The influence of dysbiotic disorders and local inflammatory reactions in the reproductive tract on the development of preterm birth
Medzhidova M.K., Tyutyunnik V.L., Kan N.E., Donnikov A.E., Mikhailova O.I.
Objective: To investigate how the state of microbiocenosis and the expression profile of immune response genes in the reproductive tract of pregnant women affect the occurrence of preterm birth.
Materials and methods: This study included 228 women who were divided into groups based on gestational age at delivery. Fifty-two patients gave birth at a gestational age of 22.0–27.6 weeks, 56 at a gestational age of 28.0–31.6 weeks, 60 at a gestational age of 32.0–33.6 weeks, and 60 at a gestational age of 34.0–37.0 weeks. Each group was divided into subgroups categorized according to whether they had preterm prelabor rupture of the membranes (PPROM) or spontaneous onset of labor (SPB) without PPROM. Subgroups included women who delivered at 22.0–27.6 weeks (PPROM n=28, SPB n=24), 28.0–31.6 weeks (PPROM n=30, SPB n=26), 32.0–33.6 weeks (PPROM n=35, SPB n=25), and 34.0–37.0 weeks (PPROM n=32, SPB n=28). A total of 125 patients with PPROM and 103 with SPB without PPROM were enrolled. The control groups consisted of pregnant women with threatened preterm birth who delivered at term (n=80) and those with a normal pregnancy who delivered at 37.0–41.0 weeks (n=72). All patients were tested for vaginal microbiocenosis using the Femoflor method with determination of local inflammatory response by measuring mRNA expression levels of cytokine genes in vaginal scrapings.
Results: There were significant differences between the groups of women with preterm birth with and without PPROM and those with a normal pregnancy. The incidence of dysbiosis combined with local inflammation was higher in women with spontaneous onset of labor (p=5.02×10-15) and with PPROM (p=2.01×10-15). Normocenosis was significantly more common in women with a normal pregnancy compared to those with spontaneous onset of labor (p=3.3×10-18) and with PPROM (p=1.2×10-21).
Conclusion: Dysbiotic disorders without local inflammation are not significant prognostic factors for preterm births. To accurately predict this risk, a comprehensive assessment of microbiocenosis and local inflammation in the vagina should be performed.
Authors' contributions: Medzhidova M.K., Tyutyunnik V.L., Donnikov A.E., Kan N.E., Mikhailova O.I. – conception and design of the study, data collection, review and analysis of relevant literature, statistical analysis, drafting and editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Medzhidova M.K., Tyutyunnik V.L., Kan N.E., Donnikov A.E., Mikhailova O.I.
The influence of dysbiotic disorders and local inflammatory reactions in the reproductive tract on the development of preterm birth.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (5): 74-81 (in Russian)
https://dx.doi.org/10.18565/aig.2024.14
Keywords
Preterm birth (PB) is a global problem in obstetrics due to a lack of understanding regarding its pathogenesis and multifaceted etiology [1–4].
Infectious factors are known to contribute to 40% of preterm births, with 80% of extremely preterm and early preterm births linked to infections [5–7]. Rupture of membranes is the most common cause of labor, accounting for 34–56% of all preterm births [1, 7–9]. Preterm prelabor rupture of membranes (PPROM) is closely associated with perinatal infection, which leads to a 10-fold increased risk of neonatal sepsis, perinatal and infant mortality, and maternal septic complications [10–14]. Some studies have suggested that dysbiotic vaginal disorders play a significant role in addition to infectious agents as they trigger a local inflammatory reaction in women with PB [5, 8, 10, 15]. It is also known that women with PB at different gestational ages exhibit immune mechanisms that lead to an increase in pro-inflammatory factors [9, 12, 14, 16–20]. However, currently, there is a lack of evidence to develop methods for correcting these changes.
This study aimed to investigate how the state of microbiocenosis and the expression profile of immune response genes in the reproductive tract of pregnant women affect the occurrence of preterm birth.
Materials and methods
This was a prospective cohort study of pregnant women admitted to the hospital with threatened PB; the study endpoint was obstetric outcome.
