Sensitivity and specificity of plasma nitric oxide metabolites for the diagnosis of intrauterine pneumonia in preterm prelabor rupture of membranes
Objective: To determine the diagnostic accuracy of nitrite (NO2–) and non-thiolate nitroso compounds (RNO) for the detection of intrauterine pneumonia (IUP) in women with preterm labor and premature rupture of membranes (PROM). Materials and methods: This cross-sectional study included 87 patients. Plasma NO2–+RNO, serum leukocyte and C-reactive protein levels were assessed in all patients on admission, and genital secretions were examined microscopically and culturally. NO2–+RNO was determined using an enzyme sensor (RF patent #2461831). In addition, birth outcomes and neonatal developmental histories were analyzed. Based on retrospective analysis, five groups were formed: Ia – ≥37 weeks without PROM (n=21); Ib – ≥37 weeks + PROM (n=12), IIa – 220–366 weeks without PROM (n=12); IIb – 220–366 weeks + PROM (without neonatal IUP) (n=14); IIc – 220–366 weeks + PROM (with neonatal IUP) (n=28). Results: In the presence of IUP, NO2–+RNO values (3.02±0.90 μM) were statistically significantly higher when compared with groups without IUP (p<0.001). There was a statistically significant direct correlation between the presence of neonatal IUP and the level of maternal venous NO metabolites (rs=0.626; p<0.001). ROC analysis showed a sensitivity of 85.2% (95% CI 66.27–95.81) and specificity of 93.33% (95% CI 83.8–98.15) for NO2–+RNO≥2.2 μM. Conclusion: Determination of maternal venous nitric oxide metabolites has a high diagnostic accuracy for the early detection of IUP in preterm birth complicated by PROM: PPV 85.2% (95% CI 68.77–93.76), NPV 93.33% (95% CI 84.97–97.20). The use of this marker for the diagnosis of IUP will allow a personalized approach to selecting appropriate obstetric management and reducing adverse perinatal outcomes associated with infection. Authors' contributions: Shalina R.I., Anankina A.A., Osipov A.N., Titov V.Yu., Spiridonov D.S. – conception and design of the study, data collection and analysis, review of the relevant literature, statistical analysis, manuscript drafting, and editing. All authors contributed equally to the study and manuscript drafting and read and approved it for submission. Conflicts of interest: The authors have no conflicts of interest to declare. Funding: There was no funding for this study. Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the Pirogov RNRMU of Minzdrav of Russia (Ref. No: 213 of 13.12.2021). Patient Consent for Publication: All patients provided informed consent for the publication of their data. Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator. For citation: Shalina R.I., Anankina A.A., Osipov A.N., Titov V.Yu., Spiridonov D.S. Sensitivity and specificity of plasma nitric oxide metabolites for the diagnosis of intrauterine pneumonia in preterm prelabor rupture of membranes. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (5): 59-67 (in Russian) https://dx.doi.org/10.18565/aig.2023.20Shalina R.I., Anankina A.A., Osipov A.N., Titov V.Yu., Spiridonov D.S.
Keywords
Congenital (intrauterine) pneumonia (IUP) develops as a result of intrauterine or intrapartum fetal infection [1]. Accumulated evidence suggests that preterm prelabor rupture of membranes (PROM) in patients with intrauterine infection is not caused by an infectious agent per se but by the body's protective response to it [2]. Inflammatory mediators are produced and a cascade of immunological reactions is launched [2, 3]. Thus, fetal infection can be both a cause and consequence of PROM [1]. The problem of IUP is due to the ambiguity of the etiology, the difficulty of antenatal diagnosis, and the multifactorial effect of the infectious agent on the fetus. Timely diagnosis is also difficult because there are no clear correlations between the severity of maternal infectious and inflammatory diseases and the degree of fetal damage [4]. According to current clinical guidelines, expectant management is indicated for PROM that occurs before the 37th week [5, 6]. However, an increase in the duration of ruptured membranes (DRM) is associated with an increased risk of complications, such as chorioamnionitis (CA), placental abruption, prolapse or compression of the umbilical cord, and postpartum complications requiring antibiotics [7, 8]. At the same time, an active management strategy increases the risk of neonatal complications associated with immaturity, including respiratory distress syndrome, and the need for mechanical ventilation and neonatal intensive care unit admission, resulting in increased perinatal morbidity and mortality [9]. Although expectant management may improve perinatal outcomes, it requires careful monitoring of the state of the mother and fetus [7]. Therefore, it is necessary to develop and implement a non-invasive diagnostic method with high sensitivity and specificity. Current methods with high diagnostic accuracy require amniocentesis, which is contraindicated in patients with threatened preterm birth (PB). Ultrasound diagnostics allows only a suggestion but is not a criterion for making a diagnosis [10]. As mentioned above, fetal intrauterine infections may be asymptomatic. In this regard, timely diagnosis of IUP with the threat of PT and expectant management in the third trimester of pregnancy remains an unresolved problem. In 2012 Titov V.Yu., Osipov A.N. et al. proposed a method for early diagnosis of inflammatory processes based on recording the content of nitrite (NO2–) and non-thiolate nitroso compounds (RNO) in blood plasma using a previously patented enzyme sensor (RF patent No. 2461831) [11]. These substances are products of the interaction of the superoxide anion produced by activated leukocytes with the deposited nitric oxide contained in the plasma [12]. In the absence of an inflammatory process, the above compounds are found in the blood in an amount of less than 0.1 μM, since non-activated leukocytes do not produce superoxide [13].
