age, the control group included 100 patients, who had no 2019-nCoV infection in pregnancy, and the absence IgG class antibodies against SARS-CoV-2 in the blood was confirmed by the serological test. There were significant differences in anthropometric data, the parity and concomitant pathology between the patients in the groups.
Inclusion criteria for the patients in the main group: confirmed diagnosis of the coronavirus disease COVID-19 in current pregnancy using polymerase chain reaction (PCR); patient’s informed consent to participate in the study.
Exclusion criteria from the main group: absence of confirmed or suspected 2019-nCoV infection in current pregnancy; severe external genital pathology at the stage of decompensation; multiple pregnancy; infection caused by human immunodeficiency virus (HIV); patient's refusal to participate in the study.
Inclusion criteria for the patients in the control group: absence of confirmed 2019-nCoV infection in current pregnancy using PCR; patient’s informed consent to participate in the study.
Exclusion criteria from the control group: confirmed or suspected 2019-nCoV infection in current pregnancy; severe external genital pathology at the stage of decompensation; multiple pregnancy; infection caused by HIV; patient’s refusal to participate in the study.
The patients underwent treatment of COVID-19 during pregnancy in the following medical facilitites: respiratory hospitals of “Medsanchast No.2/Medical and sanitary unit No.2.” (Tomsk, Mikhlenko A.B., Chief Medical Officer) and maternity hospital No. 4 (Tomsk, Chernyavskaya O.V., acting Chief Medical Officer). The women gave birth and underwent medical examination at “Regional Perinatal Center named after I.D. Evtushenko” (Tomsk, Stepanov I.A., Chief Medical Officer).
Upon admission to the obstetric department, the patients underwent ultrasound examination and Doppler test using Nemio XG device (Toshiba, Japan); and cardiotocography test during labor using Sonicaid Team device (Sonicaid Ltd/ Huntleigh Healthcare, the Great Britain) and FC 1400 device (Bionet, South Korea).
Labor and delivery was performed according to the clinical recommendations approved by the Ministry of Health of Russia: “Normal birth”; “Singleton birth”; “Cesarean birth”; “Preeclampsia. Eclampsia. Edema, proteinuria and hypertensive disorders during pregnancy, childbirth and the postpartum period”; “Fetal growth restriction”.
Newborn baby care and examination was performed according to the clinical recommendations approved by the Ministry of Health of Russia: «Routine newborn care in the delivery room and in the postnatal ward”; “Management of newborns with respiratory distress syndrome”; “Resuscitation and stabilization on newborn’s condition in the delivery room”.
Other methods of examination apart from standard methods specified in the clinical protocol
In the laboratory of the Center for Perinatal Health (Tomsk), the gene polymorphism associated with impaired hemostasis system was determined using real-time PCR in peripheral blood samples: F2 (prothrombin, coagulation factor II, 20210G>A); F5 (coagulation factor V Leiden 1691G>A); F7 (coagulation factor VII, 10976G>A); F13 (coagulation factor XIII 103G>T); FGB (beta subunit of the coagulation factor fibrinogen, coagulation factor I, -455 G>A); ITGA2 (glycoprotein Ia, VLA-2 receptor 807C>T); ITGB3 (integrin subunit beta-3, platelet fibrinogen receptor 1565T>C); SERPINE1 (PAI-I, plasminogen activator inhibitor I, -675 5G>4G) (PCR kits manufacturer LLC “DNA-technology”, Russia).
In the Central research laboratory of the Siberian State Medical University (Tomsk), the enzyme-linked immunosorbent assay (ELISA) was performed to quantify specific proteins in blood – soluble fms-like tyrosine kinase 1(sFlt1), ng/mL; placental growth factor (PLGF), ng/mL; tissue plasminogen activator (tPA), ng/mL; plasminogen activator inhibitor-1 (PAI-1), ng/mL (reagent kits manufacturer Cloud-Clone Corp., USA). Maternal blood samples were collected for laboratory tests either before delivery or at the beginning of the first stage of labor.
Statistical analysis
Data statistical analysis was performed using software programs SPSS, Version 20, and Statistica, version 6.0.
