The role of the components of the immune system in preterm birth due to placental dysfunction
Nikolaeva A.S., Tanysheva G.A., Koliado O.V., Remneva O.V., Molchanova I.V., Dyusupov A.A.
Objective: To evaluate the role of the components of the immune system in the pathogenesis of preterm birth due to severe preeclampsia and placental abruption of normally implanted placenta.
Materials and methods: A total of 80 women with induced preterm birth (the main group) were examined. The sample was divided into two subgroups. Subgroup 1 consisted of patients with abdominal delivery due to progression of severe preeclampsia. Subgroup 2 consisted of patients with preterm birth due to placental abruption of normally implanted placenta. The control group consisted of women with term uncomplicated birth. The levels of interleukin (IL)-8, IL-10, tumor necrosis factor alpha (TNF-α), human chorionic gonadotropin and pregnancy-associated plasma protein A (PAPP-A) were studied as the predictors of preterm birth.
Results: It was found that IL-8, TNF-α and PAPP-A play an important role in the pathogenesis of induced preterm birth due to severe obstetric complications. Elevation of IL-8 level by 1 unit increases the risk of preterm birth by 2.96 times; elevation of TNF-α level by 1 unit increases the risk of preterm birth by 1.56 times, while elevation of PAPP-A level by 1 unit reduces the risk of adverse outcomes by 0.04 times.
Conclusion: The components of the immune system play an important role in the pathogenesis of preterm birth and placental dysfunction. Research in this area expands knowledge of the mechanisms of severe obstetric complications and is important for prediction of adverse obstetric outcomes.
Authors' contributions: Nikolaeva A.S. – patient selection and examination, statistical data processing, Tanysheva G.A. – concept and design of the study; Kolyado O.A. – manuscript writing; Remneva O.V. – manuscript editing; Molchanova I.V. – review of publications on the topic of the article; Dyusupov A.A. – final approval of the manuscript.
Conflicts of interest: The authors confirm that they have no conflicts of interest to declare.
Funding: The study was carried out without any financial support.
Ethical Approval: The study was approved by the Ethics Committee of the Semey Medical University, Republic of Kazakhstan (2022).
Patient Consent for Publication: The patients have signed informed consent for publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Nikolaeva A.S., Tanysheva G.A., Koliado O.V., Remneva O.V., Molchanova I.V., Dyusupov A.A.
The role of the components of the immune system in preterm birth due to placental dysfunction.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (4): 79-84 (in Russian)
https://dx.doi.org/10.18565/aig.2025.28
Keywords
The issue of preterm birth (PB) still remains unsolved. This is largely due to a variety of causes and pathogenetic mechanisms leading to premature termination of pregnancy. Up to 70–80% of births before 37 weeks of gestation are spontaneous preterm births. Infection and inflammation play the main role in the genesis of SPBs. The impact of the infectious agent initiates a cascade of immune response that causes dynamic cervical changes or rupture of fetal membranes [1]. However, immune mechanisms are involved not only in the development of spontaneous preterm birth, but also in the genesis of other great obstetric syndromes, in particular, preeclampsia (PE) – the main pathology leading to induced preterm birth [2–7].
Impaired immunoregulatory functions play a role in one way or another in the processes of premature termination of pregnancy, causing labor contractions or damage to the fetoplacental complex [1, 2]. Research that expands the knowledge about the pathogenesis of PB is of great importance for finding the ways to predict PB and develop the preventive programs for high-risk groups.
The objective of the study was to evaluate the role of the components of the immune system in the pathogenesis of preterm birth due to placenta-mediated complications – severe preeclampsia (PE) or placental abruption.
Materials and methods
Eighty patients with induced and late PB were involved in the study. These women were included in the main group, which was divided into two subgroups depending on placenta-mediated pregnancy complication, that was an indication for cesarean section. Subgroup 1 consisted of 40 patients (n=40), who had abdominal delivery due to severe preeclampsia. Subgroup 2 consisted of 40 patients (n=40), who had preterm birth due to placental abruption without severe preeclampsia.