The leading role of IUP in the structure of adverse perinatal outcomes in PB and the high level of infection in pregnant and postpartum women necessitates the search for reliable methods for its diagnosis.
Our study aimed to determine the diagnostic accuracy of nitrite (NO2–) and non-thiolate nitroso compounds (RNO) for the detection of IUP in women with preterm prelabor rupture of membranes.
Our main hypotheses were: 1) The sensitivity and specificity of NO2–+RNO in the detection of IUP are higher than those of conventional markers of inflammation (leukocytes and C-reactive protein (CRP) in blood serum and the number of leukocytes in the vaginal and cervical contents); 2) The NO2–+RNO values were significantly different in patients with IUP compared to those in pregnant women with threatened PB (with or without PROM) and without fetal IUP.
Materials and methods
This cross-sectional study included patients hospitalized at the Center for Family Planning and Reproduction of the Moscow Health Department between 2021 and 2022. On admission, the examination of the patients according to order no. 1130n (n=87) was supplemented by the determination of the NO2–+ RNO level in the venous blood (before receiving any medication). One-step sampling was performed for subsequent assessment of blood plasma levels of NO2–+ RNO, leukocytes, and serum CRP, and microscopic and culture examinations of vaginal discharge. In addition, a retrospective assessment was performed after the diagnosis of IUP was made in newborns from the group with PROM that occurred at 220–366 weeks of gestation.
The cohort was divided according to gestational age, the presence of PROM, and IUP: Ia – ≥37 weeks without PROM (n=21); Ib – ≥37 weeks + PROM (n=12), IIa – 220–366 weeks without PROM (n=12); IIb – 220–366 weeks + PROM (without neonatal IUP) (n=14); IIc – 220–366 weeks + PROM (with neonatal IUP) (n=28).
Inclusion criteria were informed consent to participate in the study, singleton pregnancy, for groups Ia and Ib – ≥ 37 weeks gestation, and for groups IIa, b, c – 220–366 weeks gestation. The exclusion criteria were antenatal death, congenital malformations in the fetus, antibiotic therapy before hospitalization (≤ 7 days), and maternal acute inflammatory diseases. The observation of patients was carried out from the moment of hospitalization to delivery, for newborns, from birth to discharge from the hospital, or transfer to the second stage of nursing.
According to the clinical guidelines for IUP, approved by the Russian Association of Perinatal Medicine and the Russian Society of Neonatologists, IUP is diagnosed with clinical and radiological manifestations in the first 72 hours of a child's life. Congenital pneumonia was diagnosed if at least one main and/or three (or more) auxiliary diagnostic features were identified [1].
PROM was diagnosed using a rapid test for placental alpha-microglobulin-1 (PAMG-1) in the cervicovaginal secretions.
Registration of blood plasma NO2– and RNO was performed using an enzyme sensor [11]. To determine the NO2–+ RNO content, venous blood samples (2 ml) were collected from the study subjects in test tubes containing sodium heparin. Plasma was obtained by centrifuging the samples in a TsUM-1 centrifuge at a speed of 3000 rpm for 3 min. The supernatant was separated from erythrocytes and used to determine the NO2–+ RNO content [11–13]. Catalase activity was determined using a calorimetric method based on the control of the kinetics of heat production accompanying the enzymatic decomposition of hydrogen peroxide by catalase [12]. The staff who determined the content of NO2–+ RNO did not have clinical data of the patients and did not know about the neonatal outcomes. 0.1 μM was chosen as the reference value for NO2–+ RNO, and higher values were considered indicative of an inflammatory process [13].
