Antenatal factors associated with factors associated with brain-derived neurotrophic factor and insulin-like growth factor-1 levels in premature infants
Aim. The study was aimed at determining antenatal factors associated with brain-derived neurothophic factor and insulin-like growth factor-1 levels in premature infants. Materials and methods. The prospective study included 62 mother-infant pairs. The women and newborns were divided into two groups depending on gestational age at birth: group I included 21 mother-infant pairs with spontaneous preterm birth (PTB), the average gestational age for births was 33.3±1.93 weeks; group II included 41 mother-infant pairs with term birth (TB) at 39,4±1,04 weeks. Enzyme immunoassay (EIA) was performed for quantitative detection of brain-derived neurotrophic factor and insulin-like growth factor-1 levels in blood plasma of newborns. Results. BDNF level was high in the term birth group (р<0.0001), while IGF-1 level was low (р=0.0038). In the preterm birth group, the median BDNF value in women who had treatment for prevention of respiratory distress syndrome (RDS) in newborns was 488.5 [70.9; 2295] pg/ml, and 326.5 [133.4; 2730] pg/ml in women who did not receive RDS profilaxis, р=0,9644. In the preterm birth group, the median value of IGF-1 was 45.3 [28.3; 58.1] ng/ml in women who received RDS profilaxis, and 90.9 [57.7; 252.3] ng/ml in women who did not receive RDS profilaxis, р=0.0197. Conclusion. The specific features of the course of pregnancy affect BDNF and IGF-1 levels in blood plasma of premature infants, including antenatal prophylaxis of RDS and antibiotic therapy.Gorina K.A., Khodzhaeva Z.S., Vtorushina V.V., Krechetova L.V., Ionov O.V., Lenyushkina A.A., Timofeeva L.A., Makieva M.I., Degtyarev D.N.
Keywords
Specific features of the course of pregnancy and the initial somatic state of the expectant mother are the determining factors in the quality of fetal health. Prematurity per se is associated with increased morbidity and mortality; nevertheless, neonatal outcomes are directly related to antenatal events throughout gestation. [1], therefore, the assessment of potential risk factors before birth is one of the fundamental research challenges.
Fetal brain development is exposed to adverse effects due to a well-defined developmental trajectory against the background of ongoing transcriptional and epigenetic changes [2–4]. Disrupted sequence of expression networks may contribute to the risks of atypical development of the nervous system to a certain extent [4]. Brain-derived neurotrophic factor (BDNF) – is a neurotrophin that plays an essential role in neuronal development and differentiation in the nervous system of newborns [5]. BDNF contributes to synaptic plasticity as well as various aspects of learning, including long-term memory [6]. Insulin-like growth factor 1 (IGF-1), or somatomedin C, is an anabolic hormone with multipotent impact, including mitogenic, differentiating, anti-apoptotic and metabolic effects [7, 8]. In the prenatal period, at the stages of fetal brain development, IGF-1 is actively expressed in the regions of the brain. IGF-1 modulates permeability of the blood-brain barrier for other systemic neuroactive proteins and increases the activity of other growth factors, such as BDNF and vascular endothelial growth factor [8, 9], that allows to consider it as a full-fledged neurotrophic factor [10].
Antenatal prevention of neonatal respiratory distress syndrome (RDS) is a mandatory component of obstetric management of preterm birth (PTB) [11]; In more than 50% of patients, who antenatally received a course of glucocorticoids (GCs), pregnancy was prolonged for 7–10 days [12]. The use of antibacterial drugs for treatment of patients with the risk of PTB remains a controversial issue. [13, 14], nevertheless, this type of therapy and/or prophylaxis is actively used. Epidemiological studies show that the effect of antibiotics can increase the risk of immune and metabolic diseases in adulthood, and experimental studies show that high doses of antibiotics have adverse long-term effects on development of the central nervous system [15, 16]. Despite the obvious and undeniable short-term benefits of therapy for women during pregnancy, the long-term risks remain the subject of active controversies. The experimental studies suggest that the levels of brain-derived neurotrophic factors in the offspring’s plasma may depend on maternal corticosteroid and antibiotic treatment during pregnancy [17, 18].
The study was aimed at determining antenatal factors associated with brain-derived neurothophic factor (BDNF) and insulin-like growth factor 1 (IGF-1) levels in premature infants of different gestational age.
