Pregestational neural network prediction of fetal growth restriction or small-for-gestational-age fetus with subsequent intensive care of the newborn
Ziyadinov A.A., Novikova V.A., Radzinsky V.E.
Objective: To create and test a prototype of a neural network tool for pregestational stratification of high-risk women according to the delivery of a fetus with growth restriction (FGR) or small for gestational age (SGA) and the need for intensive care of the newborn.
Materials and methods: This was a prospective cohort study which was conducted at the Perinatal Centre of the Republic of Crimea, N.A. Semashko Republican Clinical Hospital from 2018 to 2020. The study included 611 women with singleton pregnancies complicated by FGR (n=435) and SGA (n=176). The prognosis was performed using a personal computer: Statistica 12.0 software, Automated Neural Networks module.
Results: The use of automated neural network model analysis provided prototype tools for pregestational stratification of women at risk for FGR or SGA (model 1); the need for neonatal intensive care (model 2), including respiratory support (model 3). This neural network prediction effectively (accuracy of training, testing and validation of neural networks up to 100%) provides maternal clinical and anamnestic and socio-demographic parameters (place and permanence of residence, education, occupation, marital status; age, including paternal), height-weight, characteristics of reproductive function, reproductive experience, fetal weight in previous deliveries, gravidity, pre-eclampsia in previous pregnancy, the history of previous delivery and its mode).
Conclusion: The obtained neural network models demonstrate the possibility of developing tools that provide predictive clinical and management analytics. The obtained neural network models demonstrate the possibility of developing tools that provide predictive clinical and management analytics. These tools can be used by clinicians in daily practice and help them choose optimal pregnancy management, screening and diagnosis of disorders, and timely routing of pregnant women at the institutions of the appropriate level.
Authors’ contributions: Ziyadinov A.A. – developing the concept and design of the study, data analysis, writing and editing the text, approval of the manuscript for publication; Novikova V.A. – developing the concept and design of the study, statistical processing of the data and interpretation of the results; Radzinsky V.E. – developing the concept and design of the study, editing the text, approval of the manuscript for publication.
Conflicts of interest: Authors declare lack of the possible conflicts of interest.
Funding: The study was conducted without sponsorship.
Ethical Approval: The study was approved by the Ethical Review Board of the V.I. Vernadsky Crimean Federal University.
Patient Consent for Publication: The patients provided an informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Ziyadinov A.A., Novikova V.A., Radzinsky V.E. Pregestational neural network prediction of fetal growth restriction or small-for-gestational-age fetus with subsequent intensive care of the newborn.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (10): 60-73 (in Russian)
https://dx.doi.org/10.18565/aig.2024.124
Keywords
One of the tasks of obstetric care services worldwide is to provide medical care to the mother for confirmed or suspected fetal pathology (ICD-10 code O36). Special attention should be paid to poor fetal growth (ICD code O36.5), which has two variants: fetal growth restriction (FGR) and small for gestational age (SGA); they are partly similar in risk factors but fundamentally different in pathogenesis [1]. The negative influence of poor fetal growth is not limited to the intrauterine period [2, 3]. Poor fetal growth threatens vulnerable newborns; it may cause infant morbidity, mortality, and health hazards in childhood, adolescence, and throughout later life (it may affect neurological and cognitive function, puberty and fertility, thyroid function, kidney function, bone mineral density accumulation, etc.; it may lead to the long-term cardiometabolic disorders: metabolic syndrome in adulthood, obesity, hypertension, diabetes, and ischemic heart disease) [3, 5]. In this aspect, the need for prevention of poor fetal growth is undeniable; it requires knowledge of its etiology, both fetal, placental, genetic, and parental (maternal and paternal) nature [1, 2]. In addition to screening, prediction of poor fetal growth has become increasingly important, taking into account its variants FGR and SGA. There is particular caution about FGR [1]. The following predictors of FGR are considered to be informative, but not always available: trophoblast-associated markers (human beta-chorionic gonadotropin (β-hCG), pregnancy-associated plasma protein (PAPP-A), placental growth factor (PlGF), placental protein 13 (PP-13)), DNA profiling data [6], Dopplerometry (pulse index and resistance index of the umbilical artery, middle cerebral artery, venous duct), 3D (three-dimensional) magnetic resonance imaging (features of the shape and texture of the placenta) [6, 8], systemic immune inflammation index (neutrophils × thrombocytes/lymphocytes) [9], etc. Of practical importance in predicting poor fetal growth is the evaluation of maternal clinical, social and economic data as well as the adaptation of prognostic models for widespread use [8]. Obtaining these data is not expensive and widely available. According to the available data, independent maternal risk factors for FGR include younger age (RR=0.9, CI: 0.8–0.9), inadequate weight gain during pregnancy (RR=3.0, CI: 1.6–6.1), history of abortion (RR=3.06, CI: 1.1–8.0), pregnancy-induced hypertension, especially in primigravidas (RR=10.1, CI: 1.0–23.2) [10]; African, East Asian, South Asian and mixed racial origin; maternal comorbidity including chronic arterial hypertension (CAH), diabetes mellitus (DM) and systemic lupus erythematosus and/or antiphospholipid syndrome; reproductive factors, such as in vitro fertilization; smoking; history of pre-eclampsia (PE) or stillbirth, interval between pregnancies less than 0.5 years [8]. According to the meta-analysis carried out in 2023, there is an association between FGR and seven maternal factors, most of which are manageable and potentially preventable: smoking (OR=1.62, 95% CI: 1.38–1.90), first birth (OR=1.64, 95% CI: 1.20–2.24) and pregestational body mass index (BMI) <18.5 kg/m2 (OR=1.98, 95% CI: 1.29–3.03), anemia (OR=2.01, 95% CI: 1.44–2.82), hypoproteinemia (OR=2.91, 95% CI: 1.94–4.36), pregnancy-induced hypertension (OR=3.45, 95% CI: 1.80–6.58) and inadequate weight gain in women during pregnancy (OR=2.51, 95% CI: 1.88–3.35) [11]. The public availability of predictors enables the prediction of FGR on a nationwide scale using data from social insurance bases, including parity, maternal age, insurance class, marital status, and occupation (profession) [12]. The application of machine learning and artificial intelligence (AI), including deep neural networks has enhanced the accuracy of the prediction of poor fetal growth [13–15]. This is very important because timely prediction of FGR allows clinicians to refer women carrying fetuses with poor fetal growth to the medical institution of the appropriate level and influence the birth outcome for the neonate. According to a systematic review and a meta-analysis conducted in 2023, AI and machine learning have the potential to optimise accurate, cost-effective screening of FGR and pregnancy outcomes in general, but the implementation of algorithms into routine clinical practice requires quality assessment of uniform diagnostic criteria [7]. The use of sociodemographic, clinical and anamnestic maternal characteristics has led to the development of new approaches to the prediction of FGR, which is a variant of poor fetal growth. Poor living conditions, low literacy and general lack of knowledge, low socioeconomic level are known to increase the risk of FGR [16].
However, the following factors must be considered when discussing the risks of poor fetal growth for the fetus/newborn. It is important to note that ante- and intra-natal abnormalities are the primary determinant of the severity of the condition of the growth-deficient fetus/newborn [4]. FGR carries the risk of severe conditions up to fetal death, and can cause complications such as meconium aspiration syndrome, asphyxia, respiratory distress syndrome, hypoglycemia, hypothermia, bronchopulmonary dysplasia, and intrapartum thrombosis. Unfortunately, there is a lack of data on whether pregestational maternal factors influence the birth outcome in cases of FGR or SGA (the need for intensive care, including respiratory support, which is crucial for the choice of measures for screening and prevention of poor fetal growth). This gap in knowledge was the rationale for the present study.
The objective of the study was to develop and test a prototype of a neural network tool for pregestational stratification of high-risk women according to the delivery of a fetus with growth restriction (FGR) or small for gestational age (SGA) and the need for intensive care of the newborn.
Materials and methods
This was a prospective cohort study which was conducted at the Perinatal Centre of the Republic of Crimea, N.A. Semashko Republican Clinical Hospital from 2018 to 2020. The study included 611 women with singleton pregnancies complicated by FGR (n=435) and SGA (n=176). The criteria for FGR and SGA met the current national clinical guidelines [1].
