Rationale for using clinical indicators to stratify pregnant women according to risk of preeclampsia severity
Tezikov Yu.V., Lipatov I.S., Khalitova A.I., Tyutyunnik V.L., Kan N.E., Yurasova E.V., Rudneva A.A., Garazhankin A.D.
Objective: To evaluate the feasibility of using clinical indicators to stratify pregnant women based on preeclampsia severity.
Materials and methods: Ninety-nine pregnant women with moderate preeclampsia were studied, including 48 with progression to severe preeclampsia (group I) and 51 without progression to severe preeclampsia (group II). The control group consisted of 30 healthy women with healthy pregnancies (group III). The examination was performed at hospital admission with moderate preeclampsia and included the progression of preeclampsia to severe preeclampsia. It comprises the determination of the type of 24-hour blood pressure (24h BP) profile, assessment of the frequency of nocturia (NU), periods of gestational sleep apnea, subjective evaluation of sleep characteristics, and the distribution of adipose tissue.
Results: A comparative analysis of high-risk factors for preeclampsia, timing of preeclampsia manifestation, concomitant pregnancy complications, and diagnostic criteria for preeclampsia revealed a lack of predictive capability of the indicators regarding the severity of this pregnancy complication. Analysis of the examination findings using descriptive statistics, univariate and multivariate logistic regression, ROC analysis, and clinical epidemiology tests enabled the development of a prognostic risk stratification model for identifying pregnant women at a high risk of developing severe preeclampsia. The most effective variables were the pathological types of 24-h BP and quantitative assessment of NU (AUC=0.849 –"very good" quality, 95% CI 0.735–0.923, p<0.001). Calculation of the main performance characteristics of clinical epidemiology showed a higher prognostic significance of the model compared to individual clinical criteria (Se=85.3 %, Sp=79.2 %, p<0.001).
Conclusion: A comprehensive clinical approach to predicting severe preeclampsia using the calculation of an integral indicator, has proven its validity and potential for timely diagnosis of early clinical manifestations of the pathological process. This ensures a rational choice of obstetric strategies and addresses the critical issue of preventing life-threatening obstetric complications.
Authors’ contributions: Khalitova A.I., Yurasova E.V., Rudneva A.A., Garazhankin A.D. – data collection and analysis;
Tezikov Yu.V., Lipatov I.S. – conception and design of the study, review of the relevant literature, drafting of the manuscript; Tyutyunnik V.L., Kan N.E. – data interpretation, editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the Samara State Medical University.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Tezikov Yu.V., Lipatov I.S., Khalitova A.I., Tyutyunnik V.L., Kan N.E., Yurasova E.V., Rudneva A.A., Garazhankin A.D. Rationale for using clinical indicators to stratify pregnant women according to risk of preeclampsia severity.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (1): 44-54 (in Russian)
https://dx.doi.org/10.18565/aig.2024.255
Keywords
Preeclampsia (PE) is a pressing issue in modern obstetrics. It represents a severe form of hypertensive disorder, one of the most severe complications, and is a leading cause of maternal and perinatal morbidity and mortality. According to various authors, the incidence of PE ranges from 3% to 8% of pregnancies and shows no tendency to decrease [1, 2]. Recent adverse outcome audits have suggested that the conditional preventability of maternal mortality from PE and eclampsia may reach 80.9%. A distinctive feature of PE is its unpredictability, rapid development, and atypical or latent manifestation of severe forms [3–5]. The ability to predict severe PE is crucial for timely and effective antihypertensive therapy, and if necessary, for making decisions regarding delivery or continuation of pregnancy.
Clinical practice and scientific research have confirmed the pregnant woman’s capacity for long-term compensation during the pathological course of gestation. In such cases, the quantitative parameters of laboratory tests often remain at borderline values for extended periods, which reflects the category of "norm of compensated pathology’ [6, 7]. Therefore, risk stratification of pregnant women by PE severity using clinical criteria may offer advantages over laboratory predictors, given that fluctuations in quantitative values can significantly change due to the multifaceted mechanisms of PE pathogenesis [8–10].
Moreover, modern scientific laboratory criteria for assessing the severity of PE are not always accessible in practical healthcare settings, as most are not included in the compulsory medical insurance system and not all maternity institutions possess the necessary laboratory equipment. The main clinical assessment criteria for the severity of PE, regardless of early or late manifestations, are anti-angiogenic changes that present as systemic alterations in hemodynamics (arterial hypertension) and the functional state of the kidneys (proteinuria and edema) [11]. Additionally, 24h BP fluctuations in arterial pressure (AP) and clinical characteristics of night sleep, such as gestational sleep apnea (GSA) and frequency of nocturnal awakenings for urination (nocturia), have diagnostic value [12–17].
In recent years, 24h BP has been actively studied in the context of obstetric pathology. Based on identified circadian rhythm patterns concerning AP, prognostic and early diagnostic criteria for PE, placental insufficiency, preterm birth, post-term pregnancy, and other gestational complications have been developed [18–20]. The four main types of 24h BP profiles are as follows: 1) dipper–the optimal type of circadian rhythm, occurring in 60–80% of cases, characterized by a decrease in systolic and diastolic pressure at night by 10–20%; 2) non-dipper–either no or insufficient decrease in AP at night (0–10%); 3) night-peaker–an increase in AP at night, often associated with sleep apnea and other serious disorders of autonomic regulation of sleep-wake functions; and 4) over-dipper–an excessive decrease in AP at night by an average of 20% or more. The pathological types of 24h BP include non-dipper, night-peaker, and over-dipper [12, 13].