The study included 228 women with PB at 22.0–37.0 weeks' gestation who were divided into groups according to the accepted classification: group 1 consisted of 52 women with extremely early PB (22.0–27.6 weeks); group 2 (n=56) with early PB (28.0–31.6 weeks); group 3 (n=60) with PB (32.0–33.6 weeks); group 4 (n=60) with late PB (34.0–37.0 weeks).
The control group included 100 pregnant women with threatened PB whose pregnancies were prolonged to full-term and 72 women with a physiologic pregnancy who delivered at 37.0–41.0 weeks.
Each group was divided into subgroups categorized according to whether they had preterm prelabor rupture of the membranes (PPROM) or spontaneous onset of labor (SPB) without PPROM. Subgroups included women who delivered at 22.0–27.6 weeks (PPROM n=28, SPB n=24), 28.0–31.6 weeks (PPROM n=30, SPB n=26), 32.0–33.6 weeks (PPROM n=35, SPB n=25), and 34.0–37.0 weeks (PPROM n=32, SPB n=28). A total of 125 patients with PPROM and 103 patients with SPB without PPROM were enrolled in the study.
Inclusion criteria were singleton pregnancy, threatened PB at 22.0–36.6 weeks’ gestation; first period of PB; PROM at 22.0–36.6 weeks’ gestation.
The exclusion criteria were multiple pregnancies, severe non-obstetric comorbidities, severe obstetric pathology requiring early elective delivery, placental abruption, fetal growth restriction, fetal congenital malformations, sexually transmitted infections, and HIV infection.
The study was reviewed and approved by the Research Ethics Committee of V.I. Kulakov NMRC for OG&P of the Russian Ministry of Health, all patients signed informed consent to participate in the study.
In this study, vaginal microbiocenosis was studied using the Femoflor method (DNA Technology, Russia), and the local inflammatory response was determined by the mRNA expression level of cytokine genes in vaginal scrapings (Immunoquantex, DNA Technology, Russia).
The biomaterial was taken with a cytobrush, separately from the cervical canal and vagina. To avoid mRNA degradation, the material was placed in a plastic tube containing 500 μl of RNA stabilization medium (lysis solution from the PROBA-NK isolation reagent kit (NPO DNA-Technology LLC, Russia)).
Statistical analysis
Statistical analysis was performed using SPSS Statistics 23.0. Continuous variables were expressed as medians with interquartile ranges (Me (Q25; Q75); two unpaired groups were compared using the Mann–Whitney test (U) for non-parametric data. To compare categorical data, the Pearson χ2 test was used, adjusted for continuity, or Fisher's exact test, depending on the size of the compared groups. If a zero frequency was found in any cell of two-way contingency tables, Fisher's exact test was used with Wald correction by adding 0.5 all cells of the table [21].
The Bonferroni correction was used for multiple comparisons. The critical level of significance was calculated using the formula αbaseline/n, where αbaseline=0.05, and n is the number of comparisons. For the 4 groups, the critical level was 0.05/3=0.017.