Results
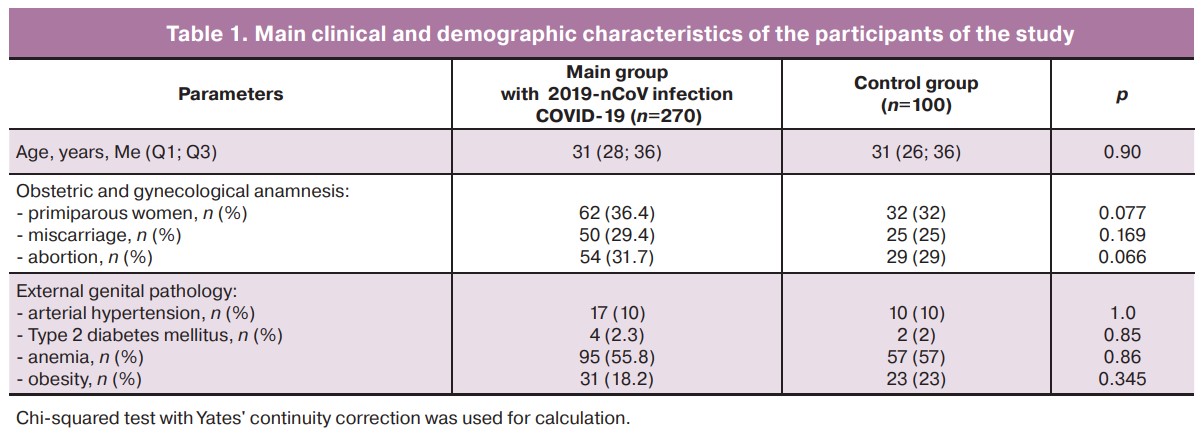
Comparison of the anthropometric, demographic and anamnestic data in patient groups showed that there were no statistically significant differences between the participants of the study. (Table 1).
In 131/170 (77%) cases, pregnant women, the residents of Tomsk and Tomsk region had mild and asymptomatic COVID-19. Moderate coronavirus disease was in 36/170 (21%) cases, and only in 4/170 (2%) cases there was severe COVID-19 infection. A more severe course of the disease was typical in cases of SARS-CoV-2 infection in late pregnancy. Pneumonia of viral etiology was diagnosed in 19/170 (11%) patients with nCoV infection.
The comparative analysis using chi-squared test with Yates' continuity correction showed statistically significant increase of the frequency of gestational and perinatal complications in the main group versus the group without COVID-19. Preterm births were in 26/270 (15%) cases versus 6/100 (6%) in the control group (p=0.015). Pregnancy complication was premature abruption of normally located placenta in 10/170 (6%) cases versus 2/100 (2%) in the control group (р=0,05). In addition, pregnancy complication was most often severe preeclampsia in 27/170 (16%) cases versus 7/100 (7%) in the control group (p=0.012). Fetal growth restriction was diagnosed in 34/170 (20%) cases versus 4/100 (2.4%) in the control group (р=0.045). Antenatal fetal death was registered in 5/170 (3%) cases versus 1/100 (1%) in the control group (р=0.29).
Perinatal complications most often were in the main group of the study. So, asphyxia was diagnosed in 14/170 (10%) newborns versus 3/100 (3%) in the control group (р=0.045). In the main group, 27/170 (16%) newborns versus 13/100 (13%) in the control group required transfer to the Resuscitation and Intensive Care Unit (р=0.568).
Taking into account the specific characteristics of pathogenesis of novel coronavirus infection and the character of identified complications, gene polymorphisms of hemostasis in patients in the main group and in the control group, the balance of the main regulatory proteins of the fibrinolytic system and the markers of placental dysfunction were further analyzed.
The patients in the main group and in the control group underwent genetic testing for detection of gene polymorphism of hemostasis. At least one polymorphism of F2, F5, F7, FGB, PAI-1, F13, ITGB3, ITGА-2 genes was identified in 220/270 (81.5%) patients. At the same time, in 37/270 (13.8%) cases, polymorphism of only one gene was detected, and in 183/270 (67.7%) pregnant women, combinations of polymorphisms of genes encoding proteins of the hemostasis system were found.
The analysis of relationship between pregnancy outcome, characteristics of the course of nCoV infection, carriage of pathological alleles of genes encoding proteins of the hemostatic system found that polymorphism of the studied genes was associated with the pathological course of pregnancy and childbirth. In this publication, we focus on the discussion of complications associated with polymorphism of genes encoding the major regulatory proteins of fibrinolysis – FXIII, PAI-1 and t-PA, since it is known that in the pathogenesis of infectious and inflammatory injury, in mechanism of tissue repair, growth and involutional changes in the placenta, plasminogen inhibitors and activators closely interact with transglutaminase FXIII. It is logical to assume that carriage of initially pathological alleles encoding protein synthesis of the fibrinolytic system (PAI-1 and FXIII) will be associated with gestational complications during COVID-19 infection.
Functional chi-squared test was used to determine the relationship between carriage of fibrinolytic gene polymorphism, the severity of 2019-nCoV infection and the risk of infection with SARS-CoV-2 Beta. The presence of genotypic variant F13(103) G/T, T/T is associated with an increased risk of infection and clinically significant disease in circulation of the most aggressive beta variant of SARS-CoV-2. In moderate to severe cause of infection, gene polymorphism in F13(103) G/T, T/T was found in 57/100 (57%) cases versus 22/67 (32.8%) cases of mild cause of infection (p=0.03). Carriage of PAI-1(675)4G allele was associated with a milder course nCoV infection, regardless of the trimester of infection (p<0.005, correlation coefficient 0.38). Functional chi-squared test was performed for analysis using the “FunChisq” R package.
