Prooxidant and antioxidant content in capillary blood in preterm birth
Objective: To determine the content of prooxidants and antioxidants in the capillary blood obtained at the same gestation stage from pregnant women who had preterm birth and normal pregnancy.Kan N.E., Salpagarova Z.Kh., Tyutyunnik V.L., Shchipitsyna V.S., Krasnyi A.M.
Materials and methods: The study included 47pregnant women. The main group consisted of 31 patients who had preterm birth and control group included 16 women with a normal pregnancy. The peripheral blood samples were obtained from the patients of both groups at the same gestation stage. The level of reactive oxygen species and antioxidant protection was determined by means of a FORM 3000 device using FORT and FORT kits (Callegari, Italy). The capillary blood was obtained and heparin was used as an anticoagulant. Statistical analysis and plotting were performed using Attestat (Russia), Statistica 10, and OriginPro 8.5 (USA) software.
Results: There was an increase in the level of reactive oxygen species (ROS) in the main group in comparison with the control group. Their content in patients with preterm birth and normal pregnancy was 2.51 (2.06; 3.03) mmol/L and 1.22 (1.22; 1.66) mmol/L (p=0.01), respectively. The area under the ROC-curve was 0.90. The content of antioxidants in capillary blood was increased in the control group, namely 1.5 (1.03;2.31) mmol/L; their content was 0.97 (0.35;1.195) mmol/L in women with preterm birth. The area under the ROC-curve was 0. 77. The combined measurement of both parameters (reactive oxygen species and antioxidants) showed the greatest significance. The area under the ROC-curve was 0.93.
Conclusion: Preterm birth is associated with a systemic inflammatory response syndrome which is known to be accompanied by the development of oxidative stress leading to increased processes of apoptosis and disorders both at the cellular and subcellular levels. It is possible to determine the level of proinflammatory response and predict the development of complications, as well as to evaluate the effectiveness of therapy aimed at prolonging pregnancy by studying the indicators of oxidative stress and antioxidants.
Keywords
Preterm birth which is one of the great obstetrical syndromes remains the main cause of perinatal morbidity and infant mortality, and its frequency has no tendency to decrease [1–3].
Preterm birth is known to be an early idiopathic activation of the normal process of childbirth which is due to inflammatory decidual activation. Its manifestation is the development of oxidative stress, acceleration of apoptosis processes at the cellular and subcellular levels [4, 5] which are accompanied by imbalanced production of reactive oxygen species (ROS) and factors of antioxidant protection [5, 6]. According to S.M. Yellon et al. [4], inflammation caused by infection and other risk factors for spontaneous preterm birth and premature rupture of membranes can cause redox imbalance which increases the release of free radicals and destroys antioxidant protection [7–9]. Oxidative stress can initiate intracellular signaling cascades that increase the production of proinflammatory mediators.
It should be noted that modifications caused by the imbalance of ROS and antioxidant status lead to the appearance of new functional groups, such as hydroxyl or carbonyl groups. As a result, there is protein fragmentation, the formation of protein-protein cross-links, damage to the tertiary structure and loss of functional activity [10–12]. Moreover, ROS have a general biological toxic effect leading to direct peroxidation of membranes, proteins, structural enzymes, as well as nucleic acids [13–15].
Therefore, measuring the level of ROS and antioxidants can be promising for improving timely diagnosis and prognosis of preterm birth.
The aim of the study is to determine the content of prooxidants and antioxidants in the capillary blood obtained at the same gestation stage from pregnant women who had preterm birth and women with normal pregnancy.
Materials and methods
The study included 47 pregnant women who were divided into two groups. The main group consisted of 31 patients who had preterm birth and control group included 16 women with a normal full-term pregnancy whose patient’s obstetric history was unremarkable. The samples were obtained from the patients of both groups at the same gestation stage.
The study was conducted at the National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow, Russia. It was approved by the local research ethics committee. All pregnant women signed an informed consent to participate in the study.
The patients with a singleton pregnancy at 220 to 366 weeks gestation with intact membranes and clinical signs of preterm birth were eligible for inclusion in the study. Generally accepted methods and results of ultrasound screening in the first trimester were used to determine the gestational age.
The exclusion criteria were multiple pregnancy, rhesus sensitization, severe extragenital pathology, fetal growth retardation, preeclampsia.
The capillary blood was used as the test material which was centrifuged at 800g for 1 min. Blood was drawn into the capillaries. The time interval between blood collection and investigation did not exceed 25 minutes.