Results and discussion
The ages of the women included in the study ranged from 18 to 41 years. Analysis of somatic and gynecological status showed that chronic cystitis was significantly more common in groups 2 (16/56, 28.5%) and 3 (18/60, 30.0%) than in groups 1 (6/52, 11.5%, p=0.033 and 0.021, respectively) and 4 (8/60, 13.3%) (p=0.065 and 0.045, respectively). Chronic pyelonephritis was significantly more common in groups 1 (17/52, 32.7%) and 2 (19/56, 33.9%) than in groups 3 (8/60, 13.3%, p=0.022 and 0.015, respectively) and 4 (6/60, 10.0%, p=0.004 and 0.003, respectively). There were no significant differences in the menstrual function characteristics between the study groups. Chronic salpingoophoritis was more common in group 2 (13/56, 23.2%) than in groups 3 (6/60, 10.0%, p=0.078) and 4 (5/60, 8.3%, p=0.039). Analysis of the incidence of infectious and inflammatory diseases showed that bacterial vaginosis and vulvovaginal candidiasis were more common in groups 1 and 2, while Ureaplasma infection was more common in group 1. However, no statistically significant differences were observed between the groups. Analysis of the reproductive history revealed that spontaneous abortion was more common in group 2 (15/56, 26.8%), which was significantly higher than that in groups 3 (6/60, 10.0%) and 4 (6/60, 10.0%) (p=0.029 and 0.029, respectively). Missed miscarriages occurred in 16/52 (30.8%) patients in group 1, 7/56 (12.5%) in group 2, 5/60 (5.8%) in group 3, and 6/60 (10.0%) in group 4, showing that it was significantly more frequent in group 1 (p=0.033, 0.003, and 0.008, respectively). A history of PB in the studied groups was reported in group 1, 14/52 (26.9%); group 2, 16/56 (28.6%); group 3, 8/60 (13.3%); and group 4, 7/60 (11.7%); this was also statistically significantly higher in groups 1 and 2 than in group 4 (p=0.052 and 0.035, respectively).
Thus, our analysis of the clinical and anamnestic data of the patients makes it possible to identify with a fairly high probability such risk factors for PB as chronic inflammatory diseases of the urinary system and pelvic organs, as well as a complicated obstetric and gynecological history (spontaneous miscarriages, missed miscarriages and PB in the medical history).
The first stage in our study was to determine the species composition of microbiocenosis and assess the local inflammatory reaction in the vagina in women with PB at 22.0–37.0 weeks with spontaneous onset of labor and PPROM.
Analysis of vaginal microbiocenosis revealed that among women with spontaneous PB at 22.0–37.0 weeks (Fig. 1a), 62/103 (60.2%) had dysbiotic changes. A deeper analysis of the structure of women with vaginal dysbiosis revealed the presence of isolated vaginal dysbiosis in 12/103 (11.6%) cases and a combination of dysbiotic changes and local inflammation in the vagina in 50/103 (48.6%) patients. In addition found that 32/103 (31.1%) women in this group had isolated local inflammation without dysbiotic changes. At the same time, normocenosis occurred in only 9/103 (8.7%) women with spontaneous PB at 22.0–37.0 weeks.
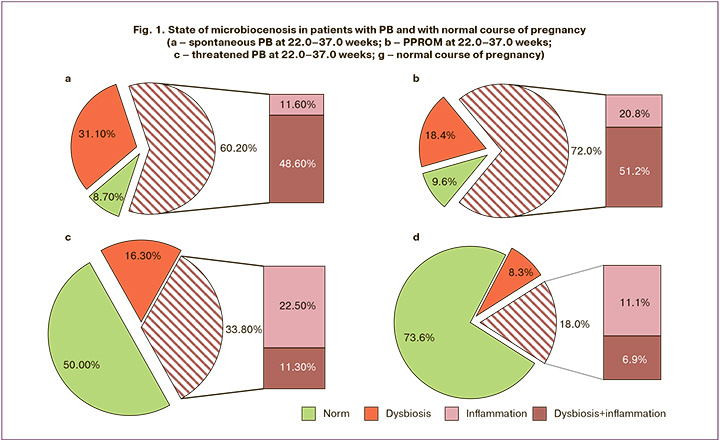
Analysis of a group of women with PPROM (Fig. 1b) at 22.0–37.0 weeks showed that 90/125 (72.0%) of the pregnant women had vaginal dysbiosis. Structural analysis of pregnant women with vaginal dysbiosis revealed the presence of isolated dysbiosis in 26/125 (20.8%) cases and a combination of local inflammation and dysbiotic changes in the vagina in 64/125 (51.2%) cases. Isolated local inflammation in the vagina without dysbiotic changes was detected in 23/125 (18.4%) pregnant women with PPROM. At the same time, normocenosis in pregnant women with PPROM and PB occurred in only 12/125 (9.6%) cases.