PAI-1 polymorphism was detected in 183/270 (68%) tested women in both groups. At the same time, PAI-1(675) 4G/4G homozygous variant had statistically significant association with the number of pregnancy complications (p=0.03), while no such association was found for the PAI-1(675) 5G/4G genotype (p=0.32).
Carriage of polymorphism in PAI-1 and/or F13 was associated with development of preeclampsia and fetal growth restriction. COVID-19 disease statistically significantly increased the risk of these complications (p=0.039 and p=0.031, respectively) (Table 2).
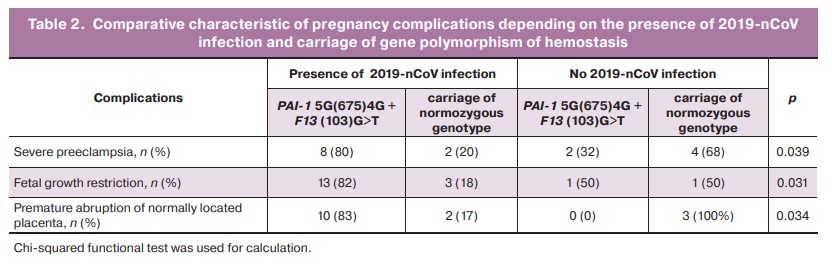
Homozygous variants and compounds of several polymorphisms occur in the group of critical complications and their rate is significantly higher compared to the population. In 8/10 (80%) cases in patients with severe preeclampsia, polymorphism of the PAI-1 gene was identified. Of them, homozygous polymorphism was detected in 4 cases. In 5/10 (50%) cases of the total number of identified severe preeclampsia, there was combined polymorphism of the PAI-1 gene with alternative variants of the F13 and ITGB3 genes. The F13(103) T allelic variant was found in 8/10 (80%) patients with premature placental abruption who had COVID-19; a homozygous variant was detected in 2/10 (25%). In the vast majority of cases, polymorphisms were simultaneously detected in FGB, ITGB3 and PAI-1.
The presence of alternative gene variant does not always lead to disease. Often the compensatory mechanisms help the functional system to maintain stability. The fibrinolytic balance in pregnant women was assessed by calculation of the t-PA/PAI-1 ratio. At the time of delivery, PAI-1 concentrations were comparable in the study groups. The concentration of t-PA was significantly higher in carriers of the F13(103) T allele (p=0.013, the Mann–Whitney U test). Novel coronavirus infection led to an increase in concentration of t-PA by 50–66%.
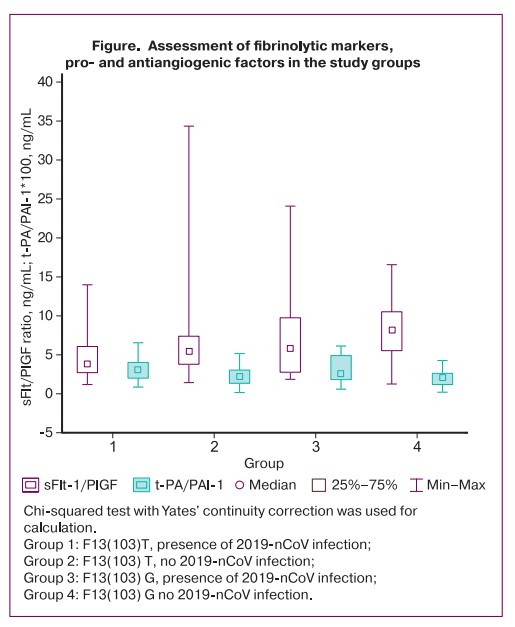
The presence and severity of placental dysfunction were determined by concentration of the main pro- and antiangiogenic factors and sflt/PlGF ratio. Among pregnant women in both groups with similar soluble receptor concentration in the blood, there was statistically significant increase in concentration of PlGF in patients with the allelic variant of F13(103) T (Figure). The comparative analysis showed that with increasing period of time from COVID-10 infection to childbirth, there was significantly increased concentration of PlGF in carriers of this polymorphism (65.2 pg/mL with shortest infectious period versus 579.8 pg/mL with the maximum period). Accordingly, by the time of birth there was progressive decreasing sflt/PlGF ratio (9.7 with the maximum infectious period to birth versus 2.5 with the shortest period) (p=0.013).
Discussion
By 2021, information on continuity of specific pathological symptoms for a long time after acute COVID-19 in the absence of the virus in the body has been accumulated [7, 8]. Chronic systemic inflammation with the autoimmune component, endothelial dysfunction of the microvasculature, subsequent thrombosis and tissue hypoxia were identified as the main pathogenetic mechanisms of post-COVID-19 syndrome [9, 10]. Novel coronavirus infection in pregnancy can be the cause of perinatal complications due to pathological processes of acute COVID-19 (up to 12 weeks) and post-COVID-19 syndrome. Comorbidities, genetic disorders of hemostasis system, innate and adaptive immunity significantly increase the risk of placental and fetal injury [11]. The studies suggest that the presence of high-risk inherited thrombophilia may increase the risk of thrombosis in pregnant women [12]. The risk may be increased in the presence of acute viral infections. Potential molecular mechanisms through which inherited thrombophilia may influence the risk of thrombosis in acute viral infections include interactions between the genetic factors and inflammatory cascade. They include increased concentrations of interleukin-6 and tumor necrosis factor, then thrombin and fibrin in combination with dysregulation of vascular tone [9, 13–16].