An assessment of the qualitative characteristics of sleep resulting from changes in central regulation shows that preeclampsia (PE) is characterized by insomnia, which manifests as increased daytime sleepiness due to disruptions in the structure of nighttime sleep and the occurrence of pronounced negative dreams [21]. Additionally, both moderate and severe forms of PE in women are associated with GSA [14, 15]. One of the persistent and early clinical manifestations of PE is nocturia, which is defined as nighttime awakenings to urinate once or more. Although this symptom has been recognized for a long time, its significance resurfaced in 2002 when the International Society for Continence of Urination compared the informational content of two symptoms – both termed "nocturia" – and decided to use only one term, "nocturia" (NU), to designate them. NU is a multifactorial symptom associated with insulin resistance and serves as a marker for hormonal dysfunction, endothelial and mitochondrial metabolic dysfunction, and disturbances in the cellular energy supply of the reproductive and urinary systems [16, 17, 22]. Similarities between PE and the structural and functional phases of metabolic syndrome have been established, with common pathogenetic mechanisms including diabetogenic changes (such as pathological insulin resistance and hyperinsulinemia), atherogenicity (dyslipidemia with an increase in atherogenic lipid fractions), and destabilization of the vascular endothelium related to insulin resistance. These factors contribute to an inflammatory, oxidative, and prothrombogenic state, driven by the primary need to supply the developing fetus with essential plastic and energy materials amidst gestational maladaptation, hereditary deviations, epigenetic dysregulation, altered lifestyles, and the presence of somatic, endocrine, and infectious pathologies [7–9, 23]. Considering the fundamental mechanisms of energy and plastic support for the fetus in PE, namely pathological insulin resistance and hyperinsulinemia, along with their connection to visceral fat deposition similar to that seen in metabolic syndrome, observations have revealed an associative relationship between pathogenetic indicators and clinical manifestations of PE, particularly regarding the dynamics of preperitoneal fat accumulation in pregnant women [24].
Undoubtedly, a key approach for optimizing gestational outcomes in PE involves the development of highly informative methods for predicting, early diagnosis, and implementing effective prevention strategies. Severe complications of PE occur primarily in its most severe form, making the identification of high-risk pregnant women essential. This selection process is extremely important and practically necessary.
This study aimed to evaluate the feasibility of using clinical indicators to stratify pregnant women based on preeclampsia severity.
Materials and methods
To identify differences between pregnant women with moderate PE who did not progress to a severe form and those who did, we compared the examination results of 99 pregnant women with moderate PE and without pronounced somatic pathology. Group 1 comprised 48 patients with moderate PE who progressed to a severe form, whereas Group 2 consisted of 51 patients with moderate PE who did not progress. Group 3, which served as a control, included 30 women with uncomplicated pregnancies and no somatic pathology.
Inclusion criteria for groups 1 and 2 included moderate PE with (Group 1) or without (Group 2) progression to a severe form, arterial pressure (AP) levels less than 130/85 mmHg, a body mass index (BMI) between 18.5 and 24.9 kg/m² at the pre-pregnancy stage, and the absence of metabolic disorders. Exclusion criteria included diabetes mellitus during pregnancy (both manifest and gestational); infectious, somatic, endocrine, and autoimmune pathologies; pregnancy resulting from assisted reproductive technologies; congenital malformations of the fetus; and failure to comply with the examination protocol.
When diagnosing PE, we considered the WHO Health Organization criteria approved by the Ministry of Health of the Russian Federation, as outlined in the current clinical guidelines (2021) [11]. Additional examinations of pregnant women were conducted upon admission to the hospital during the third trimester, when clinical manifestations of moderate PE were present, as well as during the progression from moderate to severe PE (Group 1). These examinations included determination of the type of 24h BP, assessment of the frequency of NU, evaluation of GSA periods, assessment of the qualitative characteristics of sleep, and analysis of the distribution of adipose tissue.
To identify 24h BP, the BP-Lab system (OOO "Petr Telegin,” Russia) was used. The following requirements were met during 24h BP monitoring: a minimum duration of 23 h with at least 56 successful measurements and a poor-quality record lasting no more than 1 h. Polysomnographic monitoring was conducted using a Somno Check 2 device (Weinmann, Germany) to diagnose GSA. The obtained data were assessed automatically, calculating the apnea/hypopnea index, with a diagnostic threshold for GSA set at more than five episodes per hour.
For a subjective assessment of sleep characteristics, we analyzed data collected from the questionnaire by Levin Ya.I. (1998). This questionnaire evaluated sleep quality, duration, time taken to fall asleep, dreams, and night awakenings using a scoring system, with a score of 22 or higher indicating that sleep characteristics were within the “norm”; scores between 19 and 21 were classified as “borderline,” suggesting a level between normal sleep and sleep disorders; and scores of 18 or lower indicated “insomnia” (sleep disorder) [21].
To identify the characteristics of adipose tissue deposition, the Voluson E6 GE Healthcare ultrasound system (USA) was used to measure subcutaneous and preperitoneal fat thicknesses (SCFT and PPFT). The abdominal wall fat index (AWFI), calculated as the PPFT/SCFT ratio, indicates visceral redistribution of adipose tissue when AWFI is greater than 1.0 [24]. To assess the severity of placental insufficiency, we used the classification of Strizhakov A.N. et al. (2014) [25].
Statistical analysis
The results of the clinical examinations were analyzed using IBM SPSS Statistics 25 software. The assumption of normality was assessed using the Shapiro–Wilk test. Continuous variables showing normal distribution were expressed as means (M) and standard deviation (SD); otherwise, median (Me) with the interquartile range [25th quartile (Q1) and 75th quartile (Q3)] were reported. Statistical differences between groups were identified using one-way analysis of variance (ANOVA) or Mann–Whitney U test. Intragroup dynamics of the parameters were assessed using the paired Wilcoxon test. Categorical variables were evaluated using the Pearson χ² test with Yates correction. The strength and direction of the correlation between the indicators were assessed using Spearman’s rank correlation coefficient. The predictive value of each clinical indicator was determined using univariate and multivariate logistic regression analysis. A logistic regression model was developed to classify pregnant women into risk groups, with all possible combinations of clinical signs assessed as dependent variables in the models. The independent variable was group membership of pregnant women. The quality of the prognosis was evaluated using ROC analysis. Clinical epidemiological tests were performed to assess the possible practical applications of the developed prognostic criteria. Differences were considered statistically significant at p of less than 0.05 [26, 27].