The general inclusion criteria in the subgroups of the main group were 32.0–36.6 weeks of gestation, maternal age 18–40 years.
Exclusion criteria included the following: multiple pregnancy, fetal growth restriction, premature rupture of membranes, pregnancy resulting from assisted reproductive technologies, severe somatic maternal diseases at the stage of subcompensation and decompensation, maternal traumas and acute infectious diseases during current pregnancy. The control group (n=80) consisted of patients who had term birth and had no obstetric complications.
The diagnosis of severe preeclampsia was made according to the criteria of clinical recommendations of the Ministry of Health of Russia (2021) [8]. The indications to stop watchful waiting strategy in severe preeclampsia were the following: severe refractory hypertension, the symptoms of eclampsia and other symptoms of organ failure.
The diagnosis of placental abruption was confirmed in all cases by ultrasound examination of the fetoplacental complex. Voluson E8 ultrasound system (General Electric, USA) was used for ultrasound examination.
All women gave birth at level III facility – the Perinatal Center in Semey, Republic of Kazakhstan, in 2022–2024.
One hour before delivery, blood samples from the cubital vein were collected from patients to analyze the markers of placental dysfunction and immune response: interleukin 8 (IL-8), IL-10, tumor necrosis factor alpha (TNF-α), human chorionic gonadotropin (hCG), and pregnancy-associated plasma protein A (PAAP-A). Concentrations of these markers were determined by enzyme immunoassay using immunoassay analyzer AIFR-01 “Uniplan” (Private JSC “Picon”, JSC “Vector-Best”, Novosibirsk Region, Koltsovo, Russia).
Statistical analysis
The data were analyzed using software package SPSS Statistics, version 20, and statistical programming language R, v. 4.2.3. Before performing statistical analysis, the quantitative data were tested for normality of distribution using graphical methods and the Shapiro–Wilk test. After that, the assumption about the normality of distribution of the quantitative data was rejected.
Descriptive statistics of quantitative data are presented as median (Me) and interquartile range (IQR), and qualitative data as absolute numbers and percentages.
The differences between two qualitative variables were tested using Pearson's chi-squared test. When the expected values were less than 5, Fisher’s exact test was used.
Comparison of IL-8, IL-10 and TNF-α levels between the groups was performed using the Kruskal–Wallis test. Post hoc comparisons were made using the Dunn–Bonferroni test. Spearman’s correlation was used to test relationship between the values of PAPP-A, hCG and IL-8, IL-10, TNF-α.
The differences were considered to be statistically significant at p<0.05 (after calculation of the number of comparisons).
Results
The comparison groups were comparable in age and parity. The mean age of patients in the main group was 31.1 years, in the control group – 30.5 years (р=1.00).
Assessment of the place of residence found that PB was most common among rural residents compared with women who had preterm birth in the control group (р=0.003). Assessment of somatic health showed that patients who had preterm birth due to placenta-mediated complications more often had thyroid disorders, that were mainly represented by autoimmune thyroiditis associated with subclinical hypothyroidism and multinodular goiter (р=0.023). Low levels of thyroid hormone before conception contributed to abnormal placentation and, as a result led to severe obstetric complications (Table 1).