Our study analyzed relationship between the severity of COVID-19, the presence of gene polymorphisms in the fibrinolytic system and pregnancy outcomes. The results obtained in the study show that altered expression of PAI-1 and FXIII proteins in patients leads to an increase in fibrinolytic potential and intensification of the pathological angiogenesis in the post-COVID-19 period. The presence of four guanine bases (4G) in the promoter region of the PAI-1 gene (4G) increases the expression of circulating PAI-1 by 3–5 times, and is accompanied by reduction of fibrinolytic activity of blood [5]. High concentrations of PAI-1 promote intervillous thrombosis that reduces placental perfusion. Limitation of endovascular trophoblast invasion in early pregnancy leads to placenta-mediated pregnancy complications: preeclampsia and placental insufficiency [17].
Polymorphism in the F13G(103) T gene for subunit A of transglutaminase leads to increasing the activity of the fibrin-stabilizing factor by 2.5 times. Intensive fibrin polymerization of alpha chain and gamma chain under the action of transglutaminase inhibits the lateral aggregation of fibrin fibers, that reduces weight-to-length ratio. At the same time, fibrin fibers form finer mesh-like structure, and short fibers are tightly twisted. This leads to decreased of fibrinolytic activity, since t-PA and u-PA perform better on dense fibrin mesh with large pores [18].
The additive effect of the F13(103) T allele and PAI-1 4G allele in viral infection increases the resistance of fibrin to degradation and reduces the activity of the fibrinolytic system. In the first and second trimester, this leads to impaired remodeling of the extracellular matrix and, as a consequence, to defective trophoblast invasion. In the third trimester, this leads to disruption of placental perfusion due to changes in the structure of the syncytiocapillary membranes and fibrinoid. The negative potential of this combination was proven in our sample by the increase incidence of severe preeclampsia, premature abruption of normally located placenta, and fetal growth restriction in the carriers.
The increased influence of the F13/PAI-1 polymorphism (or their compound) in fetal growth restriction and preeclampsia in 2019-nCoV infection is probably mediated through placental disorders. The gestational age at the time of the occurrence of infection may potentially influence the severity of preeclampsia and fetal growth restriction. The most unfavorable option is infection at the end of the first trimester and at the beginning of the second trimester of pregnancy.
According to literature data, the isolated effect of carriage of the PAI-1 4G allele is assessed without regard to pregnancy, as an independent predictor of prothrombotic readiness and severity of coronavirus infection.
Our study found that during pregnancy there is an inverse dependence of the severity of 2019-nCoV infection on the presence of the PAI-1 4G allele. Probably, a simultaneous increase in the level of t-PA neutralizes the negative effect of the inhibitor at the systemic level, but this is not enough to balance the vascular endothelium of the placental bed [19, 20]. A significant increase in the level of t-PA and, accordingly, t-PA/PAI-1 ratio in carriers of the F13(103) T allele against the background of infection, that was found in our study, has a logical explanation. In response to specific stimuli such as bradykinin, interleukin-6 and tumor necrosis factor-α, the endothelium produces increased amounts of both t-PA and PAI-1 [5]. The study by Whyte C.S. et al. found a significant increase in the level of tPA in patients with COVID-10 versus health control group. Moreover, the balance of fibrinolysis is disrupted. High PAI-1concentration suppresses plasmin production and fibrinolysis in COVID-19, despite a concomitant increase of t-PA [21]. Zuo Y. et al. suggested that he source of the plasminogen activator and inhibitor can be endothelial cells, which are directly destroyed by SARS-COV-2. At the same time, high concentrations of PAI-1 overcome the effects of local production of t-PA and cause a prothrombotic and hypofibrinolytic condition in the presence of 2019-nCoV infection [22]. During the post-COVID-19 period, the balance changes towards activation of fibrinolysis, that increases the risk of preterm birth and placental abruption. So far as the modulation of parietal fibrinolysis is concerned, the results of our study showed no increase in intrapartum and postpartum hemorrhage, a high level of t-PA. Probably, the same reason determines the similar level of PAI-1 in the blood of carriers of the polymorphism and the wild-type allele, regardless of the past infection. An additional amount of proteins synthesized in the cells in the presence of the 4G allele quickly binds to the glycocalyx is undetectable in the systemic circulation, at the same time is activating additional production of plasminogen activators.