Results and discussion
The mean age of the pregnant women was 32 (3.5), 31 (3.8), and 29 (3.2) years in groups I, II, and III, respectively (pI-II=0.42, pII-III=0.34, and pI-III=0.26). Analysis of high-risk factors among 99 pregnant women in groups I and II with PE showed their presence in 97% (96/99) of cases, whereas severe PE was observed in women with obligatory high-risk factors. In group I, pregnant women with severe PE were more likely to have a personal history of PE complicated by PE: 72.9% (35/48); severe PE in the history was found in 35.4% (17/48); a family history complicated by PE was detected in 16.6% (8/48); and the least common factor was being "primiparous of late reproductive age," noted in 10.4% (5/48) of women. In group II, among pregnant women without progression of moderate PE, 52.9% (27/51) had an obstetric history complicated by PE, 17.6% (9/51) had a severe course of PE in their personal history, 23.5% (12/51) had a family history complicated by PE, 13.7% (7/51) were primiparous of late reproductive age, and in 5.8% (3/51), high-risk factors were not identified. In control group III, risk factors for PE development were absent.
The frequency of the factor "medical history of PE " in groups I and II showed statistically insignificant differences (χ²=3.41, p=0.07); however, there was a tendency for the presence of this high-risk factor to increase in group I with the progression of PE severity. Data on complicated medical history of severe PE in the comparison groups showed a similar pattern (χ²=3.17, p=0.08). The results for the factor "complicated family history" in groups I and II also did not differ statistically (χ²=0.36, p=0.55), nor was there any difference for the factor "primigravida of late reproductive age" (χ²=0.04, p=0.85). Pregnant women in the comparison groups did not receive PE prophylaxis with low doses of acetylsalicylic acid.
The timing of PE manifestation was analyzed separately: in group I, early PE (gestational age from 29+5 to 33+6 weeks) and late PE (from 34+2 to 36+6 weeks) were diagnosed in 35.4% (17/48) and 64.6% (31/48), respectively; in group II, the frequency of early PE (gestational age from 29+4 to 33+5 weeks) was 29.4% (15/51), while the late phenotype (from 34+3 to 37+1 weeks) accounted for 70.6% (36/51). Despite the more frequent clinical manifestations of severe PE with early onset (35.4% vs. 29.4%), no statistical differences were observed between groups I and II (χ²=0.18, p=0.67).
In the comparative analysis, the features of the clinical course of pregnancy in groups I and II consisted of the following obstetric complications: early toxicosis of moderate severity was observed in 18.7% (9/48) of group I and 15.6% (8/51) of group II (χ²=0.02, p=0.89); the threat of pregnancy termination was noted in 31.2% (15/48) and 31.3% (16/51) (χ²=0.01, p=0.92); retrochorial hematoma occurred in 14.6% (7/48) and 11.7% (6/51) (χ²=0.04, p=0.84); preterm birth was documented in 29.2% (14/48) and 9.8% (5/51) (χ²=4.79, p=0.03); placental insufficiency was seen in 43.8% (21/48) and 19.6% (10/51) (χ²=5.63, p=0.02); fetal growth restriction was present in 35.4% (17/48) and 15.5% (8/51) (χ²=4.11, p=0.04); and premature placental abruption was found in 12.5% (6/48) and 0% (0/51) (χ²=4.77, p=0.03). In group III, gestational complications were not diagnosed. There were statistically significant differences between the comparison groups for complications such as placental insufficiency (p=0.02), fetal growth restriction (p=0.04), premature placental abruption (p=0.03), and preterm birth (p=0.03). However, this obstetric pathology was more frequently associated with the clinical manifestations of severe PE, allowing us to classify these criteria as late clinical characteristics of severe PE.
We conducted a comparative analysis of the characteristics of arterial pressure (AP) and proteinuria levels in women in groups I and II, depending on the severity of PE, upon admission in all pregnant women with moderate PE and those with progression to severe PE (Table 1). The initial parameters (on admission) did not show statistically significant differences in the levels of systolic, diastolic, mean AP, or proteinuria in single/daily portions, indicating the impossibility of predicting progression from moderate to severe PE.
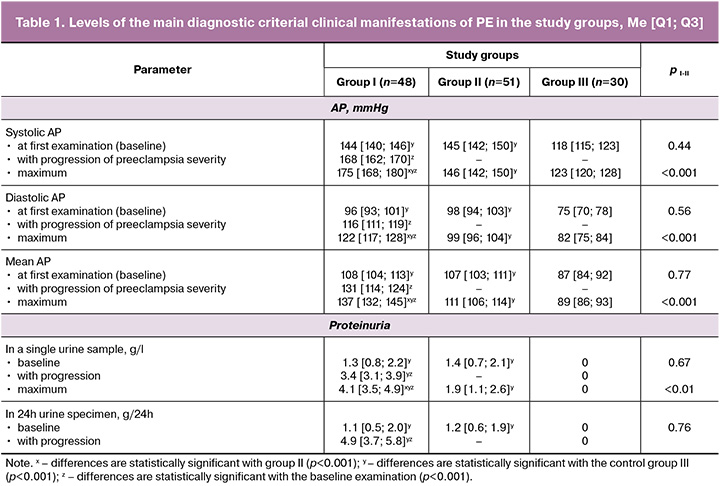
In general, a comparative analysis of data regarding the presence of high-risk factors, timing of manifestation, concomitant gestational pathology, and the PE criterion dyad revealed an absence of statistically significant indicators that could serve as predictive criteria for risk stratification of pregnant women based on PE severity.
The specificities of the studied clinical indicators in pregnant women with moderate PE progressing to severe PE, as well as in those without PE severity progression, are presented in Table 2.