In relation to the results obtained, it was interesting to study and analyze patients with a complication in the form of threatened PB whose pregnancy was prolonged to full-term (Fig. 1c). In this group of patients, a significant percentage of pregnant women with vaginal normocenosis was identified, which was 40/80 (50%), while the percentage of pregnant women with dysbiotic changes in the vagina was 27/80 (33.8%). Analysis of the structure of patients with dysbiosis in the vagina revealed that only 9/80 (11.3%) pregnant women had local inflammation in combination with dysbiosis and 18/80 (22.5%) had isolated dysbiosis without inflammatory disorders. The number of women with isolated local inflammation in this group was 13/80 (16.3%).
We also analyzed the species composition of vaginal microbiocenosis and local immunity in pregnant women with normal pregnancies and full-term deliveries with spontaneous onset of labor (Fig. 1d). The number of pregnant women with vaginal normocenosis in this group was 53/72 (73.6%). At the same time, dysbiotic vaginal changes were detected in 13/72 (18%) pregnant women. A detailed analysis of the structure of patients with vaginal dysbiosis showed that only 5/72 (6.9%) pregnant women had local inflammation in combination with dysbiosis, and 8/72 (11.1%) had isolated dysbiosis without inflammatory changes. Isolated local inflammation occurred in 6/72 patients (8.3%).
Thus, in a comparative assessment of the state of vaginal microbiocenosis in patients with threatened PB whose pregnancy was prolonged to full term and in women with a normal course of pregnancy, no statistically significant differences were found.
There were significant differences between the groups of women with PB (spontaneous PB and PPROM) and those with normal pregnancy. The incidence of dysbiosis in combination with local inflammation was higher in women with spontaneous PB (p=5.02×10-15) and PPROM (p=2.01×10-15). At the same time, normocenosis in the group of women with normal pregnancy occurred significantly more often in comparison with PPROM (p=1.2×10-21) and spontaneous PB (p=3.3×10-18). The state of vaginal microbiocenosis and the incidence of local inflammation in patients with threatened PB, which ended in timely delivery, did not differ significantly from those in patients in the comparison group (Fig. 1a-d).
Considering the data obtained, in the second stage, we decided to conduct a comparative analysis of the frequency of occurrence of various conditions of the vaginal microbiota and the presence of local inflammation in groups of women with spontaneous development of labor and PPROM at different stages of gestation in comparison with a group of women with timely birth, whose pregnancy was complicated by threatened PB and the normal course of pregnancy at similar gestational ages. The proportion of patients with corresponding conditions was compared using the χ2 test.
In patients with extremely early PB (Fig. 2a), a combination of dysbiotic vaginal changes with local inflammation was detected in 100% (28/28) of women with PPROM and in 75% (18/24) of women with spontaneous PB, which was statistically significant (p=6.6×10-3). In addition, dysbiosis in combination with local inflammation was significantly more common in the spontaneous PB group than in the threatened PB – 0/25 (0%) (p=1.1×10-8) and normal course of pregnancy – 2/ 25 (8%) (p=1.8×10-6). In the group of women with PPROM, the incidence of dysbiosis in combination with local inflammation was significantly higher than that in women with normal pregnancies (p=1.6×10-10). There were 3/24 (13%) women with normocenosis and spontaneous PB, which was significantly lower compared to pregnant women at risk for PB, where normocenosis occurred in 16/25 (64%) cases (p=2.0×10-4) and normal course of pregnancy in 17/25 (68%) cases (p=7.7×10-5). Notably, in women with extremely early PB with PPROM, no cases of normocenosis were identified. In a comparative analysis of the state of microbiocenosis and local inflammation in the vagina in women with threatened PB and those a with normal course of pregnancy, no significant differences were observed. It is noteworthy that isolated inflammation occurred in patients with threatened PB at 22.0–27.6 weeks in 3/25 (12.0%) patients and during the normal course of pregnancy in 3/25 (12%) patients.