Materials and methods
The prospective study included 62 mother-infant pairs. The women and newborns were divided into two groups depending on gestational age at birth: group I included 21 mother-infant pairs with spontaneous preterm birth (PTB), the average gestational age for births was 33.3±1.93 weeks; group II included 41 mother-infant pairs with term birth (TB) at 39,4±1,04 weeks.
Assessment of anamnestic and clinical laboratory data of all patients under the study was performed, including somatic, obstetric and gynecological anamnesis, the course of pregnancy, standard laboratory panel of peripherial blood parameters, microbial composition in lower genital tract, the methods for functional assessment of the fetal condition and fetometry. At the end of pregnancy, delivery methods and perinatal outcomes were analyzed. The condition of newborns was assessed immediately after birth using the Apgar score, during early and late neonatal periods; clinical, laboratory and instrumental data were analyzed.
Inclusion criteria in the comparison and control groups were: singleton spontaneous pregnancy: gestational age for preterm birth was 280–346 weeks; patients’ informed consent to participate in the study and to collect infants’ additional blood volume for research purposes when performing standard clinical tests. Exclusion criteria were multiple pregnancy, placenta praevia, severe extragenital and obstetric pathology. Exclusion criteria for the newborns were: birth asphyxia, antenatally diagnosed signs of fetal growth retardation, congenital malformations of the central nervous system and other organs damage. The patients excluded from the study were the women with the symptoms of severe infectious diseases and/or exacerbation of chronic diseases as well as those, with refusal to participate in the study, and severe condition of their newborns, which did not allow to collect biological material. The study was approved by the Ethics Committee of the Academician V.I. Kulkakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia.
EDTA vacutainers were used for venous blood collection from newborns within the first 12 hours after birth. Plasma separation and blood centrifugation was performed for 20 minutes (300g) at temperature of 4°C, and repeated centrifugation (14000g) was for 10 minutes at room temperature. Plasma samples were stored at temperature of -80°C for subsequent lab tests. BDNF and IGF-1 levels in plasma were quantified by enzyme immunoassay (EIA) using commercial kits produced by Research & Diagnostics Systems, Inc. (USA) and «Mediagnost» (Germany), respectively. Analytical sensitivity for BDNF detection was 20 pg/ml, and 0.09 ng/ml for IGF-1. The measurement of BDNF and IGF-1 concentrations was peformed using spectrophotometer Infinite F50 («TECAN», USA).
Statistical analysis
Statistical analysis was performed with GraphPad Prism 8.3 and IBM SPSS Statistics 22 in compliance with the general recommendations for medical and biological research. Normal distribution of quantitative variables was presented as arithmetic mean and standard deviation; the data, which were different to a normal distribution, were presented as median and quartiles. D'Agostino–Pearson's test and Anderson–Darling test, as well as the Kolmogorov–Smirnov test was used to assess the normality of data, and the Levene's test was used to assess the equality of variances. The data were analyzed using Fisher's exact test and the parametric Student's t-test for comparison of independent variables in the groups. Nonparametric Mann–Whitney U-test was used in the absence of normal data distribution. Spearman's correlation coefficient was used to measure the degree of association between the variables. Statistically significant differences for all tests were at p<0.05.
Results
The study included 62 mother-infant pairs. The age of women, weight-for-age indicators and BMI were comparable in the groups. The clinical and demographic characteristics of patients are presented in Table 1.
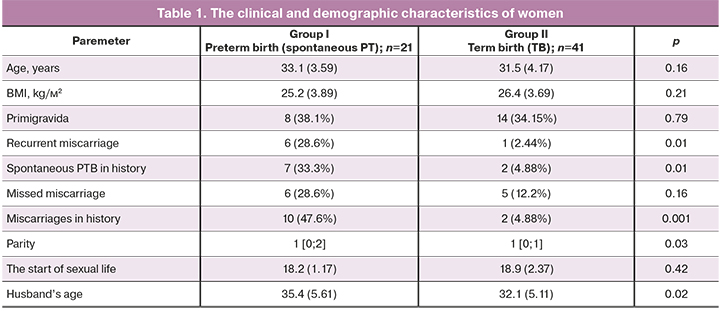
Table 1 shows the analyzed clinical and demographic parameters, which reflect specific anamnestic features for spontaneous PTB. Statistically, in patients with preterm birth in reproductive history, recurrent miscarriage (OR 3.0; 95% CI 1.54–4.88) and previous PTV (OR 2.94; 95% CI 1.51–5.06) were more often against the background of high parity. Assessment of the influence of the husband’s age showed a statistically significant increase in the age in the group of patients with PTB, which correlated with the published data [19]. The differences in such factors as smoking, as well as the presence of polycystic ovary syndrome (PCOS), endometriosis, uterine fibroids, gestational diabetes mellitus, preeclampsia and the spectrum of extragenital diseases were not statistically significant in both groups (р>0.05).