The data from a sample of pregnant women with poor fetal growth obtained from the general cohort of patients (n=24282) delivered during the period were used to build a prediction model for FGR and SGA. The sample was formed on the basis of the following inclusion criteria: women with singleton pregnancies complicated by poor fetal growth (FGR or SGA); confirmation of poor fetal growth in the postnatal period; one dominant cause of poor fetal growth; cephalic presentation and live birth. The exclusion criteria are multiple pregnancies, difference in antenatal and postnatal diagnosis of poor fetal growth, two or more competing causes of poor fetal growth.
The predictive models were validated in a sample of women at high risk of poor fetal growth who received preconception counselling and whose subsequent pregnancies were complicated by poor fetal growth (n=50).
Statistical analysis
Statistical processing of the data, construction and development of predictive models, evaluation of their performance were carried out using Statistica 12.0 software package (Automated Neural Networks module) and Microsoft Excel 2007.
The obtained data were mathematically processed with subsequent selection of the optimal set of indicators that were more likely to be characteristic of FGR or SGA.
Qualitative variables were presented as absolute and relative values (%), and were compared with each other using the chi-square test (χ2).
The correlation of study factor with outcome/group was assessed using odds ratio (OR) with 95% confidence interval (CI). In the presence of two factor attributes, the relationship with the outcome attribute was assessed based on the analysis of four-way contingency tables using the χ2 criterion; in the presence of three or more factor attributes, multi-way tables were used.
The outcome categories were modelled using neural networks. The training, testing and validation of automated neural networks with an optimal number of characteristics were conducted, after which the networks were used to classify women into the desired groups. The selection of neural networks was based on the inclusion of all available parameters, followed by the identification of the smallest number of input characteristics with the highest classification quality. The sensitivity analysis of the Statistica 12.0 software was used to assess the importance (sensitivity) of a parameter. Due to the lack of automated selection, characteristics with an importance coefficient of at least 1 and/or maximizing the quality of the classification were selected gradually. The performance of the obtained models was assessed on the basis of the confusion matrix, which represents the proportion of correctly classified objects in the training and test samples.
The informative value of prognostic models was based on the estimation of sensitivity (Se) and specificity (Sp) according to the formulae: Se=true positives/(true positives+false negatives); Sp=true negatives/(true negatives+false positives).
Results and Discussion
A total of three neural network models for the stratification of pregnant women were created on the basis of informative pregestational maternal clinical and anamnestic, as well as socio-demographic parameters:
1) poor fetal growth variant: FGR or SGA (model 1);
2) need for intensive care (IC) of the newborn: yes/no (model 2);
3) need for respiratory support (RS) during neonatal IC: yes/no (model 3).
Two types (quantitative and categorical) of independent variables (predictors) were selected in the model construction process. They were the input data of the obtained neural networks in different combinations (Table 1).
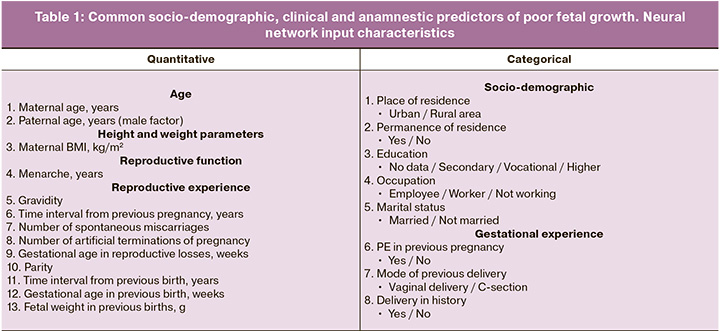
Table 1 shows that quantitative predictors predominantly reflected reproductive experience. Categorical variables were not necessarily binary, some had more than two options. Five of the eight categorical predictors were related to socio-demographic characteristics (place of residence, permanence of residence, education, occupation, marital status); reproductive predictors (history: delivery and its mode, PE) were in the minority.