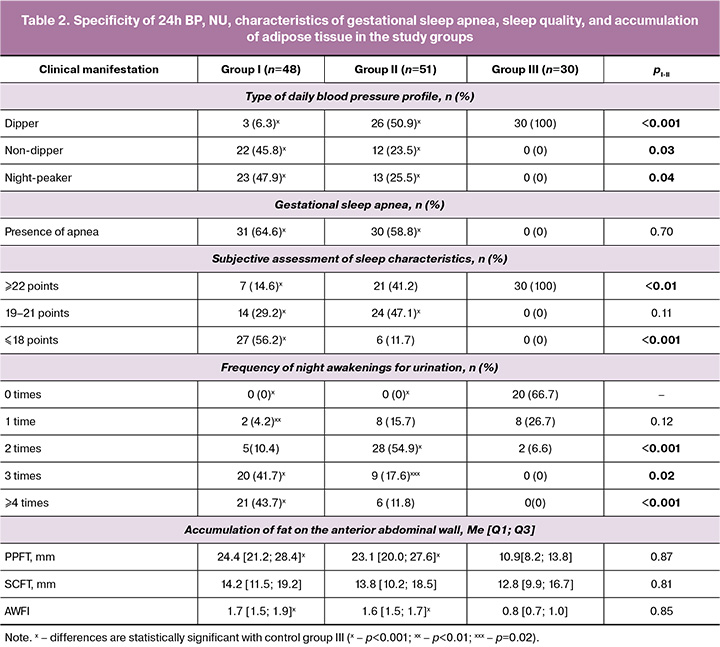
Of the four types of AP circadian rhythms, only dipper (29.3%, 29/99), non-dipper (34.3%, 34/99), and night-peakers (36.4%, 36/99) were diagnosed in 99 pregnant women with moderate PE in comparison to groups I and II; however, the pathological type of 24h BP, over-dipper, was not identified in any of the examined pregnant women with moderate PE. Intragroup analysis of 24h BP showed that the normal type (dipper) was found in 6.3% (3/48) of pregnant women in group I, 50.9% (26/51) in group II, and 100% (30/30) in control group III. When analyzing the pathological types of 24h BP, it was revealed that night-peaker and non-dipper types were more often identified in group I (93.7%, 45/48), with statistically significant differences compared to group II (49%, 25/51) [χ²=20.24, p<0.001]. The incidence of individual pathological types of 24h BP showed statistically significant differences favoring pregnant women in group I for both the non-dipper type (45.8% vs. 23.5%) [χ²=4.51, p=0.03] and the night-peaker type (47.9% vs. 25.5%) [χ²=4.45, p=0.04], indicating a 1.8 times higher prevalence of pathological types of 24h BP (93.7% vs. 49%) in pregnant women with the transformation of moderate PE into a severe course. The connection between the identified pathological types of 24h BP and the progression of PE severity can be explained by the increased destabilization of the vascular endothelium due to structural and functional remodeling of the vascular wall, which increases its rigidity. This breakdown in the mechanisms of multilevel regulation of AP in the dynamics of circadian rhythms creates a vicious circle of clinical symptoms that exacerbate one another (pathological type of 24h BP ↔ visceral fat deposition ↔ sleep apnea ↔ insomnia ↔ nocturia) [14, 16–18, 21]. Adaptive gestational changes in the respiratory system create conditions for difficulty in breathing, which can manifest as a specific complication known as gestational sleep apnea (GSA), an analog of obstructive sleep apnea syndrome. GSA is accompanied by hypoxemia/hypoxia, and consequently, a shift in the balance of redox reactions and the oxidant/antioxidant system, leading to oxidative stress, an important factor in the pathogenesis of PE [14, 15]. GSA occurred among pregnant women in comparison groups I and II in 64.5% (31/48) and 58.8% (30/51) of the cases, respectively; however, no statistically significant differences were found between the groups (χ²=0.15, p=0.70). No complications were observed in the control group.
An assessment of the qualitative characteristics of sleep revealed that women with moderate PE in both groups I (56.2%, 27/48) and II (11.7%, 6/51) experienced insomnia (χ²=20.06, p<0.001); In the control group, all pregnant women had questionnaire results corresponding to more than 22 points. Insomnia is a pathology of central genesis and is associated with disturbances in the nocturnal rhythm of systemic hemodynamics, hypoxia, disruptions in sleep structure, loss of deep sleep stages, and nocturnal awakenings for urination. These factors ultimately lead to disruption of the functional state during daytime and cognitive dysfunction [21].
Analysis of NU manifestations showed that both groups I and II had statistically significant differences in the frequency of nocturnal awakenings for urination compared to the control group of healthy women [χ²I-III=39.6, p<0.001; χ²II-III=41.6, p<0.001]. The average frequency of nocturnal awakenings per woman in group I was 3.8, 2.3, and 0.4, respectively. The most pronounced NU manifestations (three or more times) were observed in women in group I (85.4%, 41/48) compared to those in group II (29.4%, 15/51), indicating a connection between NU and PE severity (χ²=29.3, p<0.001). Night awakening for urination serves as a marker of poor-quality night sleep and is an independent stress factor. Repeated sleep disturbances due to urination are accompanied by a sharp increase in catecholamines, leading to changes in heart rate and AP, which can result in periodic hypoxia of the heart muscle, increased pathological insulin resistance, vascular and endocrine dysfunction, and a procoagulant state [9, 16, 17]. In recent decades, a pathogenetic relationship has been established between the development of arterial hypertension and visceral distribution of adipose tissue, which is a specific sign of metabolic syndrome characterized by high cardiovascular risks [7, 9]. The PPFT in women with moderate PE in groups I and II, with a normal body mass index at the pre-pregnancy stage, was 2 times higher than the control values (pI-III<0.001, pII-III<0.001). In this case, the AWFI in groups I and II of pregnant women corresponded to 1.7 [1.5; 1.9] and 1.6 [1.5; 1.7], respectively, which significantly exceeded the level in the control group (pI-III<0.001, pII-III<0.001), indicating a visceral type of fat deposition similar to metabolic syndrome. However, in the intergroup comparison, this indicator did not show statistically significant differences in pregnant women with different courses of moderate PE (p=0.85). Correlation analysis between NU and other clinical manifestations showed a moderate positive relationship with pathological types of 24h BP, coded as "0" for normal, "1" for non-dippers, and "2" for night-peakers (rho=0.64, p<0.001), with GSA manifestations (rho=0.61, p<0.001), and a moderate negative relationship with subjective sleep characteristics (rho=-0.65, p<0.001).
The results of a comprehensive clinical examination of 99 pregnant women with moderate PE in comparison to groups I and II, which were heterogeneous due to the presence of progression from moderate to severe PE in group I, identified clinical criteria with varying predictive capabilities regarding the severity of PE. Evaluation of the progression time from moderate to severe PE in group I indicated that aggravation of the PE course, considering the dyad criterion (AP≥160 mmHg, proteinuria ≥5 g/day), was observed within an interval of 3 to 10 days (M (SD) – 6 (2) days). It is important to note that, unlike laboratory parameters, clinical manifestations of the pathological process already reflect the resulting influence of different factors on the genesis of PE, taking into account individual protective mechanisms.