The analysis of the course of pregnancy (Table 2) which started on the second trimester showed an increased risk of pregnancy termination in the PTB group in 12/21 (57.1%) and in 5/41 (12.2%) women, respectively, р=0.001, OR 3.53; 95% CI 1.83–6.84). Tocolytic agents were used to reduce the risk of PTB: nifedipine in 7/21 (33.3%) and in 1/41 (2.44%) women, respectively, p=0.002; ginipral in 5/21 (23.8%) and in 0/41 (0%) women, respectively (р=0,003). It should be noted, that there was a high percentage of women who received antibacterial treatment in the group with spontaneous PTB (OR 3.45; 95% CI 1.82–6.46) due to the presence of inflammatory markers as well as after they underwent cerclage. Therapeutic regimens in the antenatal period for prevention of RDS at the gestational age of 240–346 weeks in the PTB group was a mandatory element in preparing for childbirth, however the therapeutic effect lasted only for 7 days. Our detailed analysis showed, that the average time interval from RDS prophylaxis to childbirth was 1.5 [0.4; 4.3] weeks.
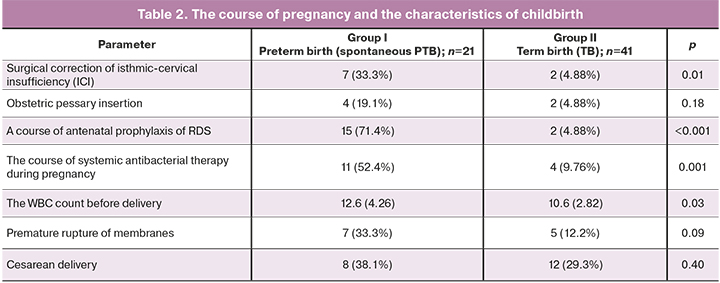
The comparative analysis of the characteristics of childbirth did not show statistically significant differences. Regardless of gestational age, vaginal delivery was a predominant method of childbirth in both groups (in 13/21 (61.9%) and 29/41 (70.7%) women, respectively, р=0.40). Nevertheless, pregnant women in the PTB group had a significantly increased amount of leukocytes (WBC, p=0.04) in the peripheral blood.
In addition to logical individual differences in newborns due to the gestational age, such as Apgar score at birth (7.0 [6; 8] and 8 [8; 8] points, р <0.0001 at the 1st minute; 8.0 [7.5; 8] and 9 [9; 9] points, р <0.001 at the 5th minute) and anthropometric data, statistically significant high prevalence of infections specific to the perinatal period was noted (11/21 (52.4%) and 0/41 (0%), р <0.001). Assessment of respiratory severity in premature infants according to the Silverman scoring system was 2.0 points [2; 3]. In addition, premature infants were characterized by high incidence of complications due to morpho-functional immaturity: RDS had 11/21 (52.4%) newborns, transient tachypnea – 6/21 (28.6%), congenital pneumonia – 9/21 (42, 7%), retinopathy 2/21 (9.5%), central nervous system (CNS) depression 7/21 (33.3%).