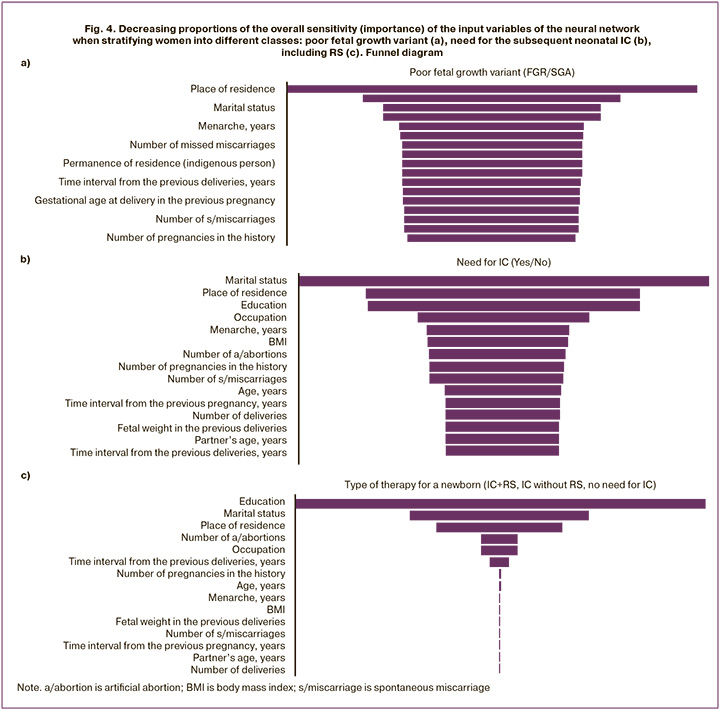
The poor fetal growth variant (model 1) predicted 20 parameters (Table 3, block A) with 100% accuracy of training, testing and validation of the five obtained neural networks (Table 2, block A). Place of residence was of primary sensitivity. Marital status and education ranked third and fourth, second only to the mode of previous delivery. Menarche, number of artificial terminations of pregnancy, number of missed miscarriages, PE in previous pregnancies and other reproductive factors were of even lower importance. Paternal age as an input (predictor) which is most often neglected in the risk assessment of poor fetal growth and its variant is noteworthy. It should be emphasized that such parameters as time interval from last delivery or pregnancy, gestational age in previous delivery, fetal weight in previous deliveries, number of pregnancies in history, number of spontaneous miscarriages and deliveries, maternal age and BMI, partner’s age had a sensitivity of less than one, but they were retained in the predictive model. Removing each of them from the neural network model significantly decreased its accuracy.