In our study, none of the patients during pregnancy had a suspicion of fetal intrauterine infection, and there were no indications for amniocentesis (in patients with threatened PB, amniocentesis is contraindicated). Markers of inflammation generally accepted in clinical practice used as reference tests in the study included the serum levels of leukocytes, CRP, and leukocyte count in vaginal and cervical secretions. The determination of these parameters is indicated for the timely diagnosis of septic complications in PROM according to the clinical guidelines for PB (2020) [6].
In vitro quantitation of CRP in human serum was performed using an enzyme-linked immunosorbent assay, referred to as the high-sensitivity C-reactive protein (hs-CRP ELISA). This test is based on the principle of indirect enzyme-linked immunosorbent assay.
Considering that there are currently no recommendations for diagnosing IUP at the antenatal stage, the threshold values for comparing the sensitivity and specificity of the index and reference tests were determined using ROC curve analysis. The results are presented in section "Methods of laboratory diagnostics and IUP.”
The study was reviewed and approved by the Research Ethics Committee of the Pirogov RNRMU, Ministry of Health of Russia (protocol No. 213 of December 13, 2021).
Statistical analysis
Statistical analyses and plotting were performed using IBM SPSS Statistics 26.0 (IBM Corp., USA). 3.0.6 (Stattech LLC, Russia) and MedCalc (MedCalc Software Ltd., USA). Pearson's χ2 test for 2×2 contingency tables was used to compare the categorical variables. Fisher’s exact test was used when the expected frequency of one or more cells was less than 10. The normality of the distribution was tested using the ShapiroWilk test. Quantitative variables showing a normal distribution were expressed as mean (M), standard deviation (SD), and 95% confidence interval (95% CI). In the absence of a normal distribution, data are presented as the median (Me) and lower and upper quartiles (Q1–Q3). Normally distributed continuous variables were compared between the two groups using Student’s and Welch t tests (unequal variance t-test). Variables that did not meet normality assumptions were compared using the Mann–Whitney U test. Normally distributed continuous variables were compared between three or more groups and tested using one-way analysis of variance followed by Tukey's post hoc tests for between-group comparisons. Variables not meeting normality assumptions were compared with the Kruskal–Wallis test followed by Dunn's post hoc test with Holm's correction. The relationship between the parameters was also assessed using the nonparametric Spearman correlation coefficient (rs), and the strength of the correlation was assessed using the Chaddock scale. The performance characteristics of diagnostic tests (sensitivity, specificity, positive predictive value, and negative predictive value) in the diagnosis of a particular outcome were assessed by constructing ROC curves. The threshold value of the quantitative tests at the cut-off point was determined by the highest value of the Youden index. The significance threshold was set at p<0.05. The performance characteristics of diagnostic tests (sensitivity, specificity, positive and negative predictive value, and 95% CI) were calculated using contingency tables (with determination of the number of true and false positives and true and false negatives) using MedCalc software. The required sample size was calculated using the Epi Info based on population size indicators (according to the Federal State Statistics Service, the number of preterm births in 2021 was 59,500) and the incidence of the disease (according to the IUP clinical guidelines, it was diagnosed in 0.98% of preterm infants with a birth weight of 1000 g or more) (with a given research power of 95% and a significance level of 0.05).
Results
General characteristics of the pregnant women and perinatal outcomes of pregnancies and childbirth
The general characteristics of the patients by age, body mass index (BMI), gestational age at the time of sampling, obstetric history, DRM, and parameters of newborns are presented in Table 1. It should be noted that patients in the IUP group were significantly more likely to have isthmic-cervical insufficiency than those in the other groups (p<0.001). Post-hoc analysis showed that parameters such as DRM, weight, and Apgar scores at 1 and 5 min were significantly different in the IUP group. However, the difference in Silverman scores was not statistically significant between the groups with and without IUP. When comparing the methods of delivery, a statistically significant difference was found between the control group (full-term delivery without PROM) and the group with PB and IUP:20 (69%) patients from the control group had vaginal delivery and 12 (46) 2%) of patients in the IUP group had an operative delivery (emergency caesarean section) (p=0.005).