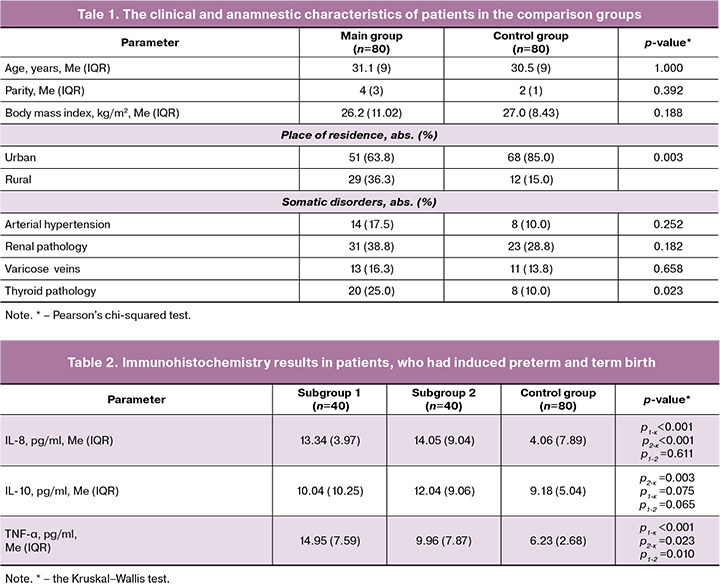
Comparison of the frequency and structure of extragenital pathology in women in both subgroups of the main group found no statistically significant differences: arterial hypertension was in 4/40 (100%) and 10/40 women (250%) (p=0.142); renal pathology was in 14/40 women (35.0%) and 17/40 women (42.5%) (р=0.492); varicose veins were in 10/40 (25.0%) and 3/40 women (7.5%) (р=0.070) in subgroups 1 and 2, respectively. The median gestational age at the time of giving birth was 34.6 (4.3) weeks in subgroup 1, and 36.2 (2.4) weeks in subgroup 2 (р=0.342).
The course of pregnancy at early gestation was significantly more often complicated by threatened spontaneous miscarriage in women in the main group compared with the control group – 34/80 (42.5%) and 14/80 (17.5%), respectively (p<0.001), and no differences were found in the subgroups between the women, who had preterm birth – 21/40 (52.5%) and 13/40 (32.5%), respectively (p=0.071).
Evaluation of serum hCG and PAPP-A levels was performed during the first biochemical screening at 11–13.6 weeks of pregnancy. Evaluation of the levels of laboratory markers reflecting the function of the fetoplacental complex, found that hCG levels were lower in patients in subgroup 1 compared with patients in subgroup 2 – 25.6 (15.56–102.00) ng/ml and 41.3 (18.40–128.00) ng/ml, respectively (p<0.001). However, the highest levels were found in patients in the control group – 57.5 (15.40–486.00) (p<0.001).
PAPP-А levels were similar in both subgroups of patients with induced PB – 0.47 (0.21–0.87) IU/L and 0.45 ( 0.06–2.64) IU/L; р=0.254, but significantly lower than in women in the control group – 0.92 ( 0.09–4.44] IU/L; р<0.001), that can indirectly indicate more pronounced placental dysfunction in patients whose pregnancies were subsequently complicated by severe PE, compared to women who had preterm birth due to placental abruption.
Immunohistochemistry results in patients in the comparison groups are represented in Table 2.
The results represented in Table 2 demonstrate that plasma levels of IL-8 in patients with PB due to placenta-mediated complications were significantly higher compared to women, who gave birth after full-term pregnancies (р<0.001). The levels of IL-10 were higher in patients in subgroup 2 (placental abruption) versus the control group (р=0.003). The levels of TNF-α were lower in women who had term birth versus women in both subgroups of the main group, who had abdominal delivery due to life threatening conditions for the mother and fetus (р<0/05). The highest levels of TNF-α were found in patients with severe preeclampsia.
There was a significant correlation between the immunological parameters. PAPP-A level had a moderate negative correlation with IL-8 level (rs=-0.449, p<0.001), as well as a weak negative correlation with TNF-α level (rs=-0.372, p<0.001). The relationship between PAPP-A and IL-10 levels was weak (rs=-0.209, p=0.008). HCG level had statistically significant weak negative correlation with IL-8 level (rs=-0.207, p=0.009) and TNF-α level (rs=-0.192, p=0.015).
Thus, the patients who had PB due to placental dysfunction, initially had failure of immune homeostasis. Further research to identify clinical and laboratory predictors may be important for construction of prognostic models of placenta-mediated pregnancy complications.