The level of reactive oxygen species and antioxidant protection was determined using FORT test (Callegari, Italy), colorimetric analysis based on the ability of transition metals, such as iron, to catalyze the decomposition of hydroperoxides (ROOH) into derivative radicals in accordance with the Fenton reaction. Dissolving in an acid buffer, hydroperoxides reacted with transition metal ions released from proteins in an acidic medium and turned into alkoxy (RO·) and peroxy (ROO·) radicals. The types of radicals which were formed as a result of the reaction interact with an additive (phenylenediamine derivative [2CrNH2]) and form a colored, rather long-lived radical cation, which can be evaluated using a spectrophotometer at a wavelength of 505 nm (reaction based on linear kinetics, 37°C). The color intensity directly correlates with the number of radical compounds and the concentration of hydroperoxides and, consequently, with the oxidative status of the sample according to the Lambert–Baer law.
R-OOH + Fe2+→ RO·+ OH-+ Fe3+
R-OOH + Fe3+→ ROO·+ H++ Fe2+
RO·+ROO·+2CrNH2→ ROO-+ RO-+ [CrNH2+·]
The results are presented in the form of equivalent concentrations of H2O2 mmol/L.
Stable and colored radicals which were previously formed were used in the FORD test. This test revealed the decrease in absorption which was proportional to the concentration of the antioxidant in the blood in the sample according to the Lambert-Behr law. In the presence of an acid buffer (pH=5.2) and an oxidizer (FeCl3), a chromogen containing 4-amino-N, N-diethylaniline sulfate forms a stable and colored cation radical detected photometrically at a wavelength of 505 nm. The antioxidant compounds in the sample reduce the chromogen radical cation and cause discoloration of the solution which is proportional to their concentration. The optical density values obtained for the samples are compared with the standard curve obtained using Trolox (6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid), a vitamin E derivative for permeable cells which is commonly used as an antioxidant.
Chromogen (without color) + Fe2+ + H+→ chromogen·+(purple)
Chromogen +(purple) + AOH → chromogen (without color) + AO
The obtained optical density values were compared with standard curves obtained for Trolox (6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid), a vitamin E derivative with increased solubility in water. The values of the FORD test are presented in Trolox equivalents, mmol/L.
Statistical analysis
The results are presented in the text as the median (Me), the upper and lower quartiles (Q1; Q3) and they are shown as box-and-whisker plots in the figures (5%, Q1, Me, Q3, 95%). Statistical significance of the differences was determined using the nonparametric Mann–Whitney test. The groups were compared for qualitative characteristics using Fisher’s exact test. The differences were considered statistically significant at p<0.05. ROC analysis was used to determine the effectiveness of the study. The ROC analysis data are presented as the area under the curve with a 95% confidence interval (CI). Statistical analysis and plotting were performed using Attestat (Russia), Statistica 10 and OriginPro 8.5 (USA) software.
Results and discussion
The main clinical and anamnestic data of the patients and characteristics of the course of pregnancy are presented in Table.
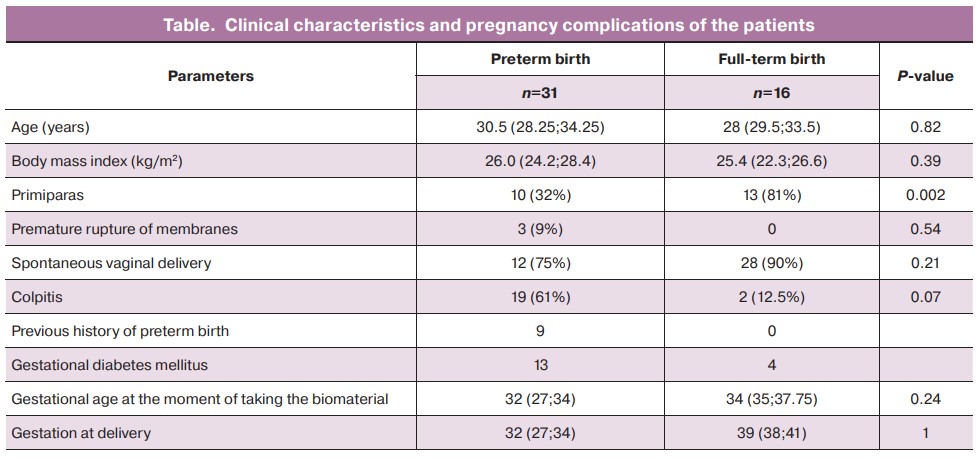
According to the data presented in Table, the age of women was not statistically significantly different between the groups and was 30.5 (28.25; 34.25) years in the group of patients who had preterm birth and 28 (29.5;33.5) years in the control group. There were no statistically significant differences in body mass index between the groups either. Preterm birth was determined to be caused by the following risk factors: parity, the previous history of preterm birth and colpitis. However, gestational diabetes mellitus and the delivery mode were not considered risk factors.