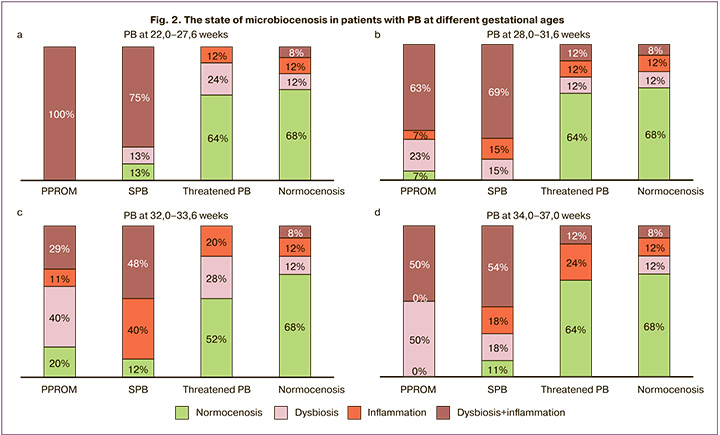
In patients with early PB (Fig. 2b), a combination of dysbiotic changes with local inflammation in the vagina was observed in 19/30 (63%) women with PPROM, which was significantly higher than that in women with a normal course of pregnancy, where such a combination occurred in 2/25 (8%) patients (p=2.6×10-5), and also when compared with patients with spontaneous PB – 18/26 (69%) cases (p=7.5×10-6). Normocenosis in women with PPROM in this group was observed in only 2/30 (7%) cases, which was significantly lower (p=1.9×10-6) than that in women with a normal course of pregnancy, where cases of normocenosis were observed in 17 of 25 (68%) patients. Among women with spontaneous PB at 28.0–31.6 weeks, no cases of normocenosis were identified. In this group of women, there were also no significant differences in the frequency of isolated dysbiosis, which occurred in 7/30 (23%) women with PPROM and in 4/26 (15%) with spontaneous PB in comparison with the control groups, where isolated dysbiosis occurred in 3/25 (12%) women with threatened PB and in 3/25 (12%) women with normal pregnancy.
The evaluation of the microbiocenosis and local immunity of the vagina in women with PPROM (Fig. 2c) compared with normal pregnant women also showed statistically significant differences. Thus, in pregnant women with PPROM, the combination of dysbiosis and local vaginal inflammation occurred in 10/35 (29%) cases, which was significantly higher than that in the normal course of pregnancy, where such a combination occurred in 2/25 (8%) women (p=4.0×10-4). In addition, significant differences were identified in the incidence of dysbiosis in combination with local inflammation between groups with spontaneous PB, including 12/25 (48%) cases and normal pregnancies (p=1.0×10-4). There were no cases of a combination of dysbiosis with local inflammation in pregnant women with threatened PB in this group, which was significantly lower than that in the group with spontaneous PB, where this combination occurred in 12/25 (48%) (p=8.6×10-5). At the same time, there were 3/25 (12%) cases of normocenosis in the group of women with spontaneous PB and 7/35 (20%) in patients with PPROM, which was also significantly less than in the group of pregnant women with normal pregnancy, where normocenosis was observed in 17/25 (68%) patients (p=5.3×10-5). Of note was the identification of patients with isolated vaginal inflammation, which occurred in 10/25 (40%) women with spontaneous PB, and in 4/35 (11%) patients with PPROM, which was statistically significant (p=9.0×10-4). In groups of patients who delivered at full term, isolated local inflammation occurred in 5/25 (20.0%) women with threatened PB and 3/25 (12%) with normal pregnancy.
No less interesting data were obtained in the group of women with late PB (Fig. 2d), where a statistically significant percentage of dysbiosis in combination with a local inflammatory reaction was observed in women with PPROM – 16/32 (50%) (p=7.1×10-4) and spontaneous PB – 5/28 (18%) (p=3.8×10-4) in women with a normal course of pregnancy, where dysbiosis in combination with local inflammation occurred in 2/25 (7%). Significant differences were identified in the incidence of normocenosis when comparing women with PPROM – 0/32 (0%) (p=7.7×10-9) and spontaneous PB – 3/28 (11%) (p=1.7×10-5) with a normal course of pregnancy, where normocenosis occurred in 17/25 (68%) women.