Endothelial and placental dysfunction is an expected condition in patients with altered transglutaminase FXIII Val34Leu, who had 2019-nCoV infection in pregnancy, since this protein is one of the main regulators of endothelial structure and function. The soluble part of the sFlt-1 receptor is produced in large amount both by normal placenta and especially in cases of placental dysfunction. The studies by Kosinska-Kaczynska K. et al. (2023) confirm that during the disease, sFlt-1/PlGF ratio was significantly higher in pregnant women with COVID-19 despite the severity of the disease compared to the normal course of pregnancy [23]. The data obtained in their study correlated with the results obtained by Giardini V. et al. (2022) [24]. The prevalence of the antiangiogenic factor in the infectious disease triggers the development of preeclampsia and fetal growth restriction.
The opposite situation was registered by us in the period of time after COVID-19 (before childbirth). Correlation analysis showed a direct dependence of the PlGF concentration (p=0.017) and inverse dependence of sFlt/PlGF ratio (p=0.005) on duration of infectious period in carriers of the F13G(103) T polymorphism. There is an obvious dissonance between the clinical and laboratory data in the studied sample. This is, firstly, simultaneous increased incidence of preeclampsia and the absence of the most sensitive biochemical marker of high significance – sFlt/PlGF ratio, and, secondly, the absence of elevated sFlt after 30 weeks’ gestation, when in normal cases there are enchanced proinflammatory and antiangiogenic characteristics of the immunity and hemostasis. The study by Yakovleva N.Yu. et al. showed that in normal course of pregnancy, PlGF concentration increases by 30–32 weeks versus the second trimester, but the balance is maintained due to the mirror elevation of sFlt [25]. In pregnancy > 32 weeks, the researchers found progressive reduction in PlGF up to the time of childbirth [26]. In our study, increased PlGF concentrations and low sFlt/PlGF ratio before delivery were likely to be due to compensation of the initial dominance of antiangiogenic factors during acute COVID-19. Thus, the situation is created, when even correct balance between supraphysiological concentrations of pro- and antiangiogenic factors, leads to gestational complications.
This phenomenon can be explained by the properties of FXIII transglutaminase. Proangiogenic properties of FXIII increase migration and proliferation of endothelial cells. The vascular endothelial growth factor (VEGF) family is the main regulator of angiogenesis. In turn, FXIII activates VEGFR-2 by cross-linking it with alpha(V)beta(3) integrin on the surface of endothelial cells, thereby stimulating formation of fetoplacental vessels [27]. Cytokine synthesis, including in the presence of 2019-nCoV infection, activates the production of Hypoxia-Inducible Factor (HIF)-1, which stimulates release of proteins of the VEGF family [28]. It has been proven that the number of regulators of angiogenesis, such as EGF-A, PlGF and FGF-2 significantly increases in blood in the presence of 2019-nCoV infection correlating with the severity of infection [29]. Given the fact that the effect of the FXIII polymorphism depends fibrinogen concentration [30], it can be assumed that in the third trimester, with natural accumulation of prothrombin and fibrinogen, placental growth factor production is high and there is imbalance between PlGF and sFlt levels.
For example, the biopsy test results of the samples of lung tissue from patients with 2019-nCoV infection showed that increased concentration of angiogenesis activators in abnormal fibrinolysis and remodeling of the extracellular matrix, that was caused by F13G(103) T polymorphism led to stimulation of intussusceptive (nonsprouting) angiogenesis angiogenesis [31]. Normal intussusceptive angiogenesis activation in placenta lasts up to 24 weeks; elongation of the existing vessels dominates at later stage of pregnancy (unbranched angiogenesis) [32]. Inappropriate angiogenesis probably plays a negative role in the pathogenesis of gestational complications in the presence of 2019-nCoV infection and in carriers of the F13G(103) T polymorphism increasing the risk of placental abruption with massive bleeding from abnormal newly formed vessels of the placental bed against the background of fibrinolysis activation. This theoretical assumption requires further study and morphological confirmation.
Conclusion
Critical complications of pregnancy are associated with the presence of genetic risk factors, in particular with hereditary thrombophilias. In women in the period of time after COVID-19 and before childbirth, an association was found between the presence of PAI-1 and FXIII gene polymorphism and an increase in the concentration of fibrinolysis activators and proangiogenic factors.
It is known that the presence of genetically factors that determine fibrinolysis can modulate the processes of inflammation, wound healing, coagulation and angiogenesis. The analysis of gestational complications in the later period after COVID-19 disease showed that carriage of polymorphism of genes encoding the main proteins of fibrinolysis against the background of an infectious process can contribute to the development of complications, such as severe preeclampsia, preterm birth and placental abruption. Revealing these mechanisms can serve as the basis to build prognostic models, develop a monitoring system for the risk group, and identify new therapeutic targets to prevent critical conditions in mother and fetus. Similar studies involving other infectious diseases are necessary to thoroughly understand the pathogenetic mechanisms.