The content of ROS and antioxidants in groups of women who had preterm birth and normal pregnancy at the same gestation stage is shown in Figure 1.
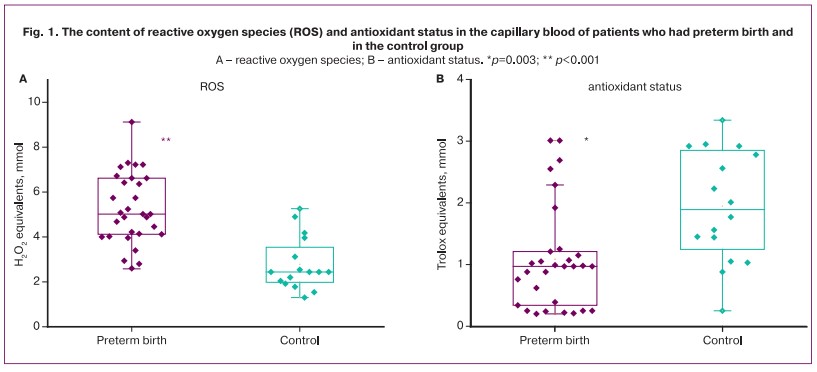
The patients with preterm birth demonstrated an increase in ROS in comparison with the patients who had full-term birth. The content of ROS was 2.51 (2.06;3.03) mmol/L and 1.22 (1.22;1.66) mmol/L (in groups, respectively) (p=0.01) (Fig. 1A). ROC analysis was statistically significant: AUC=0.90 (95% CI: 0.81–0.99), sensitivity was 100% (95% CI: 0.9–1), specificity was 69% (95% CI: 0.5–0.69), positive predictive value was 86% (95% CI: 0.77–0.86), negative predictive value was 100% (95% CI: 0.73–1) with an optimal threshold of 2.6 mmol/L. The level of antioxidants was significantly higher in the control group, it is shown in Figure 1B. Their content in the capillary blood in the control group was 1.5 (1.03; 2.31) mmol/L and it was 0.97 (0.35;1.195) mmol/L in women who had preterm birth. The area under the ROC curve (AUC) was 0.77 (95% CI: 0.63–0.91) with a sensitivity of 80% (95% CI: 0.68–0.88) and specificity of 75% (95% CI: 0.57–0.9), positive predictive value was 86% (95% CI: 0.73–0.94), negative predictive value was 61% (95% CI: 0.47–0.8) with an optimal threshold of 1.25 mmol/L.
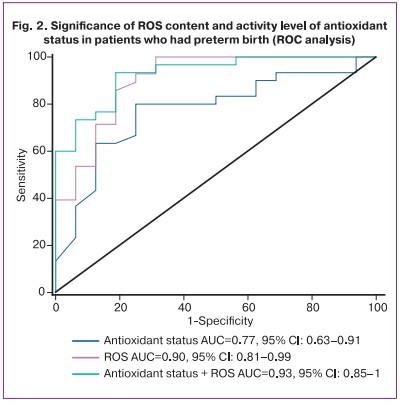
The measurement of both parameters (ROS + antioxidant status) showed the greatest significance: AUC=0.93 (95% CI: 0.85–1), sensitivity was 93% (95% CI: 0.82–0.99), specificity was 81% (95% CI: 0.61–0.91), positive predictive value was 90% (95% CI: 0.8–0.95), negative predictive value was 87% (95% CI: 0.65–0.97) with an optimal threshold of 0.55 (Fig. 2).
In the study of M. Grzesiak et al. [13], the level of oxidative stress in the peripheral blood of pregnant women was determined using a colorimetric method based on iron-mediated reactions of apparently stable products of the lipid peroxidation process (hydroperoxides); the level of the cation radical was determined as an antioxidant. According to the results, the level of oxidative stress in patients who had preterm birth was significantly higher than in women with normal pregnancy.