Using univariate analysis, we assessed the information value of various additional clinical manifestations – NU, pathological types of 24h BP (non-dipper, night-peaker), insomnia (less than 19 points), GSA, and features of fat deposition on the anterior abdominal wall – for the risk stratification of pregnant women by the severity of PE (Table 3).
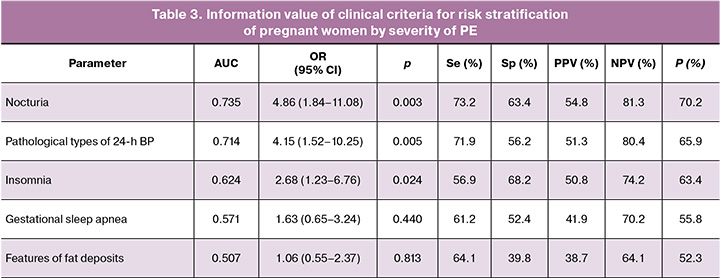
From the analyzed examination data, it follows that in the risk stratification of pregnant women by the severity of PE, the NU criterion (3 or more times; AUC=0.735) and 24h BP (pathological types of 24h BP (non-dipper, night-peaker; AUC=0.714) demonstrated the maximum information value. This suggests a significant role for these clinical manifestations in the nature of PE, primarily because of their connection with insulin resistance and the associated inflammatory, prothrombogenic, and oxidative status, as well as the compensatory capabilities of vascular tone regulation, all of which are reflected in sleep patterns, functional state of organs and systems, and circadian rhythm of the studied parameters [9, 28, 29].
However, despite the “good” informativeness quality of the clinical criteria (NU and 24h BP), the calculation of the main operational characteristics in clinical epidemiology revealed minimal clinical acceptability of the sensitivity indicators (Se) at 73.2% and 71.9%, respectively, at p<0.05, and low values of the specificity indicator (Sp) of 63.4% and 56.2%, respectively, at p<0.05. The issue of increasing the informativeness of predicting severe PE was resolved by objectifying the additive effects of the indicators.
The comprehensive application of the selected criteria for the risk stratification of pregnant women based on PE severity was reflected in the multivariate logistic regression model. The most effective variables identified were the pathological types of 24h BP (non-dipper, night-peaker) and quantitative assessment of NU (three or more night awakenings for urination) (Table 4).
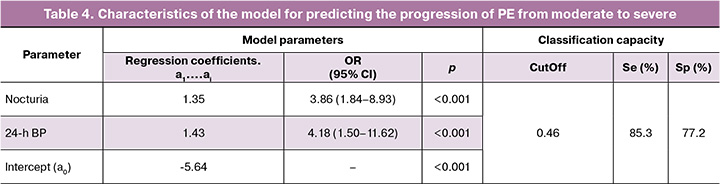
Using the clinical criteria (NU, 24h BP), the values of the classification functions were calculated using the following formula: Z = 1.35 × NU + 1.43 × 24h BP – 5.64. When determining the value of the integrative resultant (Z), the following were taken into account: NU, the number of episodes of sleep interruption for urination; presence/absence of pathological type of 24h BP (24h BP profile in pregnant women with moderate PE corresponding to non-dipper, night peaker is 1, corresponding to a normal profile (dipper) is 0). To determine the probability of moderate PE progression to severe severity (P), the obtained digital value of Z was considered in the calculation: P=1/(1+e-z), where e=const=2.72. The value of the final indicator P≥0.46 (CutOff) indicates a high risk of moderate PE progression to severe within 3-10 days; the value of the indicator P<0.46 indicates a low risk of transformation, that is, the course of PE will correspond to moderate severity in the next 3-10 days. Re-evaluation of the prognosis of PE severity should be carried out 10 days after the first examination. Assessment of the informativeness of the predictive model using ROC analysis showed “very good” quality (AUC=0.849; 95% CI, 0.735–0.923; p<0.001). Calculation of the main operational characteristics of clinical epidemiology showed a higher prognostic significance of the developed model compared to individual clinical criteria (NU, 24h BP): Se=85.3%, Sp=77.2% – with СutOff equal to 0.46. In the comparative aspect, ROC curves of the prognostic model and individual clinical criteria for the prediction of severe PE are presented in the figure.
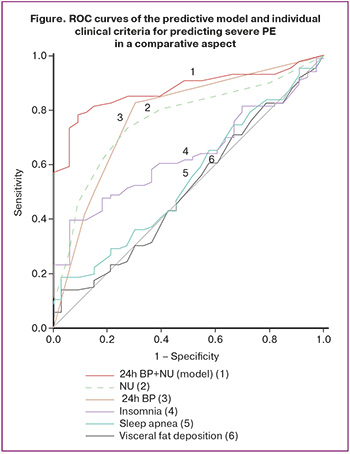
The informativeness of additional clinical criteria in predicting the severity of PE can be explained by the fact that in moderate PE transitioning to a severe degree, the mutual influence of pathological types of 24h BP characterized by either insufficient decrease or increase in AP at night (93.7%) is associated with borderline or negative characteristics of nighttime sleep (85.4%) and pronounced manifestations of NU (85.4%). This occurs against the backdrop of the basic diagnostic criterion dyad (AP levels and proteinuria), which is consistent with moderate PE. This indicates instability in the clinical condition with a significant impairment of compensatory-adaptive capabilities, which is subsequently reflected in the progression of PE severity. Achieving a positive effect from the predictive assessment of clinical criteria for risk stratification of pregnant women by the severity of PE is linked to a comprehensive evaluation of previously unused clinical parameters that are characterized by non-invasiveness, safety for the "mother-fetus" functional system, and accessibility for modern obstetric institutions.
Several studies dedicated to predicting severe PE have attempted to forecast its occurrence from the first trimester of pregnancy using anamnestic factors (such as heredity and history of cardiovascular pathology), clinical data (such as the presence of anemia), and laboratory findings for programming [30]. Additionally, predictions of severe PE have been made considering biochemical markers of the pro- and anti-angiogenic state (including vascular endothelial growth factor and fms-soluble tyrosine kinase-1) [10], as well as through the values of predictor indices involving insulin and placental growth factor levels [9, 22]. However, these studies demonstrated insufficient informativeness of predictive criteria (Se, Sp) due to the need for extensive laboratory examinations at the early stages of gestation, often in the absence of significant changes in characteristic indicators. In this study, risk stratification was applied to already existing moderate PE with extensive pathogenesis and clinical manifestations. The prognostic value of this approach is represented by a model of the individual risk for the development of severe PE.