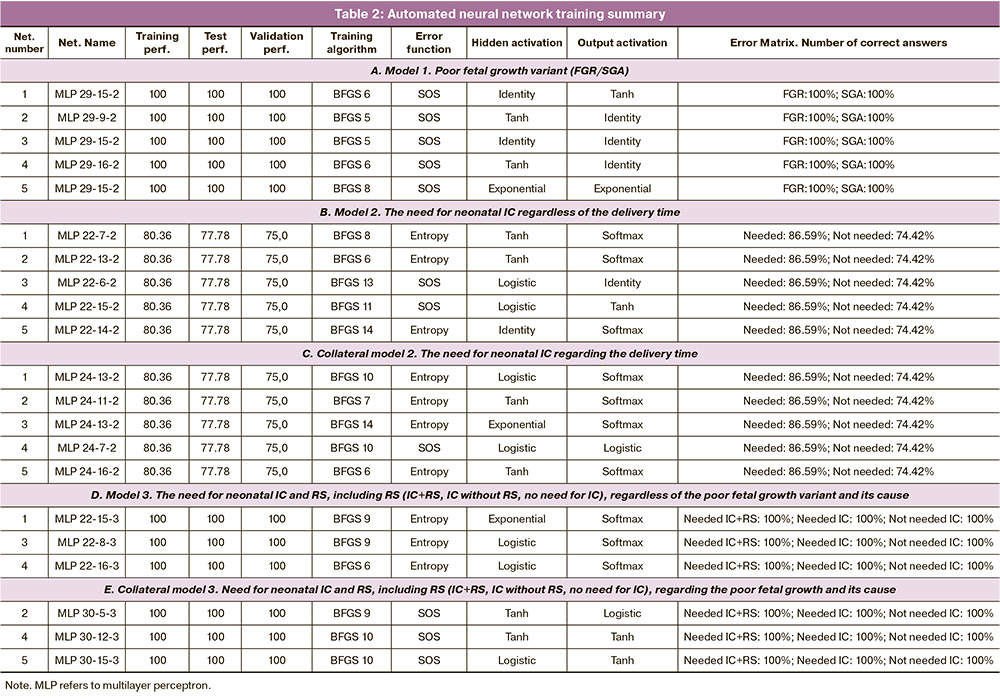
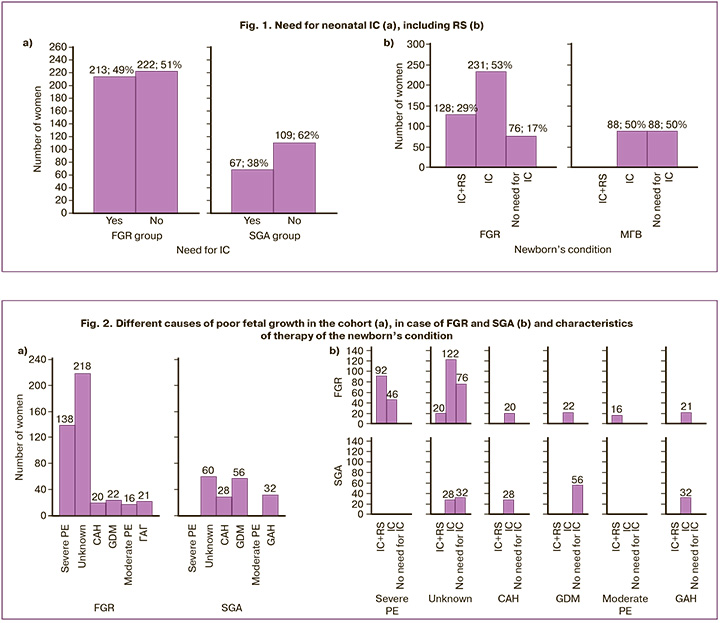
There was a successful attempt to relate the poor fetal growth variant to pregnancy and neonatal outcome, specifically the need for IC (model 2). FGR was found to be more characterized by the need for IC (213/435 vs 67/176, χ2=101.42, p<0.001) (Fig. 1a) and to be exclusive for RS (128/435 vs 0/176, χ2=63.75, p<0.001) (Fig. 1b).
The five neural networks which were obtained (Table 2, block B) showed high training accuracy (80.36%), slightly lower accuracy in testing (77.78%) and validation (75%). The scoring matrix of correct answers showed a higher proportion of neonates requiring IC (86.59%) compared to those who did not need IC (74.42%). The association of FGR or SGA with neonatal IC need was determined by 15 maternal predictors, the first four of which were socio-demographic factors, namely marital status, place of residence, education and occupation (Table 3, block B), followed by menarche and BMI. Reproductive factors ranked seventh and lower. The age of the partner was identified as a more significant factor in the model than the time interval from the last delivery.