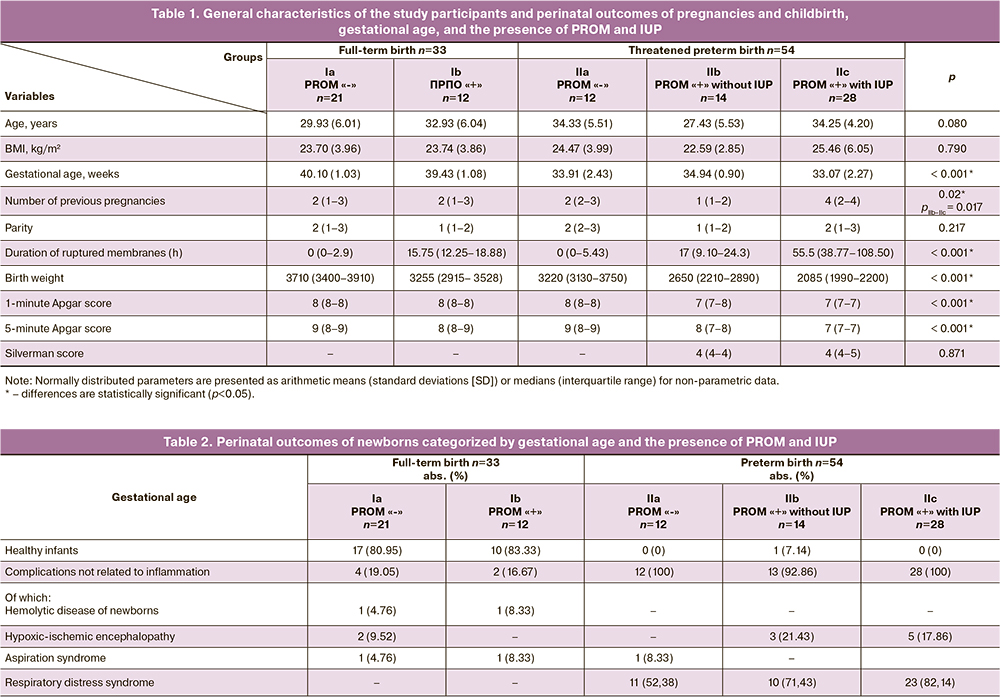
The perinatal neonatal outcomes are presented in Table 2. In the full-term delivery group, the majority of children were born healthy with no perinatal complications (66.67 and 75% in the absence and presence of PROM, respectively). The remaining children were born without any signs of IUP. In the PB group without PROM, all complications were associated with lung immaturity. Only 1 out of 42 patients with PROM had a healthy baby who did not need additional respiratory support and was further transferred to the second stage of nursing. It should be noted that Table 2 describes the complications that were set as the main diagnosis, however, most children from the group with prematurity had a combination of complications associated with prematurity. In the IUP group, complications other than pneumonia were observed.
Methods of laboratory diagnostics and IUP
The data obtained using various laboratory methods are presented in Table 3.
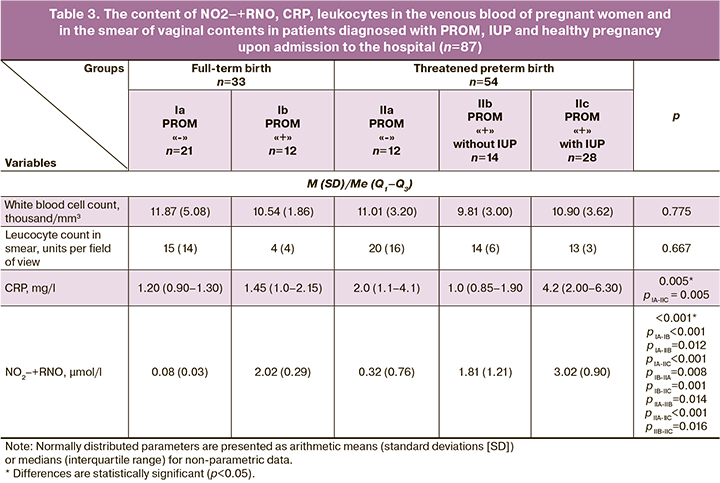
During expectant management in patients with PROM and threatened PB, inflammatory markers, such as leukocytes and CRP in venous blood, were evaluated over time to exclude the development of CA. The mean values were calculated based on the changes in the parameters. The mean values of leukocytes (p1) and CRP (p2) in venous blood were not significantly different between the absence and presence of IUP (p1=0.939; p2=0.136).