Conclusion
Given the unpredictable nature of PE and the challenges in assessing its severity, a comprehensive approach to predict severe PE involves risk stratification of pregnant women who exhibit moderate symptoms of this condition. By calculating a unified indicator, we can utilize existing clinical criteria, such as severe NU and pathological 24h BP, to enhance the predictive capabilities. This methodology allows for the timely diagnosis of early clinical manifestations of the severity of the condition and supports a rational choice of obstetric strategies.
The reduced accuracy of predicting severe PE based on individual clinical manifestations (such as the severity of NU, type 24h BP, GSA, and insomnia) without calculating the integral indicator arises because these parameters contribute differently to the course of PE in each specific clinical situation. This variability complicates the interpretation of the examination results.
Evaluating the clinical signs proposed for predicting severe PE in conjunction with an integral indicator offers a timely opportunity to assess the nature of disease progression. This assessment reflects the maladaptation of a pregnant woman's body to the gestational process. The comprehensively evaluated clinical manifestations characterize the primary mechanism of PE development: endothelial dysfunction associated with pathological insulin resistance, systemic and intracellular inflammation, oxidative stress, and cellular metabolism disorders leading to multiple organ failure. This approach is justified for stratifying pregnant women into risk groups based on the severity of PE and objectifying the choice of appropriate medical tactics.
The scientific and practical direction aimed at developing methods for predicting the nature of PE using accessible and clinically significant early criteria addresses the critical issue of preventing life-threatening obstetric complications.
References
- Серов В.Н., Нестерова Л.А. Особенности современного акушерства. Акушерство и гинекология. 2022; 3: 5-11. [Serov V.N., Nesterova L.A. Features of modern obstetrics. Obstetrics and Gynecology. 2022; (3): 5-11 (in Russian)]. https://dx.doi.org/10.18565/aig.2022.3.5-11.
- Testo A.A., McBride C., Bernstein I.M., Dumas J.A. Preeclampsia and its relationship to pathological brain aging. Front. Physiol. 2022; 13: 979547. https://dx.doi.org/10.3389/fphys.2022.979547.
- Федеральная служба государственной статистики. Здравоохранение в России. Статистический сборник 2023. М.: Росстат; 2023. 179 с. [Federal State Statistics Service. Healthcare in Russia. Statistical collection 2023. Moscow: Rosstat; 2023. 179 p. (in Russian)].
- Сидорова И.С., Никитина Н.А., Гусева Е.В. Результаты конфиденциального аудита материнской смертности от преэклампсии и эклампсии в России в 2017-2018 гг. Акушерство и гинекология. 2020; 1: 119-27. [Sidorova I.S., Nikitina N.A., Guseva E.V. The results of a confidential audit of maternal mortality due to preeclampsia and eclampsia in Russia in 2017-2018. Obstetrics and Gynecology. 2020; (1): 119-27. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.1.119-127.
- Севостьянова О.Ю., Мартиросян С.В., Салимова И.В., Савельева Е.В., Коровникова О.В., Перевозкина О.В. Результаты аудита клинического протокола по предупреждению преэклампсии у беременных женщин группы риска в перинатальном центре. Уральский медицинский журнал. 2020; 6(189): 34-8. [Sevostyanova O.Y., Martirosyan S.V., Salimova I.V., Savelyeva E.V., Korovnikova O.V., Perevozkina O.V. Results of the audit of the clinical protocol for the prevention of preeclampsia in pregnant women of risk group in the perinatal center. Ural Medical Journal. 2020; 6(189): 34-8. (in Russian)]. https://dx.doi.org/10.25694/ URMJ.2020.06.09.
- Le Y., Ye J., Lin J. Expectant management of early-onset severe preeclampsia: a principal component analysis. Ann. Transl. Med. 2019; 7(20): 519. https://dx.doi.org/10.21037/atm.2019.10.11.
- Липатов И.С., Тезиков Ю.В., Шмаков Р.Г., Азаматов А.Р., Мартынова Н.В. «Беременность – естественная модель метаболического синдрома»: результаты динамического исследования физиологической гестации. Акушерство и гинекология. 2020; 9: 88-96. [Lipatov I.S., Tezikov Yu.V., Shmakov R.G., Azamatov A.R., Martynova N.V. Pregnancy as a natural model of metabolic syndrome: results of a dynamic study of physiological gestation. Obstetrics and Gynecology. 2020; (9): 88-96. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.9.88-96.
- Ходжаева З.С., Ошхунова М.С., Муминова К.Т., Горина К.А., Холин А.М. Прогнозирование и ранняя диагностика преэклампсии: научные перспективы и клинические возможности. Акушерство и гинекология. 2022; 12: 57-65. [Khodzhaeva Z.S., Oshkhunova M.S., Muminova K.T., Gorina K.A., Kholin A.M. Prediction and early diagnosis of preeclampsia: scientific perspectives and clinical opportunities. Obstetrics and Gynecology. 2022; (12): 57-65. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.218.
- Липатов И.С., Тезиков Ю.В., Азаматов А.Р., Шмаков Р.Г. Общность клинических проявлений преэклампсии и метаболического синдрома: поиск обоснования. Акушерство и гинекология. 2021; 3: 81-9. [Lipatov I.S., Tezikov Yu.V., Azamatov A.R., Shmakov R.G. Identity of preeclampsia and metabolic syndrome clinical manifestations: searching for substantiation. Obstetrics and Gynecology. 2021; (3): 81-9. (in Russian)]. https://dx.doi.org/10.18565/aig.2021.3.81-89.
- Игнатко И.В., Флорова В.С., Кузнецов А.С., Кузина Е.Ю. Роль биохимических маркеров в стратификации риска развития преэклампсии: взгляд клинициста. Архив акушерства и гинекологии им. В.Ф. Снегирева. 2017; 4(4): 181-6. [Ignatko I.V., Florova V.S., Kuznetsov A.S., Kuzina E.Yu. The role of biochemical markers in the risk stratification for development of preeclampsia: the clinician's view. Archives of Obstetrics and Gynecology named after V.F. Snegirev. 2017; 4(4): 181-6. (in Russian)]. https://dx.doi.org/10.18821/2313-8726-2017-4-4-181-186.