It is noteworthy that in the groups of women with preterm and late PB in comparison with extremely early and early PB there was a decrease in the number of cases of a combination of dysbiotic disorders with a local inflammatory reaction in all cases of PB (PPROM, spontaneous PB). However, at the same time, there was a significant increase in the number of cases with isolated local inflammatory reaction in the vagina against the background of normocenosis and isolated dysbiosis without the presence of an inflammatory reaction.
Conclusion
Based on the results of this study, the following conclusions can be drawn:
- It is recommended that vaginal microbiocenosis and the expression profile of immune response genes be evaluated in patients at risk of PB.
- Normocenosis without local inflammation is a positive prognostic factor. Among patients hospitalized with threatened PB, 52–64% who eventually carried their pregnancy to full term showed no signs of microbiocenosis disturbance or local inflammatory reaction. In patients with normal pregnancy and timely delivery, normocenosis was observed in 68% of cases.
- In patients with threatened PB who eventually delivered at full term, the state of microbiocenosis and local inflammation is similar to that in those with a normal course of pregnancy and timely delivery.
- A combination of vaginal dysbiotic disorders and local inflammation indicates the most unfavorable prognosis. This combination is more common among all groups of PB compared to patients whose pregnancy was complicated by threatened PB but carried to full term, as well as women with a normal pregnancy and timely delivery.
- In women with extremely early and early PB, the combination of dysbiotic disorders with local inflammation was significantly higher than that in women with premature and late PB. Additionally, the incidence of isolated inflammation and isolated vaginal dysbiosis is significantly higher in women with preterm and late PB.
- Dysbiotic disorders without local inflammation are not significant predictors of PB. For a more reliable prognosis, comprehensive assessment of vaginal microbiocenosis and local inflammation should be conducted.
- A significant number of patients with PB, as well as pregnant women with threatened PB, exhibit signs of isolated local inflammation in the presence of vaginal normocenosis, which requires further research.
References
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Преждевременные роды. М.; 2020. 54 с. [Ministry of Health of the Russian Federation. Clinical guidelines. Preterm birth. Moscow; 2020. 54 p. (in Russian)].
- Dauengauer-Kirlienė S., Domarkienė I., Pilypienė I., Žukauskaitė G., Kučinskas V., Matulevičienė A. Causes of preterm birth: Genetic factors in preterm birth and preterm infant phenotypes. J. Obstet. Gynaecol. Res. 2023; 49(3): 781-93. https://dx.doi.org/10.1111/jog.15516.
- da Fonseca E.B., Damião R., Moreira D.A. Preterm birth prevention. Best Pract. Res. Clin. Obstet. Gynaecol. 2020; 69: 40-9. https://dx.doi.org/10.1016/j.bpobgyn.2020.09.003.
- Schoenmakers S., Aagaard K., Borenstein-Levin L., Kawaza K., van der Meeren L.E., Mol B.W. et al. Editorial: Preterm birth and placental pathology. Front. Endocrinol (Lausanne). 2023; 14: 1168185. https://dx.doi.org/10.3389/fendo.2023.1168185.
- Couture C., Brien M.E., Boufaied I., Duval C., Soglio D.D., Enninga E.A.L. et al. Proinflammatory changes in the maternal circulation, maternal-fetal interface, and placental transcriptome in preterm birth. Am. J. Obstet. Gynecol. 2023; 228(3): 332.e1-332.e17. https://dx.doi.org/10.1016/j.ajog.2022.08.035.
- Белоусова В.С., Стрижаков А.Н., Свитич О.А., Тимохина Е.В., Кукина П.И., Богомазова И.М., Пицхелаури Е.Г. Преждевременные роды: причины, патогенез, тактика. Акушерство и гинекология. 2020; 2: 82-7. [Belousova V.S., Strizhakov A.N., Svitich O.A., Timokhina E.V., Kukina P.I., Bogomazova I.M., Pitskhelauri E.G. Preterm birth: causes, pathogenesis, and management. Obstetrics and Gynecology. 2020; (2): 82-7. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.2.82-87.