Discussion
Successful parturition is largely determined by the normal functioning of the fetoplacental complex. Impairment of trophoblast invasion and placental angiogenesis is the basis of major obstetric syndromes [2, 9–11]. In recent years, understanding of the pathophysiology of placental dysfunction was significantly extended, largely due to an understanding of the processes at the molecular level [2, 7]. Immune cells, cytokines and interferons are key mediators that ensure the process of pregnancy from implantation to delivery. Communication between mother and fetus, physiological course of pregnancy and successful pregnancy outcomes depend on interaction coordination of their signaling pathways [9].
TNF is a cytokine that plays a central role in regulation of inflammation. According to Zak Р. et al. (2019), in preeclampsia, elevated levels of TNF were found both in maternal serum and placental tissues [5]. The exact mechanism of TNF-α-mediated reduced trophoblast cell invasion is not yet well understood. However, it is believed that TNF inhibits trophoblast integration into endothelial cell networks and suppresses human trophoblast cell invasion. Literature review on exploration of the role placental macrophages, that was conducted by the researchers at the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia (2022), concluded that abnormal maternal immune response in PE is expressed by changes in the functional activity of the monocyte-macrophage system – the most important component of the innate immune system. The cause of defective placentation underlying PE can be impaired functioning of placental immune cells, namely, macrophages, which depending on their functional state can both produce pro-inflammatory cytokines and reactive oxygen/nitrogen species (activated M1 macrophages), and suppress inflammation by producing anti-inflammatory cytokines (M2 macrophages) [10].
In any case, persistent endothelial dysfunction in patients with early PE in history determines the need for personalized pregravid preparation [11].
Immune response plays an important role in occurrence of placental abruption. Bączkowska M. et al. (2021) reported that in placental abruption of normally implanted placenta, there are the signs of chronic non-infectious inflammation and enhanced cytotoxic immune response [12].
The physiological role of PAPP-A is still being explored. A meta-analysis by Tzanaki I. et al. (2025) showed that PAPP-A levels in the first trimester of pregnancy are significantly lower in women with early PE versus women without preeclampsia [13]. Côté M. et al. (2024) reported that a combination of low levels of PAPP-A and human placental growth factor (PlGF) is associated with the highest risk of placenta-mediated complications, such as PE, fetal growth restriction, placental abruption. However, assessment is important only when a complex of indicators is evaluated [14].
Analysis of pregnancy complicated by different types pathology, that was done by Shcherbakov V.I. et al. (2020) taking into account the same immune indicators showed commonality and specificity of their response. In different complocations of pregnancy, different functional loads can be carried by the same cytokines [15].
The results obtained in our study coincide with the results in the majority of similar studies, and demonstrate that in women with induced PB caused by PE and placental abruption, there is impaired immune regulation, in particular, high plasma levels of IL-8, TNF-α and low levels of PAPP-A.
Conclusion
Thus, immune response is important in the pathogenesis of induced preterm birth. Indicators of IL-8, TNF-α and РАРР-А levels have a promising prospect for the complex model building to predict the occurrence of placenta-mediated complications. However, further search for reliable predictive markers of severe course of PE and trigger mechanisms of placental abruption are necessary.
References
- Daskalakis G., Psarris A., Koutras A., Fasoulakis Z., Prokopakis I., Varthaliti A. et al. Maternal infection and preterm birth: from molecular basis to clinical implications. Children (Basel). 2023; 10(5): 907. https://dx.doi.org/10.3390/children 10050907.
- Hoffman M.K. The great obstetrical syndromes and the placenta. BJOG. 2023; 130(3): 8-15. https://dx.doi.org/10.1111/1471-0528.17613.
- Boulanger H., Bounan S., Mahdhi A., Drouin D., Ahriz-Saksi S., Guimiot F. et al. Immunologic aspects of preeclampsia. AJOG Glob. Rep. 2024; 4(1): 100321. https://dx.doi.org/10.1016/j.xagr.2024.100321.