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Преэклампсия. Эклампсия. Отеки, протеинурия и гипертензивные расстройства во время беременности, в родах и послеродовом периоде. М.; 2024. 74 с. [Ministry of Health of the Russian Federation. Clinical guidelines. Pre-eclampsia. Eclampsia. Edema, proteinuria and hypertensive disorders during pregnancy, childbirth and postpartum period. Moscow; 2024. 74 р. (in Russian)].
- Мирошниченко А.И., Иванов К.М. Влияние ночного повышения артериального давления на ремоделирование сердца у пациентов с артериальной гипертонией. Аспирантский вестник Поволжья. 2019; 1-2: 65-9. [Miroshnichenko A.I., Ivanov K.M. The effect of nocturnal increase in blood pressure on remodeling of the heart in patients with arterial hypertension. Aspirantskii vestnik Povolzh'ya. 2019; (1-2): 65-9. (in Russian)]. https://dx.doi.org/ 10.17816/2072-2354.2019.19.1.65-69.
- Altikardes Z.A., Kayikli A., Korkmaz H., Erdal H., Baba A.F., Fak A.S. A novel method for dipper/non-dipper pattern classification in hypertensive and non-diabetic patients. Technol. Health Care. 2019; 27(S1): 47-57. https://dx.doi.org/ 10.3233/THC-199006.
- Osman A.M., Carter S.G., Carberry J.C., Eckert D.J. Obstructive sleep apnea: current perspectives. Nat. Sci. Sleep. 2018; 10: 21‐34. https://dx.doi.org/ 10.2147/NSS.S124657.
- Калачин К.А., Пырегов А.В., Шмаков Р.Г. Гестационное сонное апноэ. Связь беременности и преэклампсии с синдромом обструктивного апноэ сна. Альманах клинической медицины. 2019; 47(3): 266-75. [Kalachin K.A., Pyregov A.V., Shmakov R.G. Gestational sleep apnea. The relationship of pregnancy and preeclampsia with obstructive sleep apnea syndrome. Almanac of Clinical Medicine. 2019; 47(3): 266-75. (in Russian)]. https://dx.doi.org/10.18786/2072-0505-2019-47-031.
- Gordon D.J., Emeruwa C.J., Weiss J.P. Management strategies for nocturia. Curr. Urol. Rep. 2019; 20(11): 75. https://dx.doi.org/10.1007/s11934-019-0940-2.
- Lombardo R., Tubaro A., Burkhard F. Nocturia: the complex role of the heart, kidneys, and bladder. Eur. Urol. Focus. 2020; 6(3): 534-6. https://dx.doi.org/10.1016/j.euf.2019.07.007.
- Гасанова Б.М., Полина М.Л., Оразмурадов А.А. Влияние кардиальных фенотипов на течение и исходы беременности при гипертензивных расстройствах. Акушерство и гинекология: новости, мнения, обучение. 2019; 7(3): 31-40. [Gasanova B.M., Polina M.L., Orazmuradov A.A. The effect of cardiac phenotypes on the course and outcomes of pregnancy among patients with hypertensive disorders. Obstetrics and Gynecology: News, Opinions, Training. 2019; 7(3): 31-40. (in Russian)]. https://dx.doi.org/10.24411/2303-9698-2019-13904.
- Муминова К.Т., Ходжаева З.С., Шмаков Р.Г. Особенности течения беременности у пациенток с гипертензивными расстройствами. Доктор.Ру. 2019: 11(166): 14-21. [Muminova K.T., Khodzhaeva Z.S., Shmakov R.G. Specifics of pregnancy in patients with hypertensive disorders. Doctor.Ru. 2019; 11(166): 14-21. (in Russian)]. https://dx.doi.org/10.31550/1727-2378-2019-166-11-14-21.
- Боровкова Л.В., Колобова С.О., Боровкова Н.Ю., Полякова И.В., Некрасов А.А., Тимощенко Е.С., Андосова Л.Д., Шахова К.А., Тихомирова Ю.Р., Лазарькова А.Д. Современные методы прогнозирования гестационных осложнений у беременных с артериальной гипертензией. Женское здоровье и репродукция. 2020; 3(46). [Borovkova L.V., Kolobova S.O., Borovkova N.Yu., Polyakova I.V., Nekrasov A.A., Timoschenko E.S., Andosova L.D., Shakhova K.A., Tikhomirova Yu.R., Lazarkova A.D. Modern methods in forecasting gestational complications in pregnant women with arterial hypertension. Women's Health and Reproduction. 2020; 3(46). (in Russian)].
- Ляшенко Е.А., Левин О.С. Расстройства сна в клинической практике. Справочник поликлинического врача. 2017; 4: 57-61. [Lyashenko E.A., Levin O.S. Sleep disorders in clinical practice. Handbook for Practitioners Doctors. 2017; (4): 57-61. (in Russian)].
- Тезиков Ю.В., Липатов И.С., Зуморина Э.М., Азаматов А.Р., Тютюнник В.Л., Кан Н.Е., Чекаловец А.Л., Борисова А.И., Голоднова А.М. Клинико-патогенетическое обоснование двухэтапной профилактики преэклампсии у женщин высокого риска с применением инсулиносенситайзера для преконцепционной подготовки. Акушерство и гинекология. 2023; 9: 60-71. [Tezikov Yu.V., Lipatov I.S., Zumorina E.M., Azamatov A.R., Tyutyunnik V.L., Kan N.E., Chekalovets A.L., Borisova A.I., Golodnova A.M. Clinical and pathogenetic rationale for two-stage prevention of preeclampsia in high-risk women using an insulin sensitizer for preconception preparation. Obstetrics and Gynecology. 2023; (9): 60-71. (in Russian)]. https://dx.doi.org/10.18565/aig.2023.139.
- Серов В.Н. Метаболический синдром (нейрообменно-эндокринный синдром). Medica mente. Лечим с умом. 2015; 1: 16-9. [Serov V.N. Metabolic syndrome (neuro-endocrine syndrome). Medica mente. Lechim s umom. 2015; (1): 16-9. (in Russian)].