H.E. Soydinc et al. [15] determined the level of total oxidative status and total antioxidant capacity in vaginal flora; they came to the conclusion that the so-called oxidative stress index is a predictor of preterm birth.
In the case control study conducted by R. Xu et al. [14], the level of 8-oxo-2'-deoxyguanosine in the venous blood was determined as a marker of oxidative stress. There were no significant differences between the level of 8-oxo-2'-deoxyguanosine in the blood of women with preterm birth compared to women who had a normal pregnancy.
Such contradictory data are probably due to differences in the materials that were studied, methods for determining markers of oxidative stress and gestational age. Also, the level of oxidative stress may vary depending on race, place of living and quality of life.
Our study revealed an increased level of ROS (H2O2) in patients with preterm birth with decreased antioxidant activity. Our results are consistent with the data of other researchers and confirm the role of oxidative stress in the development of preterm birth.
Conclusion
Preterm birth is associated with a systemic inflammatory response syndrome which is known to be accompanied by the development of oxidative stress leading to increased processes of apoptosis and disorders both at the cellular and subcellular levels. It is possible to determine the level of proinflammatory response and predict the development of complications, as well as to evaluate the effectiveness of therapy aimed at prolonging pregnancy by studying the indicators of oxidative stress and antioxidants.
References
- American College of Obstetricians and Gynecologists, Society for Maternal-Fetal Medicine. ACOG Obstetric Care Consensus No. 3: Periviable birth. Obstet. Gynecol. 2015; 126(5): e82-e94. https://dx.doi.org/10.1097/AOG.0000000000001105.
-
Di Renzo G.C., Tosto V., Giardina I. The biological basis and prevention of preterm birth. Best Pract. Res. Clin. Obstet. Gynaecol. 2018; 52: 13-22.
https://dx.doi.org/10.1016/j.bpobgyn.2018.01.022.
-
Belousova V.S., Strizhakov A.N., Svitich O.A., Timokhina E.V., Kukina P.I., Bogomazova I.M., Pitskhelauri E.G. Premature birth: causes, pathogenesis, tactics. Akusherstvo i ginekologiya/Obstetrics and Gynecology. 2020; 2: 82-7. (in Russian).
https://dx.doi.org/10.18565/aig.2020.2.82-87.
- Yellon S.M., Oshiro B.T., Chhaya T.Y., Lechuga T.J., Dias R.M., Burns A.E., Force L., Apostolakis E.M. Remodeling of the cervix and parturition in mice lacking the progesterone receptor B isoform. Reprod. 2011; 85(3): 498-502. 10.1095/biolreprod.111.091983.
- Kurchakova T.A., Tyutyunnik V.L., Kan N.E., Medzhidova M.K., Sirotkina E.A. Pro- and antioxidant system for preterm labor. Akusherstvo i ginekologiya/Obstetrics and Gynecology. 2016; 5: 20-24. (in Russian). https://dx.doi.org/10.18565/aig.2016.5.20-24.
- Fogel I., Pinchuk I., Kupferminc M.J., Lichtenberg D., Fainaru O. Oxidative stress in the fetal circulation does not depend on mode of delivery. Am. J. Obstet. Gynecol. 2005; 193(1): 241-6. https://dx.doi.org/10.1016/j.ajog.2004.10.637.
- Sultana Z., Maiti K., Aitken J., Morris J., Dedman L., Smith R. Oxidative stress, placental ageing-related pathologies and adverse pregnancy outcomes. Am. J. Reprod. Immunol. 2017; 77(5). https://dx.doi.org/10.1111/aji.12653.
-
Dutta E.H., Behnia F., Boldogh I., Saade G.R., Taylor B.D., Kacerovský M.,
Menon R.
Oxidative stress damage-associated molecular signaling pathways differentiate spontaneous preterm birth and preterm premature rupture of the membranes. Mol. Hum. Reprod. 2016; 22(2): 143-57.https://dx.doi.org/10.1093/molehr/gav074.
- Radzinsky V.E., Orazmuradov A.A., Savenkova I.V., Damirova K.F., Haddad H. Preterm labor – an unsolved problem of the XXI century. Kuban Scientific Medical Bulletin. 2020; 27(4): 27-37. (in Russian).