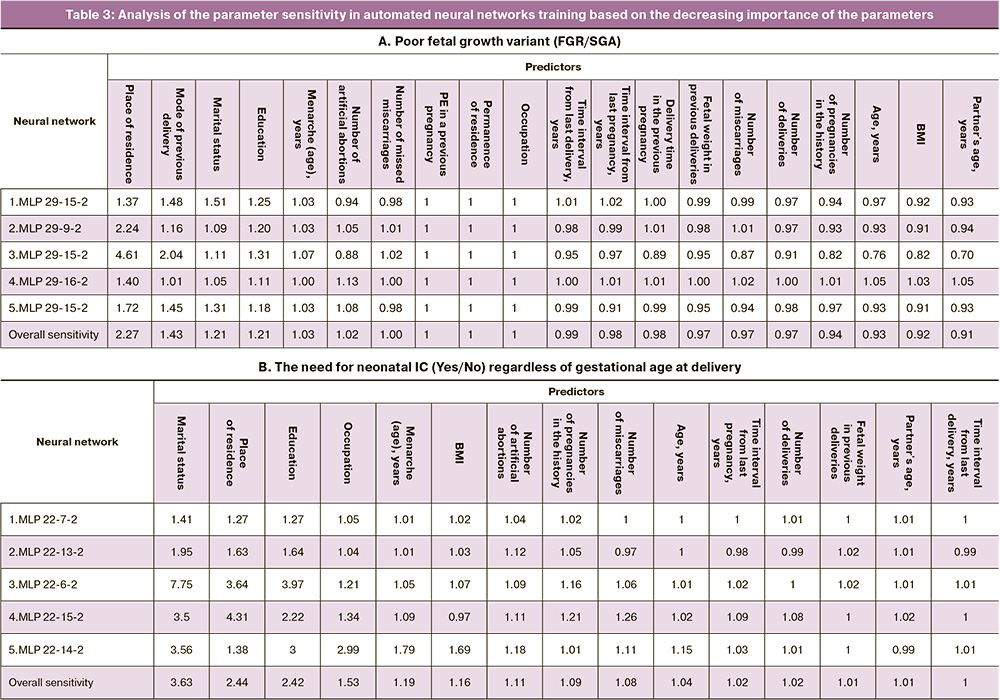
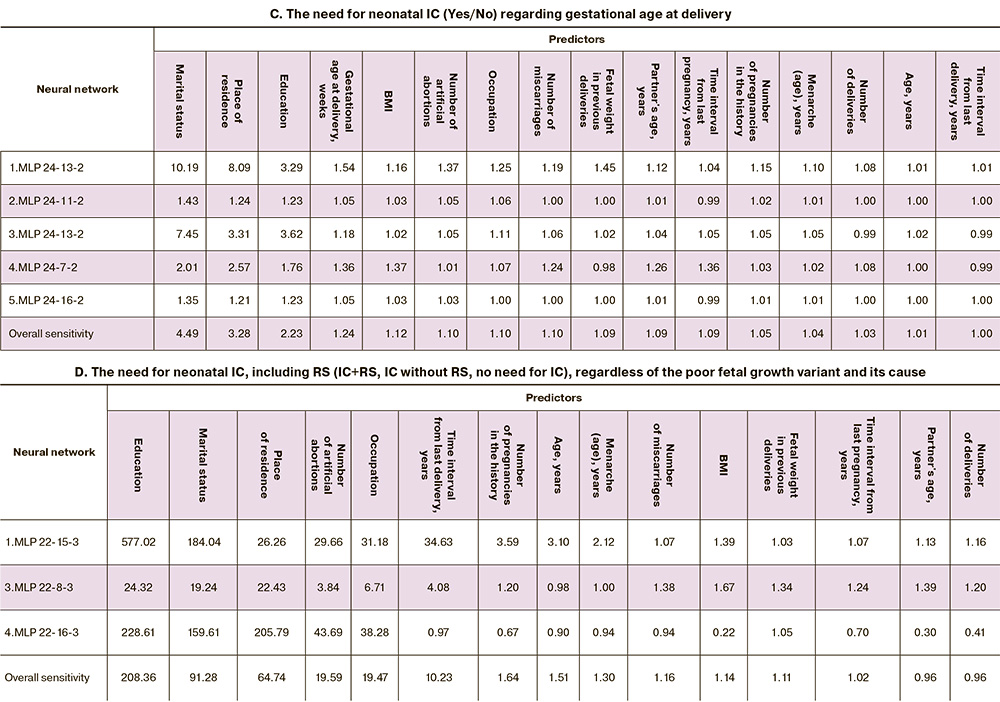
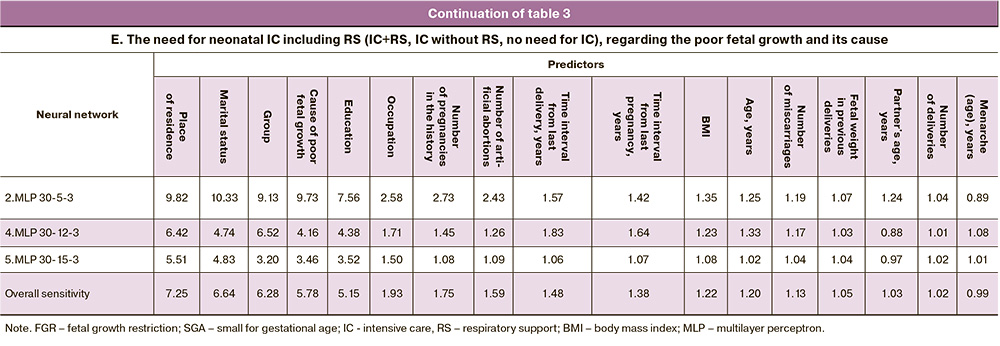
In developing the neural network model, our objective was to identify pregestational predictors. Consequently, we deliberately excluded the delivery time ignoring the potential risks of iatrogenic prematurity. However, understanding that the need for IC of the neonates born to women whose pregnancies were complicated by FGR or SGA is primarily determined by gestational age, we deviated from the main objective and included this parameter in the neural network model. Surprisingly, this did not change the accuracy of the newly obtained five neural network models (Table 2, block C); the socio-demographic predictors (input parameters), namely marital status, place of residence and education (Table 3, block C), remained the most important ones in predicting the need for neonatal IC. Gestational age at delivery was not of primary importance, but ranked fourth, leaving 12 parameters behind.