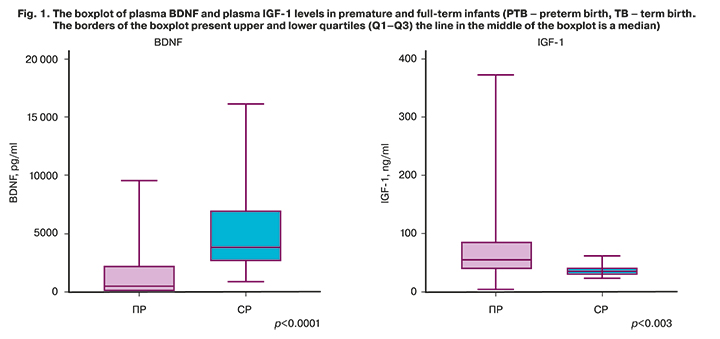
Comparative assessment of BDNF and IGF-1 levels in preterm and full-term infants found statistically significant differences (Fig.1). BDNF level was high in the TB group, while IGF-1 level was low. The median value of BDNF level in the PTB group was 476.5 [72.7; 2219] pg/ml, in the TB group – 3785 [2707; 6915] pg/ml, р <0.0001. Median value of IGF-1 level in the PTB group was 52.8 [38.1; 85.3] ng/ml, in the TB groupв 35.5 [30.4; 41.5] ng/ml, р=0.004. Figure 1 shows plasma BDNF and plasma IGF-1levels in premature and full-term infants. The analysis of the relationship between BDNF and IGF-1 levels based on the Spearman's rank-order correlation method, did not found statistically significant correlations in both groups.
At the same time multiple correlation analysis based on Spearman’s method revealed that there was statistically significant positive correlation between the level of IGF-1 and the weight of the premature infants (r=0.5; р=0.04), as well as the gestational age (r=0,5; р=0,04) in the PTB group. There was an interesting finding with regard to a positive correlation between the level of IGF-1 and the absolute number of stab neutrophils in peripheral blood (r=0.5; р=0.05). There was a negative correlation between BDNF values and the number of bed-days spent by newborns in the intensive care unit (r=-0.5; р=0.03).
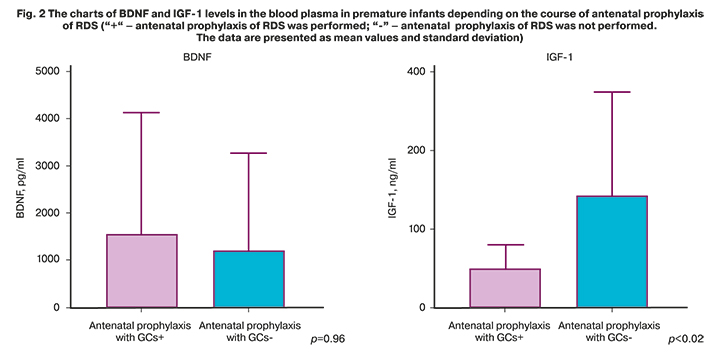
The issue of the rational indication of antenatal prophylaxis of RDS, especially the first course of preventive therapy and exclusively at imminent risk of preterm labor is being actively discussed and requires further studies. Considering numerous published data on the impact of GCs used for prenatal prophylaxis of RDS on long-term outcomes in newborns [20, 21], we analyzed BDNF and IGF-1 levels in premature infants depending on the use of the course of antenatal prophylaxis of RDS. 15/21 (71.4%) pregnant women with the risk of PTB received GCs. The median value of BDNF level in the PTB group, who received preventive therapy of PTB was 488.5 [70.9; 2295] pg/ml, and without preventive therapy – 326.5 [133.4; 2730] pg/ml, р=0.96. The median value of IGF-1 level in the PTB group who received preventive therapy of PTB was 45.3 [28.3; 58.1] ng/ml, and without preventive therapy – 90.9 [57.7; 252.3] ng/ml, р=0.02. Figure 2 shows the charts of BDNF and IGF-1 levels in the blood plasma of premature infants depending on the course of antenatal prophylaxis of RDS.