References
- Bollyky T.J., Castro E., Aravkin A.Y., Bhangdia K., Dalos J., Hulland E.N. et al. Assessing COVID-19 pandemic policies and behaviours and their economic and educational trade-offs across US states from Jan 1, 2020, to July 31, 2022: an observational analysis. Lancet. 2023; 401(10385):1341-60. https://dx.doi.org/10.1016/S0140-6736(23)00461-0.
- Overton E.E., Goffman D., Friedman A.M. The epidemiology of COVID-19 in pregnancy. Clin. Obstet. Gynecol. 2022; 65(1): 110-22. https://dx.doi.org/10.1097/GRF.0000000000000674.
- Министерство здравоохранения Российской Федерации. Временные методические рекомендации. Профилактика, диагностика и лечение новой коронавирусной инфекции (COVID-19). Версия 18 (26.10.2023). [Ministry of Health of the Russian Federation. Interim guidelines. Prevention, diagnosis and treatment of a new coronavirus infection (COVID-19). Version 18 (26.10.2023). (in Russian)].
- Аксенова Е.И., Безымянный А.С., Гавриленко О.Ф., Камынина Н.Н., Кирасирова Е.А., Крюков А.И., Кунельская Н.Л., Оленев А.С., Пивоварова О.А., Плавунов Н.Ф., Старшинин А.В., Товмасян А.С., Хисамов А.Б., Чернова Е.А. COVID-19: Анализ лучших управленческих практик. М.: ГБУ «НИИОЗММ ДЗМ»; 2021. 150 с. [Aksenova E.I., Bezymyanny A.S., Gavrilenko O.F., Kamynina N.N., Kirasirova E.A., Kryukov A.I., Kunelskaya N.L., Olenev A.S., Pivovarova O.A., Plavunov N.F., Starshinin A.V., Tovmasyan A.S., Hisamov A.B., Chernova E.A. COVID-19: Analysis of the best management practices. M.; 2021. 150 p. (in Russian)].
- Morrow G.B., Whyte C.S., Mutch N.J. A serpin with a finger in many PAIs: PAI-1's central function in thromboinflammation and cardiovascular disease. Front. Cardiovasc. Med. 2021; 8: 653655. https://dx.doi.org/10.3389/fcvm.2021.653655.
- Ji H.L., Zhao R., Matalon S., Matthay M.A. Elevated plasmin(ogen) as a common risk factor for COVID-19 susceptibility. Physiol. Rev. 2020; 100(3): 1065-75. https://dx.doi.org/10.1152/physrev.00013.2020.
- Proal A.D., VanElzakker M.B. Long COVID or post-acute sequelae of COVID-19 (PASC): An overview of biological factors that may contribute to persistent symptoms. Front. Microbiol. 2021; 12: 698169. https://dx.doi.org/10.3389/fmicb.2021.698169.
- Чазова И.Е., Блинова Н.В., Жернакова Ю.В., Кисляк О.А., Невзорова В.А., Савенков М.П., Ощепкова Е.В., Остроумова О.Д., Бойцов С.А. Консенсус экспертов Российского медицинского общества по артериальной гипертонии: артериальная гипертония и постковидный синдром. Системные гипертензии. 2022; 19(3): 5-13. [Chazova I.E., Blinova N.V., Zhernakova J.V., Kisliak O.A., Nevzorova V.A., Savenkov M.P., Oshchepkova E.V., Ostroumova O.D., Boytsov S.A. Russian medical society expert consensus on arterial hypertension: arterial hypertension and Post-COVID syndrome. Systemic Hypertension. 2022; 19(3): 5-13. (in Russian)]. https://dx.doi.org/10.38109/2075-082X-2022-3-5-13.
- Haffke M., Freitag H., Rudolf G., Seifert M., Doehner W., Scherbakov N. et al. Endothelial dysfunction and altered endothelial biomarkers in patients with post-COVID-19 syndrome and chronic fatigue syndrome (ME/CFS). J. Transl. Med. 2022; 20(1): 138. https://dx.doi.org/10.1186/s12967-022-03346-2.
- Lomova N., Dolgushina N., Tokareva A., Chagovets V., Starodubtseva N., Kulikov I. et al. Past COVID-19: the impact on IVF outcomes based on follicular fluid lipid profile. Int. J. Mol. Sci. 2022; 24(1): 10. https://dx.doi.org/10.3390/ijms24010010.
- Frankevich N., Tokareva A., Chagovets V., Starodubtseva N., Dolgushina N., Shmakov R. et al. COVID-19 infection during pregnancy: disruptions in lipid metabolism and implications for newborn health. Int. J. Mol. Sci. 2023; 24(18): 13787. https://dx.doi.org/10.3390/ijms241813787.
- Hadid T., Kafri Z., Al-Katib A. Coagulation and anticoagulation in COVID-19. Blood Rev. 2021; 47: 100761. https://dx.doi.org/10.1016/j.blre.2020.100761.