-
Mert I., Oruc A.S., Yuksel S., Cakar E.S., Buyukkagnici U., Karaer A.,
Danisman N
. Role of oxidative stress in preeclampsia and intrauterine growth restriction. J. Obstet. Gynaecol. Res. 2012; 38(4): 658-64.https://dx.doi.org/10.1111/j.1447-0756.2011.01771.x.
-
Chiba T., Omori A., Takahashi K., Tanaka K., Kudo K., Manabe M. et al.
Correlations between the detection of stress-associated hormone/oxidative stress markers in umbilical cord blood and the physical condition of the mother and neonate. J. Obstet. Gynaecol. Res. 2010; 36(5): 958-64.
https://dx.doi.org/10.1111/j.1447-0756.2010.01292.x.
- Simon-Szabo Z., Fogarasi E., Nemes-Nagy E., Denes L., Croitoru M., Szabo B. Oxidative stress and peripartum outcomes (Review). Exp. Ther. Med. 2021; 22(1): 771. https://dx.doi.org/10.3892/etm.2021.10203.
-
Grzesiak M., Gaj Z., Kocyłowski R., Suliburska J., Oszukowski P., Horzelski W. et al. Oxidative stress in women treated with atosiban for impending preterm birth. Oxid. Med. Cell. Longev. 2018; 2018: 3919106.
https://dx.doi.org/10.1155/2018/3919106.
-
Xu R., Meng X., Pang Y., An H., Wang B., Zhang L. et al. Associations of maternal exposure to 41 metals/metalloids during early pregnancy with the risk of spontaneous preterm birth: Does oxidative stress or DNA methylation play a crucial role? Int. 2021; 158: 106966. https://dx.doi.org/10.1016/
j.envint.2021.106966.
- Soydinc H.E., Sak M.E., Evliyaoglu O., Evsen M.S., Turgut A., Özler A. et al. Prolidase, matrix metalloproteinases 1 and 13 activity, oxidative-antioxidative status as a marker of preterm premature rupture of membranes and chorioamnionitis in maternal vaginal washing fluids. J. Med. Sci. 2013; 10(10): 1344-51. https://dx.doi.org/10.7150/ijms.4802.
Received 22.11.2021
Accepted 10.12.2021
About the Authors
Natalia E. Kan, Professor, MD, PhD, Deputy Director for Science, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, +7(926)220-86-55, kan-med@mail.ru, Researcher ID: B-2370-2015, SPIN-код: 5378-8437, Authors ID: 624900, Scopus Author ID: 57008835600, https://orcid.org/0000-0001-5087-5946, 117997, Russia, Moscow, Ac. Oparina str., 4.Zalina Kh. Salpagarova, postgraduate student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology,
Ministry of Health of the Russian Federation, +7(925)853-96-22, z.salpagarova1990@yandex.ru, 117997, Russia, Moscow, Ac. Oparina str. 4.
Victor L. Tyutyunnik, Professor, MD, PhD; Leading Researcher of Research and Development Service, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, +7(903)969-50-41, tioutiounnik@mail.ru, Researcher ID: B-2364-2015,
SPIN-код: 1963-1359, Authors ID: 213217, Scopus Author ID: 56190621500, https://orcid.org/0000-0002-5830-5099, 117997, Russia, Moscow, Ac. Oparina str., 4.
Valeriya S. Shchipitsyna, Junior Researcher of the Cytology Laboratory, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, +7(495)438-22-72, v_shchipitsyna@oparina4.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Aleksey M. Krasnyi, PhD, Head of the Cytology Laboratory, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, +7(495)438-22-72, alexred@list.ru, 117997, Russia, Moscow, Ac. Oparina str., 4.
Authors’ contributions: Kan N.E., Salpagarova Z.Kh., Tyutyunnik V.L., Shchipitsyna V.S., Krasnyi A.M. - developing the design of the study, obtaining the data for analysis, review of the literature on the subject of the article, statistical analysis of the obtained data, article writing.
Conflicts of interest: The authors declare that they have no competing interests.
Funding: The work was supported by the Grants of the President of the Russian Federation for state support of leading scientific schools of the Russian Federation No. NSh-4566.2018.7.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Kan N.E., Salpagarova Z.Kh., Tyutyunnik V.L., Shchipitsyna V.S., Krasnyi A.M. Prooxidant and antioxidant content in capillary blood in preterm birth. Akusherstvo i Ginekologiya/ Obstetrics and Gynecology. 2022; 1:56-61 (in Russian) https://dx.doi.org/10.18565/aig.2022.1.56-61