- Paquette A.G., MacDonald J., Bammler T., Day D.B., Loftus C.T., Buth E. et al. Placental transcriptomic signatures of spontaneous preterm birth. Am. J. Obstet. Gynecol. 2023; 228(1): 73.e1-73.e18. https://dx.doi.org/10.1016/j.ajog.2022.07.015.
- Dudley D.J., Ennen C.S. The vexing problem of preterm birth prevention. JAMA. 2023; 330(4): 323-5. https://dx.doi.org/10.1001/jama.2023.7244.
- Moosa Y., Kwon D., de Oliveira T., Wong E.B. Determinants of vaginal microbiota composition. Front. Cell. Infect. Microbiol. 2020; 10: 467. https://dx.doi.org/10.3389/fcimb.2020.00467.
- Lamont R.F. Spontaneous preterm labour that leads to preterm birth: An update and personal reflection. Placenta. 2019; 79: 21-9. https://dx.doi.org/10.1016/j.placenta.2019.03.010.
- Kindschuh W.F., Baldini F., Liu M.C., Liao J., Meydan Y., Lee H.H. et al. Preterm birth is associated with xenobiotics and predicted by the vaginal metabolome. Nat. Microbiol. 2023; 8(2): 246-59. https://dx.doi.org/10.1038/s41564-022-01293-8.
- Rowlands S., Danielewski J.A., Tabrizi S.N., Walker S.P., Garland S.M. Microbial invasion of the amniotic cavity in midtrimester pregnancies using molecular microbiology. Am. J. Obstet. Gynecol. 2017; 217(1): 71.e1-71.e5. https://dx.doi.org/10.1016/j.ajog.2017.02.051.
- Nkhalamba L., Hampton T., Mulwafu W. Defining the neurologic consequences of preterm birth. N. Engl. J. Med. 2023; 389(19): 1826-7. https://dx.doi.org/10.1056/NEJMc2310222.
- Кан Н.Е., Салпагарова З.Х., Тютюнник В.Л., Щипицына В.С., Красный А.М. Содержание про- и антиоксидантов в капиллярной крови при преждевременных родах. Акушерство и гинекология. 2022; 1: 56-61. [Kan N.E., Salpagarova Z.Kh., Tyutyunnik V.L., Shchipitsyna V.S., Krasnyi A.M. Prooxidant and antioxidant content in capillary blood in preterm birth. Obstetrics and Gynecology. 2022; (1): 56-61 (in Russian)]. https://dx.doi.org/10.18565/aig.2022.1.56-61.
- Тютюнник В.Л., Кан Н.Е., Высоких М.Ю., Кокоева Д.Н., Донников А.Е., Сарибекова А.Г., Меджидова М.К. Возможности прогнозирования преждевременных родов путем определения содержания митохондриальной ДНК и структурно-функционального белка VDAC1. Акушерство и гинекология. 2021; 3: 58-65. [Tyutyunnik V.L., Kan N.E.,
- Vysokikh M.Yu., Kokoeva D.N., Donnikov A.E., Saribekova A.G., Medzhidova M.К. Possibilities of predicting preterm birth using mitochondrial DNA and VDAC1 protein. Obstetrics and Gynecology. 2021; (3): 58-65 (in Russian)]. https://dx.doi.org/10.18565/aig.2021.3.58-65.
- Высоких М.Ю., Тютюнник В.Л., Кан Н.Е., Курчакова Т.А. Суханова Ю.А., Володина М.А., Тарасова Н.В., Цвиркун Д.В., Меджидова М.К., Арушанова А.Г. Диагностическая значимость определения содержания малонового диальдегида и активности каталазы при преждевременных родах. Акушерство и гинекология. 2017; 4: 62-7. [Vysokikh M.Y., Tyutyunnik V.L., Kan N.E., Kurchakova T.A., Sukhanova I.A., Volodina M.A., Tarasova N.V., Tsvirkun D.V., Medzidova M.K., Arushanova A.G. Diagnostic significance of malondialdehyde level and catalase activity evaluation in women with preterm labor. Obstetrics and Gynecology. 2017; (4): 62-7. (in Russian)]. https://dx.doi.org/10.18565/aig.2017.4.62-67.