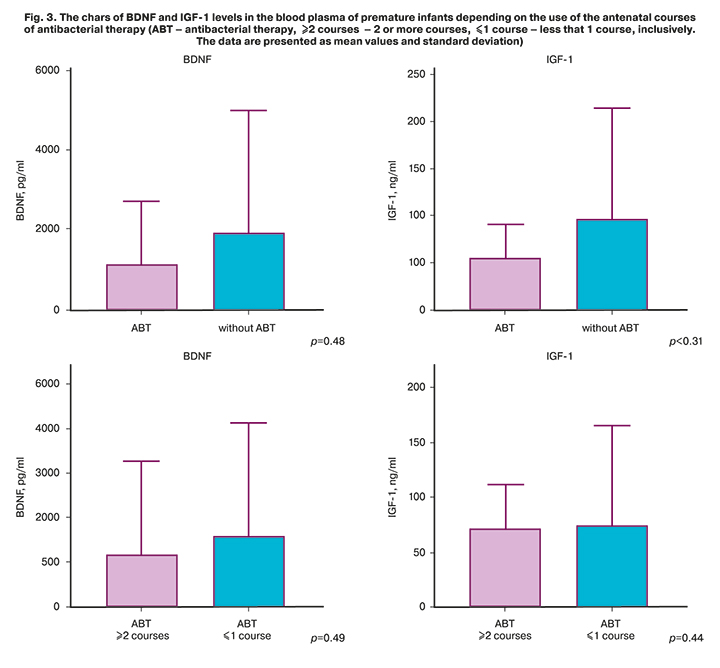
Analysis of specific features of the course of pregnancy (Table 2) showed a high percentage of women who received antibacterial treatment in the PTB group. Antibacterial therapy was used in 11/21 (52.4%) pregnant women with PTB, at the same time, indication of two or more courses during the total gestation period was used in 5/21 (23.8%) women in the PTB group. Figure 3 shows the charts of BDNF and IGF-1 levels in the blood plasma of premature infants depending on the use of the antenatal courses of antibacterial therapy. Despite the fact, that there was no statistically significant differences, it is clearly seen, that BDNF and IGF-1 levels are low against the background of antibacterial therapy compared to the patients, who did not receive treatment.
Discussion
The concept of DOHaD (Developmental Origins of Health and Disease) proposed by David Baker and based on association between infant mortality rate in the period from 1921 to 1925 and the rate of coronary heart disease from 1968 to 1978, confirmed the hypothesis of the fetal origins of adult diseases [22]. This obligates obstetricians and gynecologists actively to study the perinatal origins of the pathological conditions after birth, taking into account the gestation period.
Assessment of antenatal risk factors associated with preterm birth demonstrated specific nosological indicators: parity, recurrent miscarriage/spontaneous PTB in history, husband’s age and the risk of pregnancy termination [11, 19]. Statistically, along with the use of tocolytic therapy, antibiotic therapy was used significantly more often in pregnant women with spontaneous PTB (OR 3.45; 95% CI 1.82–6.46) due to the presence of inflammatory markers, as well as after cerclage. It should be noted, that combined use of tocolytic and antibiotic therapy probably contributed to prolongation of pregnancy.вероятно At the same time, numerous published data on the long-term effects of antibacterial therapy, in particular the large-scale ORACLE study [23], which assessed the perinatal outcomes, showed a number of adverse outcomes. Our study did not show significant differences between the neutrophins level in premature infants depending on the use of antenatal antibacterial therapy, and this is probably due to insufficient research power. Nevertheless, the data presented in Figure 3 clearly show low BDNF level in the blood plasma of premature infants after antenatal antibiotic therapy. Most likely, this fact can be explained as an additional risk factor for dysbiosis caused by antibiotic treatment, which contributes to reduced BDNF mRNA expression, as described in a number of studies [17, 24]. Assessment of IGF level showed the same tendency – the reduced IGF-1 level against the background of antibacterial therapy, and this correlates with the results of experimental research, which demonstrated that intestinal microbiota induces IGF-1 secretion [25].
Glucocorticoids are important regulators during intrauterine life that determine many aspects of fetal programming [26]. The course of antenatal prophylaxis of RDS improves the outcomes in premature infants, and the necessity to use it is beyond doubt [11]. Однако узкое терапевтическое окно, сохраняющегося эффекта от терапии (7 дней!) However, narrow therapeutic window for therapeutic effect, which lasted only 7 days, and the data on long-term adverse effects in newborns [27–29] require a thorough analysis of all risk factors for PTB, which indicate that preterm birth is inevitable, especially in indication of the first therapeutic course. In our study, assessment of the impact of the course of RDS prophylaxis on neutrophins levels showed increased BDNF level due to acceleration potential of GCs, however, it was not statistically significant. Statistically significant reduction in IGF-1 level after the course of antenatal prophylaxis of RDS is open to interpretation, nevertheless, the data in scientific literature do not contradict the obtained results. Several studies showed that GCs can reduce IGF-1 levels in premature infants by increasing gene expression responsible for the synthesis of the family of proteins that bind insulin-like growth factors I and II (IGFBP) [18, 30].