The levels of nitric oxide metabolites were significantly different between groups with and without PROM and between groups with IUP and PROM without IUP. It should be noted that there was no statistically significant difference when comparing NO2–+RNO levels in patients with PROM, depending on gestational age.
A comparison of the values of nitric oxide metabolites and the presence of PROM, IUP, and gestational age is presented in Figure 1.
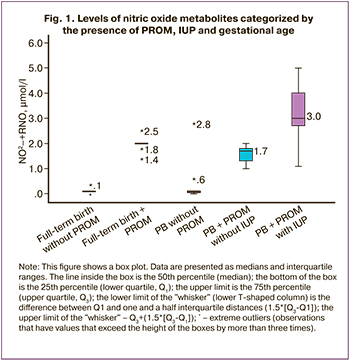
A statistically significant direct correlation was found between neonatal IUP and the level of NO metabolites in the mother's venous blood (rs=0.626; p<0.001). This relationship was strongly correlated according to the Chaddock scale.
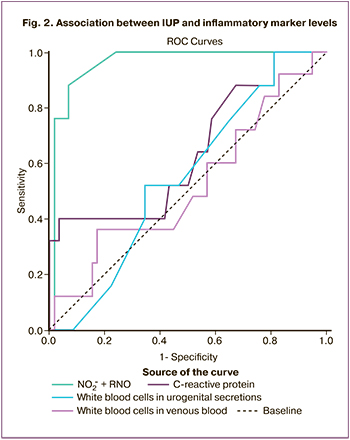
ROC analysis was performed to determine the cut-off value for nitric oxide metabolites for the diagnosis of IUP. The area under the ROC curve was 0.961 (0.021), with a 95% CI of 0.920–1.000. The resulting model was statistically significant (p<0.001). The threshold value of the NO2–+RNO index at the cutoff point, which corresponded to the highest value of the Youden index, was 2.2 µM.
In addition, the threshold values of inflammation markers widely used in practice were determined: leukocytes in venous blood, leukocyte count in a vaginal and cervical canal smear, and CRP. The pooled results of ROC analysis are shown in Figure 2. Based on the ROC curve analysis, there was no statistically significant relationship between IUP and leukocyte levels in the venous blood (p=0.978) or between vaginal and cervical discharge (p=0.657). The CRP-IUP relationship model was statistically significant (p=0.052).
Based on the obtained threshold values, a contingency table was constructed to determine the number of true and false positives and true and false negatives. Taking into account the obtained results, the following diagnostic accuracy characteristics were determined: sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). The results are presented in Table 4.
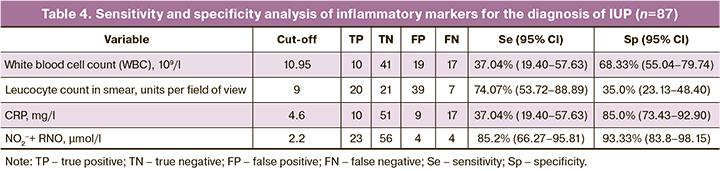
PPV of the NO2–+RNO value for diagnosing IUP was 85.2% (95% CI 68.77–93.76), and NPV was 93.33% (95% 84.97–97.20).
Assessment of indicators of predictive significance of positive and negative results for CRP: PPV – 52.63% (95% CI 33.80–70.74), NPV – 75.0% (95% CI 68.79–80.33).
Culture of vaginal and cervical contents showed that the following bacterial species were significantly more common in IUP patients (n=28) than in the other groups: Escherichia coli (p=0.0038, OR 2.93, 95% CI 1.41–6.06) and Candida albicans (p=0.0382, OR 1.05–4.83, 95% CI 1.05–4.83). Only one patient whose newborn developed IUP showed the dominance of Lactobacillus spp. According to our data, the predominance of Lactobacillus spp. in the vaginal microbiota of pregnant women reduces the chances of developing IUP in the neonate by a factor of 12.4 (p=0.001, OR 0.08, 95% CI 0.02–0.36).
Discussion
The importance of methods for the early diagnosis of IUP is unquestionable, given its significant impact on neonatal perinatal outcomes. The feasibility of diagnosis during pregnancy is of particular interest. When comparing standard markers of inflammation, such as WBC count in venous blood and vaginal discharge, we found no statistically significant differences between the groups with and without neonatal IUP.