- Fara A., Mitrev Z., Rosalia R.A., Assas B.M. Cytokine storm and COVID-19: a chronicle of pro-inflammatory cytokines. Open Biol. 2020; 10(9): 200160. https://dx.doi.org/10.1098/rsob.200160.
- Hu B., Huang S., Yin L. The cytokine storm and COVID-19. J. Med. Virol. 2021; 93(1): 250-6. https://dx.doi.org/10.1002/jmv.26232.
- Iba T., Connors J.M., Levy J.H. The coagulopathy, endotheliopathy, and vasculitis of COVID-19. Inflamm. Res. 2020; 69(12): 1181-9. https://dx.doi.org/10.1007/s00011-020-01401-6.
- Marín R., Pujol F.H., Rojas D., Sobrevia L. SARS- CoV-2 infection and oxidative stress in early-onset preeclampsia. Biochim. Biophys. Acta Mol. Basis Dis. 2022; 1868(3): 166321. https://dx.doi.org/10.1016/j.bbadis.2021.166321.
- Ye Y., Vattai A., Zhang X., Zhu J., Thaler C.J., Mahner S. et al. Role of plasminogen activator inhibitor type 1 in pathologies of female reproductive diseases. Int. J. Mol. Sci. 2017; 18(8): 1651. https://dx.doi.org/10.3390/ijms18081651.
- Joksic I., Mikovic Z., Filimonovic D., Munjas J., Karadzov O.N., Egic A. et al. Combined presence of coagulation factor XIII V34L and plasminogen activator inhibitor 1 4G/5G gene polymorphisms significantly contribute to recurrent pregnancy loss in Serbian population. J. Med. Biochem. 2020; 39(2): 199-207. https://dx.doi.org/10.2478/jomb-2019-0028.
- Воробьева Н.А., Воробьева А.И., Воронцова А.С. Прогнозирование риска развития протромбогенной готовности при инфекции COVID-19 с использованием генетического тестирования. Анализ риска здоровью. 2023; 2: 130-9. [Vorobyeva N.A., Vorobyeva A.I., Vorontsova A.S. Predicting risks of prothrombotic readiness under COVID-19 using genetic testing. Health Risk Analysis. 2023; (2): 130-9. (in Russian)]. https://dx.doi.org/10.21668/health.risk/2023.2.12.
- Николаева Л.И., Стучинская М.Д., Дедова А.В., Шевченко Н.Г., Хлопова И.Н., Кружкова И.С., Меркулова Л.Н., Кистенева Л.Б., Колобухина Л.В., Мукашева Е.А., Краснослободцев К.Г., Трушакова С.В., Крепкая А.С., Куприянов В.В., Никитенко Н.А., Хадорич Е.А., Бурмистров Е.М., Тюрин И.Н., Антипят Н.А., Бурцева Е.И. Ассоциация полиморфных вариантов генов системы гемостаза с течением COVID-19. Вопросы вирусологии. 2023; 68(5): 445-53. [Nikolaeva L.I., Stuchinskaya M.D., Dedova A.V., Shevchenko N.G., Khlopova I.N., Kruzhkova I.S., Merkulova L.N., Kisteneva L.B., Kolobukhina L.V., Mukasheva E.A., Krasnoslobodtsev K.G., Trushakova S.V., Krepkaya A.S., Kuprianov V.V., Nikitenko N.A., Khadorich E.A., Burmistrov E.M., Tyurin I.N., Antipyat N.A., Burtseva E.I. Association of polymorphic variants of hemostatic system genes with the course of COVID-19. Problems of Virology. 2023; 68(5): 445-53. (in Russian)]. https://dx.doi.org/10.36233/0507-4088-197.
- Whyte C.S., Simpson M., Morrow G.B., Wallace C.A., Mentzer A.J., Knight J.C. et al. The suboptimal fibrinolytic response in COVID-19 is dictated by high PAI-1. J. Thromb. Haemost. 2022; 20(10): 2394-406. https://dx.doi.org/10.1111/jth.15806.
- Zuo Y., Warnock M., Harbaugh A., Yalavarthi S., Gockman K., Zuo M. et al. Plasma tissue plasminogen activator and plasminogen activator inhibitor-1 in hospitalized COVID-19 patients. Sci. Rep. 2021; 11(1): 1580. https://dx.doi.org/10.1038/s41598-020-80010-z.
- Kosinska-Kaczynska K., Malicka E., Szymusik I., Dera N., Pruc M., Feduniw S. et al. The sFlt-1/PlGF ratio in pregnant patients affected by COVID-19. J. Clin. Med. 2023; 12(3): 1059. https://dx.doi.org/10.3390/jcm12031059.
- Giardini V., Ornaghi S., Gambacorti-Passerini C., Casati M., Carrer A., Acampora E. et al. Imbalanced angiogenesis in pregnancies complicated by SARS-CoV-2 infection. Viruses. 2022; 14(10): 2207. https://dx.doi.org/10.3390/v14102207.