- Melchiorre K., Giorgione V., Thilaganathan B. The placenta and preeclampsia: villain or victim? Am. J. Obstet. Gynecol. 2022; 226(2S): S954-S962. https://dx.doi.org/ 10.1016/j.ajog.2020.10.024.
- Zak P., Soucek M. Correlation of tumor necrosis factor alpha, interleukin 6 and interleukin 10 with blood pressure, risk of preeclampsia and low birth weight in gestational diabetes. Physiol. Res. 2019; 68(3): 395-408. https://dx.doi.org/ 10.33549/physiolres.934002.
- Vilotić A., Nacka-Aleksić M., Pirković, A., Bojić-Trbojević, Ž., Dekanski D. et al. IL-6 and IL-8: an overview of their roles in healthy and pathological pregnancies. Int. J. Mol. Sci. 2022; 23(23): 14574. https://dx.doi.org/ 10.3390/ijms232314574.
- Лазарева А.Ю., Фаткуллина И.Б. Иммунологические механизмы преждевременной отслойки нормально расположенной плаценты. Архив акушерства и гинекологии им. В.Ф. Снегирёва. 2024; 11(1): 35-9. [Lazareva A.Y., Fatkullina I.B. Immunological mechanisms of preterm abruption of the normally located placenta. V.F. Snegirev Archives of Obstetrics and Gynecology. 2024; 11(1): 35-9 (in Russian)]. https://dx.doi.org/10.17816/2313-8726-2024-11-1-35-39.
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Преэклампсия. Эклампсия. Отеки, протеинурия и гипертензивные расстройства во время беременности, в родах и послеродовом периоде. 2021. [Ministry of Health of the Russian Federation. Clinical guidelines. Preeclampsia. Eclampsia. Edema, proteinuria and hypertensive disorders during pregnancy, labor and the postpartum period. 2021. (in Russian)].
- Белокриницкая Т.Е., Витковский Ю.А., Фролова Н.И. Роль цитокинов и интерферонов при беременности. Фундаментальная и клиническая медицина. 2024; 9(3): 98-108. [Belokrinitskaya T.E., Vitkovsky Y.A., Frolova N.I. The role of cytokines and interferons during pregnancy. Fundamental and Clinical Medicine. 2024; 9(3): 98-108. (in Russian)]. https://dx.doi.org/10.23946/2500-0764-2024-9-3-98-108.
- Вишнякова П.А., Ельчанинов А.В., Киселева В.В., Муминова К.Т., Ходжаева З.С., Еремина И.З., Фатхудинов Т.Х. Роль плацентарных макрофагов при физиологической беременности и преэклампсии. Акушерство и гинекология. 2022; 4: 5-12. [Vishnyakova P.A., Elchaninov A.V., Kiseleva V.V., Muminova K.T., Khodzhaeva Z.S., Eremina I.Z., Fatkhudinov T.Kh. The role of placental macrophages in physiological pregnancy and preeclampsia. Obstetrics and Gynecology. 2022; (4): 5-12 (in Russian)]. https://dx.doi.org/10.18565/aig.2022.4.5-12.
- Терехина В.Ю., Николаева М.Г., Момот А.П., Кудинов А.В., Строганова Е.В., Хорева Л.А., Ясафова Н.Н., Бубликов Д.С. Сохраняющаяся дисфункция эндотелия у пациенток с ранней преэклампсией в анамнезе и особенности прегравидарной подготовки. Бюллетень медицинской науки. 2022; 3(27): 65-73. [Terekhina V.Yu., Nikolaeva M.G., Momot A.P., Kudinov A.V., Stroganova E.V., Khoreva L.A., Yasafova N.N., Bublikov D.S. Delayed endothelial dysfunction in patients with a history of early preeclampsia and features of pregravidary preparation. Bulletin of Medical Science. 2022; 3(27): 65-73. (in Russian)]. https://dx.doi.org/10.31684/25418475_2022_3_65.