The change in CRP level was not clinically significant. Similar results were reported by Hamadyanov et al. (14). According to the authors, there are currently no specific noninvasive diagnostic methods that can suggest intrauterine infection with a high degree of certainty; indirect methods (ultrasound and Doppler) can only provide a presumptive diagnosis.
The PB Clinical Guidelines (2020) recommend the investigation of white blood cell count and serum CRP levels in PROM for the timely diagnosis of septic complications [6]. The study referred to in this recommendation was a meta-analysis (12 studies, 3617 patients, 3628 children). In only one study, the authors determined the venous blood leukocyte count, which was used as one of the criteria for diagnosing CA in the presence of clinical symptoms of developed inflammation [15]. Leukocyte count does not have diagnostic value in the absence of clinical manifestations in a pregnant woman, which is comparable with our data.
The PB Clinical Guidelines suggest that CRP has a sensitivity of 68.7% and specificity of 77.1% for diagnosing septic complications [6]. This sensitivity is not sufficiently high for a test aimed at the prevention and timely detection of infection.
The study revealed a statistically significant difference in the levels of NO metabolites in maternal venous blood between groups with and without IUP. NO2− +RNO levels were significantly higher in patients with PROM than in those with intact membranes. At the same time, there was no significant difference in NO2− +RNO with respect to gestational age. Changes in nitric oxide metabolite levels, regardless of gestational age, indicated that this marker is not associated with threatened PROM in general, but has a role in the mechanisms underlying the pathogenesis of PROM. This is of great practical importance because there are currently few markers that change depending on the presence of PROM and not with PB in general. These markers include abnormal ratios of matrix metalloproteinases (MMP) and tissue-specific inhibitors of MMP (TIMP) (MMP/TIMP) in the amniotic fluid and the concentration of 3-nitrotyrosine in fetal membranes [16]. However, these markers require invasive intervention to determine them. We have shown the high sensitivity (85.2%) and specificity (93.33%) of the method for determining the level of nitric oxide metabolites in maternal venous blood for the timely diagnosis of neonatal IUP.
In our study, the vaginal and cervical culture results were comparable to the results of Brown et al. (2019). The authors showed that the prevalence of Lactobacillus spp. after 24 weeks reduced the relative risk of developing PROM [3]. Our results are similar to those of Szubert M. et al. (2021), who reported that in a group with abundant growth of aerobic bacteria and fungi, PB and signs of neonatal perinatal infection were more common [17]. Gorina K.A. et al. (2020) showed in their study that opportunistic microorganisms of facultative anaerobic origin – Staphylococcus aureus (p=0.0365) and/or Klebsiella pneumoniae (p=0.0217) were statistically significantly more often detected in patients with PB; at the same time, the growth of obligate anaerobes (Bacteroides spp.) was more modest (p=0.0416) [18].
Conclusions
1. The method for determining nitric oxide metabolites in maternal venous blood has a high diagnostic accuracy for the timely detection of IUP in PB complicated by PROM, with a PPV of 85.2% (95% CI 68.77–93.76) and NPV of 93.33% (95% CI 84.97–97.20). The sensitivity and specificity were 85.2% (95% CI 66.27–95.81) and 93.33% (95% CI 83.8–98.15), respectively.
2. The addition of NO2−+RNO to the comprehensive IUP diagnosis will improve the accuracy of the diagnosis, which will help optimize management strategies for pregnant women with threatened PB and PROM, and reduce adverse perinatal outcomes associated with infection.
References
- Антонов А.Г., Байбарина Е.Н., Балашова Е.Н., Дегтярев Д.Н., Зубков В.В., Иванов Д.О., Ионов О.В., Карпова А.Л., Киртбая А.Р., Крохина К.Н., Крючко Д.С., Ленюшкина А.А., Ли А.Г., Малютина Л.В., Мебелова И.И., Никитина И.В., Петренко Ю.В., Рындин А.Ю., Рюмина И.И., Романенко А.В. Врожденная пневмония (клинические рекомендации). Неонатология: новости, мнения, обучение. 2017; 4: 133-48. [Antonov A.G., Baybarina E.N., Balashova E.N., Degtyarev D.N., Zubkov V.V. et al. Congenital pneumonia (clinical practice guidelines). Neonatology: News, Opinions, Training. 2017; (4): 133-48. (in Russian)]. https://dx.doi.org/10.24411/2308-2402-2017-00049.