- Яковлева Н.Ю., Васильева Е.Ю., Шелепова Е.С., Рябоконь Н.Р., Хазова Е.Л., Буравлева К.Р., Кузнецова Л.В., Зазерская И.Е. Изучение динамики концентраций факторов ангиогенеза на протяжении физиологической беременности. Акушерство и гинекология. 2016; 8: 49-53. [Yakovleva N.Yu., Vasilyeva E.Yu., Shelepova E.S., Ryabokon N.R., Khazova E.L., Buravleva K.R., Kuznetsova L.V., Zazerskaya I.E. Investigation of time course of changes in the concentrations of angiogenic factors on the length of physiological pregnancy. Obstetrics and Gynecology. 2016; (8): 49-53. (in Russian)]. https://dx.doi.org/10.18565/aig.2016.8.49-53.
- Creswell L., O'Gorman N., Palmer K.R., da Silva Costa F., Rolnik D.L. Perspectives on the use of Placental Growth Factor (PlGF) in the prediction and diagnosis of pre-eclampsia: recent insights and future steps. Int. J. Womens Health. 2023; 15: 255-71. https://dx.doi.org/10.2147/IJWH.S368454.
- Gemmati D., Vigliano M., Burini F., Mari R., El Mohsein H.H., Parmeggiani F. et al. Coagulation factor XIIIA (F13A1): Novel perspectives in treatment and pharmacogenetics. Curr. Pharm. Des. 2016; 22(11): 1449-59. https://dx.doi.org/10.2174/1381612822666151210122954.
- Джалилова Д.Ш., Макарова О.В. Молекулярно-биологические механизмы взаимосвязи гипоксии, воспалительных и иммунных реакций. Иммунология. 2019; 40(5): 97-105. [Dzhalilova D.Sh., Makarova O.V. Molecular-biological mechanisms of connection between hypoxia, inflammatory and immune reactions. Immunologiya. 2019; 40(5): 97-105 (in Russian)]. https://dx.doi.org/10.24411/0206-4952-2019-15011.
- Smadja D.M., Mentzer S.J., Fontenay M., Laffan M.A., Ackermann M., Helms J. et al. COVID-19 is a systemic vascular hemopathy: insight for mechanistic and clinical aspects. Angiogenesis. 2021; 24(4): 755-88. https://dx.doi.org/10.1007/s10456-021-09805-6.
- Kattula S., Bagoly Z., Tóth N.K., Muszbek L., Wolberg A.S. The factor XIII-A Val34Leu polymorphism decreases whole blood clot mass at high fibrinogen concentrations. J. Thromb. Haemost. 2020; 18(4): 885-94. https://dx.doi.org/10.1111/jth.14744.
- Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F. et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020; 383(2): 120-8. https://dx.doi.org/10.1056/NEJMoa2015432.
- LeGallo R. Placental vasculogenesis/angiogenesis. In: McManus L.M., Mitchell R.N., eds. Pathobiology of human disease. A dynamic encyclopedia of disease mechanisms. Academic Press; 2014.
Received 14.02.2024
Accepted 28.05.2024
About the Authors
Ekaterina M. Matusevich, Teaching Assistant at the Department of Obstetrics and Gynecology, Siberian State Medical University, Ministry of Health of Russia,634050, Russia, Tomsk, Moskovsky Trakt str., 2, +7(3822)533-309, e.matusevich@bk.ru
Sergey Yu. Yuryev, Dr. Med. Sci., Professor at the Department of Obstetrics and Gynecology, Siberian State Medical University, Ministry of Health of Russia,
634050, Russia, Tomsk, Moskovsky Tract str., 2, +7(3822)533-309.
Marina G. Nikolaeva, Dr. Med. Sci., Professor at the Department of Obstetrics and Gynecology, Altai State Medical University, Ministry of Health of Russia,
656038, Russia, Altai Territory, Barnaul, Lenin Ave., 40; Senior Researcher, Altai Branch of the National Medical Research Center of Hematology, Ministry of Health of Russia, 656024, Russia, Barnaul, Lyapidevsky str., 1, +7(3852)36-85-87.
Vladimir E. Frankevich, Dr. Sci. (Physico-mathematical), Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, Perinatology,
Ministry of Health of Russia, 117997, Russia, Moscow, Academician Oparin str., 4, +7(495)438-22-92.
Natalya A. Frankevich, PhD, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, Perinatology, Ministry of Health of Russia,
117997, Russia, Moscow, Academician Oparin str., 4, +7(495)438-22-92.
Irina S. Popova, PhD, Researcher at the Central Research Laboratory, Siberian State Medical University, Ministry of Health of Russia,
634050, Russia, Tomsk, Moskovsky Tract str., 2, +7(3822)533-309.
Tatyana N. Nemtseva, obstetrician-gynecologist, Professor Medical Center, Siberian State Medical University, Ministry of Health of Russia,
634050, Russia, Tomsk, Moskovsky Tract str., 2, +7(3822)533-309.