Conclusion
The rational indication of any type of therapy, especially in the obstetric practice, should be based on the relationship between the potential benefits and possible risks. The data obtained in the course of our study on different levels of neurotrophins in premature infants, depending on antenatal characteristics, confirm the classical medical canons and the postulate of modern perinatology: “Do not do any harm”. Antibacterial treatment should be carried out only in the presence of a number of objective factors, indicating the infectious-associated etiology of PTB in the gestation period. In this regard, the results of amniotic fluid analysis the samples of which were obtained during transabdominal amniocentesis are verification of the infection and inflammation process. Prophilaxis of RDS improves perinatal outcomes. Nevertheless, in order to provide that the benefit prevails the adverse outcomes, it should be used only when PTB is inevitable. This defines the search for the markers for PTB. Given the small sample size, further research is necessary to confirm the findings of this study.
References
- Workalemahu T., Grantz K.L., Grewal J., Zhang C., Louis G.M.B., Tekola-Ayele F. Genetic and environmental influences on fetal growth vary during sensitive periods in pregnancy. Sci. Rep. 2018; 8(1): 7274. https://dx.doi.org/10.1038/s41598-018-25706-z.
- Kim D.R., Bale T.L., Epperson C.N. Prenatal programming of mental illness: current understanding of relationship and mechanisms. Curr. Psychiatry Rep. 2015; 17(2): 5. https://dx.doi.org/10.1007/s11920-014-0546-9.
- Spiers H., Hannon E., Schalkwyk L.C., Smith R., Wong C.C.Y., O’Donovan M.C. et al. Methylomic trajectories across human fetal brain development. Genome Res. 2015; 25(3): 338-52. https://dx.doi.org/10.1101/gr.180273.114.
- Fitzgerald E., Hor K., Drake A.J. Maternal influences on fetal brain development: The role of nutrition, infection and stress, and the potential for intergenerational consequences. Early Hum. Dev. 2020; 150: 105190. https://dx.doi.org/10.1016/j.earlhumdev.2020.105190.
- Antonakopoulos N., Iliodromiti Z., Mastorakos G., Iavazzo C., Valsamakis G., Salakos N. et al. Association between Brain-Derived Neurotrophic Factor (BDNF) levels in 2nd trimester amniotic fluid and fetal development. Mediators Inflamm. 2018; 2018: 8476217. https://dx.doi.org/10.1155/2018/8476217.
- Nassar M.F., Younis N.T., El-Arab S.E., Fawzi F.A. Neuro-developmental outcome and brain-derived neurotrophic factor level in relation to feeding practice in early infancy. Matern. Child Nutr. 2011; 7(2): 188-97. https://dx.doi.org/10.1111/j.1740-8709.2010.00252.x.
- Hellström A., Ley D., Hansen‐Pupp I., Hallberg B., Löfqvist C., Marter L. et al. Insulin‐like growth factor 1 has multisystem effects on foetal and preterm infant development. Acta Paediatr. 2016; 105(6): 576-86. https://dx.doi.org/10.1111/apa.13350.
- Hellström A., Ley D., Hansen-Pupp I., Hallberg B., Ramenghi L.A., Löfqvist C. et al. Role of insulinlike growth factor 1 in fetal development and in the early postnatal life of premature infants. Am. J. Perinatol. 2016; 33(11): 1067-71. https://dx.doi.org/10.1055/s-0036-1586109.
- Torres-Aleman I. Toward a comprehensive neurobiology of IGF-I. Dev. Neurobiol. 2010; 70(5): 384-96. https://dx.doi.org/10.1002/dneu.20778.
- D’Ercole J.A., Ye P. Expanding the mind: insulin-like growth factor I and brain development. Endocrinology. 2008; 149(12): 5958-62. https://dx.doi.org/10.1210/en.2008-0920.
- National Institute for Health and Care Excellence. Preterm labour and birth: NICE guideline (NG25). 20 November 2015.
- Briceño-Pérez C., Reyna-Villasmil E., Vigil-De-Gracia P. Antenatal corticosteroid therapy: historical and scientific basis to improve preterm birth management. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019; 234: 32-7. https://dx.doi.org/10.1016/j.ejogrb.2018.12.025.
- Flenady V., Hawley G., Stock O.M., Kenyon S., Badawi N. Prophylactic antibiotics for inhibiting preterm labour with intact membranes. Cochrane Database Syst. Rev. 2013; (12): CD000246. https://dx.doi.org/10.1002/14651858.CD000246.pub2.
- Lamont R.F. Advances in the prevention of infection-related preterm birth. Front. Immunol. 2015; 6: 566. https://dx.doi.org/10.3389/fimmu.2015.00566.