- Menon R., Fortunato S.J. Infection and the role of inflammation in preterm premature rupture of the membranes. Best Pract. Res. Clin. Obstet. Gynaecol. 2007; 21(3): 467-78. https://dx.doi.org/10.1016/j.bpobgyn.2007.01.008.
- Brown R.G., Al-Memar M., Marchesi J.R., Lee Y.S., Smith A., Chan D. et al. Establishment of vaginal microbiota composition in early pregnancy and its association with subsequent preterm prelabor rupture of the fetal membranes. Transl. Res. 2019; 207: 30-43. https://dx.doi.org/10.1016/j.trsl.2018.12.005.
- Лазарева Г.А., Ведощенко Т.В. Восходящее инфицирование как причина преждевременных родов. Актуальные проблемы медицины. 2013; 25:132-6. [Lazareva G.A., Vedoschenko I.V. Ascending infection is the cause of preterm birth. Current Problems of Medicine. 2013; (25): 132-6. (in Russian)].
- Prelabor Rupture of Membranes: ACOG Practice Bulletin, Number 217. Obstet. Gynecol. 2020; 135(3): e80-e97. https://dx.doi.org/10.1097/AOG.0000000000003700.
- Российское общество акушеров-гинекологов (РОАГ), Ассоциация акушерских анестезиологов-реаниматологов (АААР). Клинические рекомендации «Преждевременные роды». 2020. [Russian Society of Obstetricians and Gynecologists, Association of Obstetric Anesthesiologists and Resuscitators. Clinical Guidelines «Preterm Birth». 2020. (in Russian)].
- UpToDate. Preterm prelabor rupture of membranes: management and outcome. Available at: https://www.uptodate.com/contents/preterm-prelabor-rupture-of-membranes-management-and-outcome
- Morris J.M., Roberts C.L., Bowen J.R., Patterson J.A., Bond D.M., Algert C.S. et al. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016; 3 87 (10017): 444-52. https://dx.doi.org/10.1016/S0140-6736(15)00724-2.
- Bond D.M., Middleton P., Levett K.M., van der Ham D.P., Crowther C.A., Buchanan S.L. et al. Planned early birth versus expectant management for women with preterm prelabour rupture of membranes prior to 37 weeks' gestation for improving pregnancy outcome. Cochrane Database Syst. Rev. 2017; 3(3): CD004735. https://dx.doi.org/10.1002/14651858.CD004735.pub4.
- UpToDate. Cytomegalovirus infection in pregnancy. Available at: https://www.uptodate.com/contents/cytomegalovirus-infection-in-pregnancy?search=Cytomegalovirus%20infection%20in%20pregnancy%20&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
- Титов В.Ю., Осипов А.Н., Горский В.А., Агапов М.А., Иванова А.В., Балякин Ю.В., Иванова А.В., Камчатнов П.Р., Жданова С.Г. Способ диагностики острого неспецифического воспалительного процесса. ГОУ ВПО «Российский государственный медицинский университет Федерального агентства по здравоохранению и социальному развитию», патентообладатель. Патент РФ №2461831 от 20.09.2012. [Titov V.Yu., Osipov A.N., Gorsky V.A., Agapov M.A., Balyakin Yu.V. Ivanova A.V., Kamchatnov P.R., Zhdanova S.G. A method for the diagnosis of acute nonspecific inflammatory process. Russian State Medical University of the Federal Agency for Healthcare and Social Development, patentee. Patent of the Russian Federation №2461831 from 20.09.2012. (in Russian)].
- Титов В.Ю., Осипов А.Н., Крейнина М.В., Ванин А.Ф. Особенности метаболизма оксида азота в норме и при воспалении. Биофизика. 2013; 58(5): 857-70. [Titov V.Y., Osipov A.N., Kreinina M.V., Vanin A.F. Features of the metabolism of nitric oxide in normal state and inflammation. Biophysics. 2013; 58(5): 857-70. (in Russian)].
- Titov V., Osipov A., Vanin A. The ability of blood plasma to inhibit catalase in the presence of chloride is a highly sensitive indicator of deposited nitric oxide and leukocyte activation. Curr. Enzyme Inhibition. 2020; 16(2): 172-80.https://dx.doi.org/10.2174/1573408016999200429123919.