- Bączkowska M., Zgliczyńska M., Faryna J., Przytuła E., Nowakowski B., Ciebiera M. Molecular changes on maternal-fetal interface in placental abruption - a systematic review. Int. J. Mol. Sci. 2021; 22(12): 6612. https://dx.doi.org/10.3390/ijms22126612.
- Tzanaki I., Makrigiannakis A., Lymperopoulou C., Al-Jazrawi Z., Agouridis A.P. Pregnancy-associated plasma protein A (PAPP-A) as a first trimester serum biomarker for preeclampsia screening: a systematic review and meta-analysis. J. Matern. Fetal. Neonatal. Med. 2025; 38(1): 2448502. https://dx.doi.org/10.1080/ 14767058.2024.2448502
- Côté M., Giguère Y., Forest J., Audibert F., Johnson J.A., Okun N. et al. First-trimester PIGF and PAPP-A and the risk of placenta-mediated complications: prediction prospective study. J. Obstet. Gynaecol. Can. 2024; 47(2): 1701-2163. https://dx.doi.org/10.1016/j.jogc.2024.102732.
- Щербаков В.И., Поздняков И.М., Ширинская А.В., Волков М.В. Роль провоспалительных цитокинов в патогенезе преждевременных родов и преэклампсии. Российский вестник акушера-гинеколога. 2020; 20(2): 15-21. [Shcherbakov V.I., Pozdnyakov I.M., Shirinskaya A.V., Volkov M.V. Role of pro-inflammatory cytokines in the pathogenesis of preterm birth and preeclampsia. Russian Bulletin of Obstetrician-Gynecologist. 2020; 20(2): 15-21 (in Russian)]. https://dx.doi.org/10.17116/rosakush20202002115.
Received 11.02.2025
Accepted 17.04.2025
About the Authors
Anna S. Nikolaeva, Researcher at the A.A. Kozbagarov Department of Obstetrics and Gynecology, Semey Medical University, 071400, Republic of Kazakhstan, Semey,Abay str., 103, +7(777)477-82-12, avealokinanna@mail.ru, https://orcid.org/0000-0002-2642-5141
Gulyash A. Tanysheva, PhD, Associate Professor, Head of the A.A. Kozbagarov Department of Obstetrics and Gynecology, Semey Medical University, 071400, Republic of Kazakhstan, Semey, Abay str., 103, +7(777)153-53-57, gulyash1965@mail.ru, https://orcid.org/0000-0001-9531-5950
Olga V. Koliado, PhD, Teaching Assistant at the Department of Obstetrics, Gynecology with course of additional professional education, Altai State Medical University, Ministry of Health of Russia, 656038, Russia, Barnaul, Lenin str., 40, +7(913)0842530, olga.kolyado@yandex.ru, https://orcid.org/0000-0002-5812-4925
Olga V. Remneva, Dr. Med. Sci., Professor, Head of the Department of Obstetrics, Gynecology with course of additional professional education, Altai State Medical University, Ministry of Health of Russia, 656038, Russia, Barnaul, Lenin str., 40, +7(913)2500280, rolmed@yandex.ru, https://orcid.org/ 0000-0002-5984-1109
Irina V. Molchanova, PhD, Associate Professor at the Department of Obstetrics, Gynecology with course of additional professional education, Altai State Medical University, Ministry of Health of Russia, 656038, Russia, Barnaul, Lenin str., 40, +7(903)9491064, molcanova2008@yandex.ru, https://orcid.org/0000-0002-0741-8974
Altay A. Dyusupov, Dr. Med. Sci., Professor, Rector, Semey Medical University, 071400, Republic of Kazakhstan, Semey, Abay str., 103, +7(7222)52-22-51,
altay.dyusupov@smu.edu.kz, https://orcid.org/0000-0003-0875-1020
Corresponding author: Olga V. Remneva, rolmed@yandex.ru