- Leclercq S., Mian F.M., Stanisz A.M., Bindels L.B., Cambier E., Ben-Amram H. et al. Low-dose penicillin in early life induces long-term changes in murine gut microbiota, brain cytokines and behavior. Nat. Commun. 2017; 8: 15062. https://dx.doi.org/10.1038/ncomms15062.
- O’Connor R., Moloney G.M., Fulling C., O’Riordan K.J., Fitzgerald P., Bastiaanssen T.F.S. et al. Maternal antibiotic administration during a critical developmental window has enduring neurobehavioural effects in offspring mice. Behav. Brain Res. 2021; 404: 113156. https://dx.doi.org/10.1016/j.bbr.2021.113156.
- Bistoletti M., Caputi V., Baranzini N., Marchesi N., Filpa V., Marsilio I. et al. Antibiotic treatment-induced dysbiosis differently affects BDNF and TrkB expression in the brain and in the gut of juvenile mice. PLoS One. 2019; 14(2): e0212856. https://dx.doi.org/10.1371/journal.pone.0212856.
- Verhaeghe J., Vanstapel F., Van Bree R., Van Herck E., Coopmans W. Transient сatabolic state with reduced IGF-I after antenatal glucocorticoids. Pediatr. Res. 2007; 62(3): 295-300. https://dx.doi.org/10.1203/PDR.0b013e318123f72f.
- Khandwala Y.S., Baker V.L., Shaw G.M., Stevenson D.K., Lu Y., Eisenberg M.L. Association of paternal age with perinatal outcomes between 2007 and 2016 in the United States: population based cohort study. BMJ. 2018; 363: k4372. https://dx.doi.org/10.1136/bmj.k4372.
- Eriksson L., Haglund B., Ewald U., Odlind V., Kieler H. Short and long-term effects of antenatal corticosteroids assessed in a cohort of 7,827 children born preterm. Acta Obstet. Gynecol. Scand. 2009; 88(8): 933-8. https://dx.doi.org/10.1080/00016340903111542.
- Ilg L., Klados M., Alexander N., Kirschbaum C., Li S.-C. Long-term impacts of prenatal synthetic glucocorticoids exposure on functional brain correlates of cognitive monitoring in adolescence. Sci. Rep. 2018; 8(1): 7715. https://dx.doi.org/10.1038/s41598-018-26067-3.
- Wadhwa P., Buss C., Entringer S., Swanson J. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin. Reprod. Med. 2009; 27(5): 358-68. https://dx.doi.org/10.1055/s-0029-1237424.
- Kenyon S., Brocklehurst P., Jones D., Marlow N., Salt A., Taylor D. MRC ORACLE Children Study. Long term outcomes following prescription of antibiotics to pregnant women with either spontaneous preterm labour or preterm rupture of the membranes. BMC Pregnancy Childbirth. 2008; 8(1): 14. https://dx.doi.org/10.1186/1471-2393-8-14.
- Fröhlich E.E., Farzi A., Mayerhofer R., Reichmann F., Jačan A., Wagner B. et al. Cognitive impairment by antibiotic-induced gut dysbiosis: Analysis of gut microbiota-brain communication. Brain Behav. Immun. 2016; 56: 140-55. https://dx.doi.org/10.1016/j.bbi.2016.02.020.
- Yan J., Herzog J.W., Tsang K., Brennan C.A., Bower M.A., Garrett W.S. et al. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc. Natl. Acad. Sci. USA. 2016; 113(47): E7554-63. https://dx.doi.org/10.1073/pnas.1607235113.
- Fowden A.L., Forhead A.J. Glucocorticoids as regulatory signals during intrauterine development. Exp. Physiol. 2015; 100(12): 1477-87. https://dx.doi.org/10.1113/EP085212.
- Murphy K.E., Hannah M.E., Willan A.R., Hewson S.A., Ohlsson A., Kelly E.N. et al.; MACS Collaborative Group. Multiple courses of antenatal corticosteroids for preterm birth (MACS): a randomised controlled trial. Lancet. 2008; 372(9656): 2143-51. https://dx.doi.org/10.1016/S0140-6736(08)61929-7.