- Хамадьянов У.Р., Русакова Л.А., Хамадьянова А.У., Тихонова Т.Ф., Хамадьянова С.У., Галимов А.И., Иваха В.И. Внутриутробное инфицирование плода: современный взгляд на проблему. Российский вестник акушера-гинеколога. 2013; 13(5): 16-20. [Khamad'ianov U.R., Rusakova L.A., Khamad'ianova A.U., Tikhonova T.F., Khamad'ianova S.U., Galimov A.I., Ivakha V.I. Intrauterine fetal infection: the present view of the problem. Russian Bulletin of Obstetrician-Gynecologist. 2013; 13(5): 16-20. (in Russian)].
- Eroiz-Hernández J., Trejo-Acuña M.A., Alvarez-Tarín M.H. Conservative management of premature membrane rupture in pregnancy of 28-34 weeks. Aleatory clinical trial. Ginecol. Obstet. Mex. 1997; 65: 43-7. ( in Spanish).
- Menon R., Richardson L.S. Preterm prelabor rupture of the membranes: A disease of the fetal membranes. Semin. Perinatol. 2017; 41(7): 409-19.https://dx.doi.org/10.1053/j.semperi.2017.07.012.
- Szubert M., Weteska M., Zgliczynska J., Olszak O., Zgliczynska M., Kalinka J. et al. The association between imbalances in vaginal microflora and duration of pregnancy as well as selected maternal and neonatal parameters. Ginekol. Pol. 2021; 92(9); 624-30. https://dx.doi.org/10.5603/GP.a2021.0035.
- Горина К.А., Ходжаева З.С., Муравьева В.В., Муминова К.Т., Донников А.Е., Припутневич Т.В. Роль микробиоты кишечника матери при спонтанных преждевременных родах. Акушерство и гинекология. 2020; 8: 64-71. [Gorina K.A., Khodzhaeva Z.S., Muravieva V.V., Muminova К.Т., Donnikov A.E., Priputnevich T.V. The role of maternal gut microbiota in spontaneous preterm birth. Obstetrics and Gynecology. 2020; (8): 64-71. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.8.64-71.
Received 26.01.2023
Accepted 13.03.2023
About the Authors
Raisa I. Shalina, Dr. Med. Sci., Professor at the Academician G.M. Savelyeva Department of Obstetrics and Gynecology, Faculty of Pediatrics, N.I. Pirogov Russian National Research Medical University, Ministry of Health of the Russian Federation, +7(495)718-34-72, raisa.shalina@gmail.ru, https://orcid.org/0000-0001-7121-1663,117997, Russia, Moscow, Ostrovityanov str., 1.
Aliya A. Anankina, postgraduate student at the Academician G.M. Savelyeva Department of Obstetrics and Gynecology, Faculty of Pediatrics, N.I. Pirogov Russian National Research Medical University, Ministry of Health of the Russian Federation, +7(495)718-34-72, kuzina.aliya@yandex.ru, https://orcid.org/0000-0002-0223-0868,
117997, Russia, Moscow, Ostrovityanov str., 1.
Anatoliy N. Osipov, Corresponding Member of RAS, Dr. Bio. Sci., Professor, Head of the Department of General and Medical Biophysics, Medicobiologic Faculty, N.I. Pirogov Russian National Research Medical University, Ministry of Health of the Russian Federation, +7(495)434-11-74, anosipov@yahoo.com, https://orcid.org/0000-0001-7244-2818, 117997, Russia, Moscow, Ostrovityanov str., 1.
Vladimir Yu. Titov, Dr. Bio. Sci., Leading Researcher at the Department of Medical Biophysics, Institute of Translational Medicine, N.I. Pirogov Russian National Research Medical University, Ministry of Health of the Russian Federation, +7(495)718-34-72, vtitov43@yandex.ru, https://orcid.org/0000-0002-2639-7435,
117997, Russia, Moscow, Ostrovityanov str., 1.
Dmitrii S. Spiridonov, PhD, Associate Professor at the Academician G.M. Savelyeva Department of Obstetrics and Gynecology, Faculty of Pediatrics, N.I. Pirogov Russian National Research Medical University, Ministry of Health of the Russian Federation, +7(495)718-34-72, spiridonov_ds@rsmu.ru, https://orcid.org/0000-0001-8391-7436, 117997, Russia, Moscow, Ostrovityanov str., 1.
Corresponding author: Aliya A. Anankina, kuzina.aliya@yandex.ru