- Красный А.М., Кан Н.Е., Тютюнник В.Л., Садекова А.А., Сарибекова А.Г., Кокоева Д.Н., Салпагарова З.Х., Меджидова М.К., Вторушина В.В., Кречетова Л.В. Прогнозирование преждевременных родов путем комбинированного определения цитокинов и внеклеточной ДНК. Акушерство и гинекология. 2019; 1: 86-91. [Krasnyi A.M., Kan N.E., Tyutyunnik V.L., Sadekova A.A., Saribekova A.G., Kokoeva D.N., Salpagarova Z.Kh., Medzhidova M.K., Vtorushina V.V., Krechetova L.V. Predicting preterm labor by combined testing of cytokines and cell-free DNA. Obstetrics and Gynecology. 2019; (1): 86-91. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.1.86-91.
- Khan W., Zaki N., Ghenimi N., Ahmad A., Bian J., Masud M.M. et al. Predicting preterm birth using explainable machine learning in a prospective cohort of nulliparous and multiparous pregnant women. PLoS One. 2023; 18(12): e0293925. https://dx.doi.org/10.1371/journal.pone.0293925.
- Areia A.L., Moura P., Mota-Pinto A.; PROSPERO Nº CRD42018089859. The role of innate immunity in spontaneous preterm labor: a systematic review. J. Reprod. Immunol. 2019; 136: 102616. https://dx.doi.org/10.1016/j.jri.2019.102616.
- Радзинский В.Е., Оразмурадов А.А., Савенкова И.В., Дамирова К.Ф., Хаддад Х. Преждевременные роды – нерешенная проблема XXI века. Кубанский научный медицинский вестник. 2020; 27(4): 27-37. [Radzinsky V.E., Orazmuradov A.A., Savenkova I.V., Damirova K.F., Haddad H. Preterm labour: an open problem in XXI century. Kuban Scientific Medical Bulletin. 2020; 27(4): 27-37. (in Russian)]. https://doi.org/10.25207/1608-6228-2020-27-4-27-37.
- Abramson J.H. WINPEPI updated: computer programs for epidemiologists, and their teaching potential. Epidemiol. Perspect. Innov. 2011; 8(1): 1. https://dx.doi.org/10.1186/1742-5573-8-1.
Received 21.01.2024
Accepted 09.04.2024
About the Authors
Marzhanat К. Medzhidova, PhD, Doctoral Student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, +7(926)381-17-10, marzhana-m@yandex.ru, Researcher ID: GRR-7195-2022,SPIN-code: 5942-2320, Authors ID: 116298611:49, Scopus Author ID: 57191960453, https://orcid.org/0000-0001-6938-4207
Victor L. Tyutyunnik, Professor, Dr. Med. Sci., Leading Researcher at the Center for Scientific and Clinical Research, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, +7(903)969-50-41, tioutiounnik@mail.ru, Researcher ID: B-2364-2015, SPIN-code: 1963-1359, Authors ID: 213217, Scopus Author ID: 56190621500, https://orcid.org/0000-0002-5830-5099
Natalia E. Kan, Professor, Dr. Med. Sci., Deputy Director of Science, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, +7(926)220-86-55, kan-med@mail.ru, Researcher ID: B-2370-2015,
SPIN-code: 5378-8437, Authors ID: 624900, Scopus Author ID: 57008835600, https://orcid.org/0000-0001-5087-5946
Andrey E. Donnikov, PhD, Head of the Laboratory of Molecular Genetic Methods, Academician V.I. Kulakov National Medical Research Center for Obstetrics,
Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, +7(903)684-52-47, donnikov@dna-technology.ru,
https://orcid.org/0000-0003-3504-2406
Olga I. Mikhailova, PhD, Researcher, 2nd Obstetric Physiology Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, +7(926)564-68-13, omikhaylova@gmail.com, https://orcid.org/0000-0001-7569-8704
Corresponding author: Marzhanat К. Medzhidova, marzhana-m@yandex.ru