- Asztalos E., Willan A., Murphy K., Matthews S., Ohlsson A., Saigal S. et al. Association between gestational age at birth, antenatal corticosteroids, and outcomes at 5 years: multiple courses of antenatal corticosteroids for preterm birth study at 5 years of age (MACS-5). BMC Pregnancy Childbirth. 2014; 14(1): 272. https://dx.doi.org/10.1186/1471-2393-14-272.
- Ходжаева З.С., Горина К.А. Антенатальная профилактика респираторного дистресс-синдрома плода: взгляд в будущее. Акушерство и гинекология. 2019; 5: 12-8. [Khodzhaeva Z.S., Gorina K.A. Antenatal prevention of fetal respiratory distress syndrome: a glimpse into the future. Obstetrics and Gynecology. 2019; 5: 12-8. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.5.12-18.
- Bloomfield F.H., Knight D.B., Breier B.H., Harding J.E. Growth restriction in dexamethasone-treated preterm infants may be mediated by reduced IGF-I and IGFBP-3 plasma concentrations. Clin. Endocrinol. 2001; 54(2): 235-42. https://dx.doi.org/10.1046/j.1365-2265.2001.01219.x.
Received 14.07.2021
Accepted 27.07.2021
About the Authors
Ksenia A. Gorina, Junior researcher at the Department of Pregnancy Pathology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecologyand Perinatology, Ministry of Healthcare of Russian Federation. Tel.: +7(926)649-77-32. E-mail: k_gorina@oparina4.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Zulfiya S. Khodzhaeva, Dr. Med. Sci, Professor, Deputy Director for Research of Obstetrics Institute, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of Russian Federation. Tel.: +7(916)407-75-6. E-mail: zkhodjaeva@mail.ru.
117997, Russia, Moscow, Ac. Oparina str., 4.
Valentina V. Vtorushina, PhD, clinical laboratory diagnostics doctor of the Laboratory of Clinical Immunology, Academician V.I. Kulakov National Medical Research Center
for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of Russian Federation. Tel.: +7(495)438-11-83. E-mail: vtorushina@inbox.ru.
117997, Russia, Moscow, Ac. Oparina str., 4.
Liubov V. Krechetova, Dr. Med. Sci., Head of the Laboratory of Clinical Immunology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of Russian Federation. Tel.: +7(495)438-11-83. E-mail: l_krechetova@oparina4.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Oleg V. Ionov, MD, PhD, Head of the of the NICU named after professor Antonov A.G. of the Institute of Neonatology and Pediatrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of Russian Federation. Tel.: +7(495)438-22-77. E-mail: o_ionov@oparina4.ru. ORCID: 0000-0002-4153-133X. 117997, Russia, Moscow, Ac. Oparina str., 4.
Anna A. Lenyushkina, PhD, Head of the Neonatal Intensive Care Unit No. 2 of the Institute of Neonatology and Pediatrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of Russian Federation. Tel.: +7(495)531-44-44 (ex. 2700, 2697).
E-mail: a-lenushkina@yandex.ru. ORCID: 0000-0001-8929-2991. 117997, Russia, Moscow, Ac. Oparina str., 4.
Leyla A. Timofeeva, PhD, Head of the Department of Newborns No. 1, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of Russian Federation. Tel.: +7(495)438-11-83. Е-mail: leilatimofeeva@mail.ru, l_timofeeva@oparina4.ru.
117997, Russia, Moscow, Ac. Oparina str., 4.
Mzia I. Makieva, Head of the Department of Newborns No. 2, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of Russian Federation. Tel.: +7(495)438-11-83. Е-mail: m_makieva@oparina4.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
Dmitriy N. Degtyarev, Dr. Med. Sci., Professor, Deputy Director for Research, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Healthcare of Russian Federation; Head of the Department of Neonatology, I.M. Sechenov First MSMU, Ministry of Healthcare of Russian Federation (Sechenov University). Tel.: +7(495)438-25-33. E-mail: d_degtiarev@oparina4.ru. 117997, Russia, Moscow, Ac. Oparina str., 4.
For citation: Gorina K.A., Khodzhaeva Z.S., Vtorushina V.V., Krechetova L.V., Ionov O.V., Lenyushkina A.A., Timofeeva L.A., Makieva M.I., Degtyarev D.N.
Antenatal factors associated with factors associated with brain-derived neurotrophic factor and insulin-like growth factor-1 levels in premature infants.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 8: 102-110 (in Russian)
https://dx.doi.org/10.18565/aig.2021.8.102-110