- Чабанова Н.Б., Василькова Т.Н. Особенности жирового обмена у беременных в зависимости от срока гестации, массы тела и характера жироотложения. Современные проблемы науки и образования. 2018; 5: 27-9. [Chabanova N.B., Vasil'kova T.N. Features of fat metabolism in pregnant women depending on gestational age, body weight and the nature of fat deposition. Modern Problems of Science and Education. Surgery. 2018; (5): 27-9. (in Russian)].
- Стрижаков А.Н., Тезиков Ю.В., Липатов И.С., Шарыпова М.А.. Анпилогова И.В., Азизов К.У., Костянова Е.В. Стандартизация диагностики и клиническая классификация хронической плацентарной недостаточности. Вопросы гинекологии, акушерства и перинатологии. 2014; 13(3): 5-12. [Strizhakov A.N., Tezikov Yu.V., Lipatov I.S., Sharypova M.A., Anpilogova I.V., Azizov K.U., Kostyanova E.V. Diagnostic standardization and clinical classification of chronic placental insufficiency. Issues of Gynecology, Obstetrics and Perinatology. 2014; 13(3): 5-12. (in Russian)].
- Ланг Т., Альтман Д. Основы описания статистического анализа в статьях, публикуемых в биомедицинских журналах. Руководство «Статистический анализ и методы в публикуемой литературе (САМПЛ)». Медицинские технологии. Оценка и выбор. 2014; 1(15): 11-6. [Lang T., Altman D. Basic statistical reporting for articles published in clinical medical journals: the SAMPL guidelines. Medical technologies. Evaluation and selection. 2014; 1(15): 11-6. (in Russian)].
- de Moel-Mandel C. Understanding and communicating epidemiological measures of risk and benefit. Fam. Pract. 2023; 40(2): 423-5. https://dx.doi.org/10.1093/fampra/cmac117.
- Chen X., Stein T.P., Steer R.A., Scholl T.O. Individual free fatty acids have unique associations with inflammatory biomarkers, insulin resistance and insulin secretion in healthy and gestational diabetic pregnant women. BMJ Open Diabetes Res. Care. 2019; 7(1): e000632. https://dx.doi.org/10.1136/bmjdrc-2018-000632.
- Макацария А.Д., Бицадзе В.О., Акиньшина С.В. Тяжелые формы преэклампсии как проявление тромботической микроангиопатии. Акушерство и гинекология. 2017; 4: 21-6. [Makatsaria A.D., Bitsadze V.O., Akinshina S.V. Severe forms of preeclampsia as a manifestation of thrombotic microangiopathy. Obstetrics and Gynecology. 2017; (4): 21-6. (in Russian)]. https://dx.doi.org/10.18565/aig.2017.4.21-6.
- Кудрявцева У.В., Ковалев В.В., Баранов И.И. Способ прогнозирования тяжелой преэклампсии. Патент на изобретение RU 2739694 C1. 2020. 14с. [Kudryavtseva U.V., Kovalev V.V., Baranov I.I. Method for predicting severe preeclampsia. Patent RU 2739694 S1. 2020. 14p. (in Russian)].
Received 18.10.2024
Accepted 18.12.2024
About the Authors
Yurii V. Tezikov, Professor, Dr. Med. Sci., Head of the Department of Obstetrics and Gynecology of the Institute of Clinical Medicine, Samara State Medical University,Ministry of Health of Russia, 443099, Russia, Samara, Chapaevskaya str., 89, +7(846)958-24-18, yra.75@inbox.ru, Researcher ID: С-6187-2018, SPIN: 2896-6986,
Author ID: 161372, Scopus Author ID: 6603787595, https://orcid.org/0000-0002-8946-501X
Igor S. Lipatov, Professor, Dr. Med. Sci., Professor at the Department of Obstetrics and Gynecology of the Institute of Clinical Medicine, Samara State Medical University, Ministry of Health of Russia, 443099, Russia, Samara, Chapaevskaya str., 89, +7(846)958-24-18, i.lipatoff2012@yandex.ru, Researcher ID: С-5060-2018, SPIN: 9625-2947, Author ID: 161371, Scopus Author ID: 6603787595, https://orcid.org/0000-0001-7277-7431
Anastasia I. Khalitova, 6 th year student of the Institute of Clinical Medicine, Samara State Medical University, Ministry of Health of Russia, 443099, Russia, Samara, Chapaevskaya str., 89, +7(846)958-24-18, nsts.nvk@yandex.ru, SPIN: 6245-2050, Authors ID: 1146911, https://orcid.org/0000-0003-4604-9099
Victor L. Tyutyunnik, Professor, Dr. Med. Sci., Leading Researcher at Research and Development Service, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, +7(903)969-50-41, tioutiounnik@mail.ru,
Researcher ID: B-2364-2015, SPIN: 1963-1359, Authors ID: 213217, Scopus Author ID: 56190621500, https://orcid.org/0000-0002-5830-5099
Natalia E. Kan, Professor, Dr. Med. Sci., Deputy Director of Science, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, +7(926)220-86-55, kan-med@mail.ru, Researcher ID: B-2370-2015,
SPIN: 5378-8437, Authors ID: 624900, Scopus Author ID: 57008835600, https://orcid.org/0000-0001-5087-5946
Ekaterina V. Yurasova, 4 th year student of the Institute of Clinical Medicine, Samara State Medical University, Ministry of Health of Russia, 443099, Russia, Samara, Chapaevskaya str., 89, +7(846)958-24-18, kate163ura@gmail.com, https://orcid.org/0009-0009-2077-6301
Anastasia A. Rudneva, 5 th year student of the Institute of Clinical Medicine, Samara State Medical University, Ministry of Health of Russia, 443099, Russia, Samara, Chapaevskaya str., 89, +7(846)958-24-18, rudneva2002nastia@yandex.ru, https://orcid.org/0009-0002-6867-6894
Anton D. Garazhankin, 6 th year student of the Institute of Clinical Medicine, Samara State Medical University, Ministry of Health of Russia, 443099, Russia, Samara, Chapaevskaya str., 89, +7(846)958-24-18, garazankin2012@yandex.ru, https://orcid.org/0009-0002-8863-3242