We found a non-linear relationship between maternal pregestational risk factors and the need for respiratory support (RS) during IC (model 3). Three neural networks were obtained with a training, testing and validation accuracy of 100% (Table 2, block D), where the highest sensitivity was again demonstrated by the socio-demographic predictors, namely education, marital status and place of residence (Table 3, block D). Occupation also showed high sensitivity (19.47), second to the only reproductive factor, that is number of artificial abortions (19.59). Time interval from last delivery showed significant importance, 10 times higher than that for all remaining parameters.
There was a natural collateral interest in the potential association of the type of neonatal therapy with not only the poor fetal growth variant but also its cause. A total of six major causes of poor fetal growth were identified in the study cohort; five of them were related to cardiometabolic complications of pregnancy, namely hypertensive conditions [chronic (CAH; n=48; 7.86%) and gestational (GAH; n=53; 8.67%) arterial hypertension, PE (moderate (n=16; 2.62%) and severe (n=138; 22.59%)] and gestational DM (GDM; n=78; 12.77%). It should be noted that the cause of poor fetal growth was not clear in 45.5% (n=278) of the women. The cause of poor fetal growth was found to be correlated with poor fetal growth variant, namely FGR or SGA (χ2=185.84, p<0.001), the need for neonatal IC (χ2=62.97, p<0.001) (Fig. 2a), including RS (χ2=460.84, p<0.001) (Fig. 2b).
Creating a neural network model to predict the characteristics of neonatal therapy with additional input parameters for the FGR or SGA group and the cause of poor fetal growth, three neural networks with 100% training, testing and validation accuracy were obtained (Table 2, block E). Both entered parameters had high sensitivity (it was 6.28 for the group, and 5.78 for the cause of poor fetal growth), but ranked third and fourth in terms of significance (Table 3, block E). Two socio-demographic factors, namely place of residence and marital status, again showed primary importance. Two more socio-demographic factors, education (sensitivity=5.15) and occupation (sensitivity=1.93), were next on the list, and they had higher importance than the remaining 11 parameters.
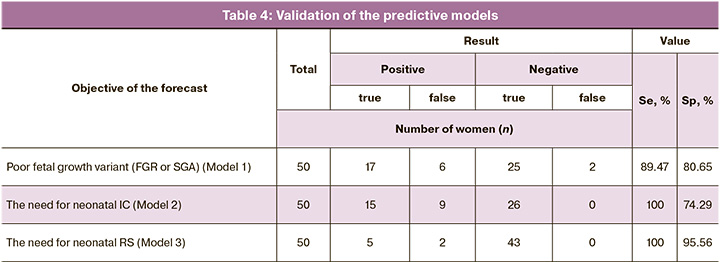
Thus, pregestational maternal socio-demographic as well as clinical and anamnestic characteristics were informative predictors of poor fetal growth variant (FGR or SGA) in subsequent pregnancy, the need for neonatal IC, including RS. The obtained neural network algorithms made it possible to stratify women into the desired classes when random or real data were substituted (Fig. 3a-c).
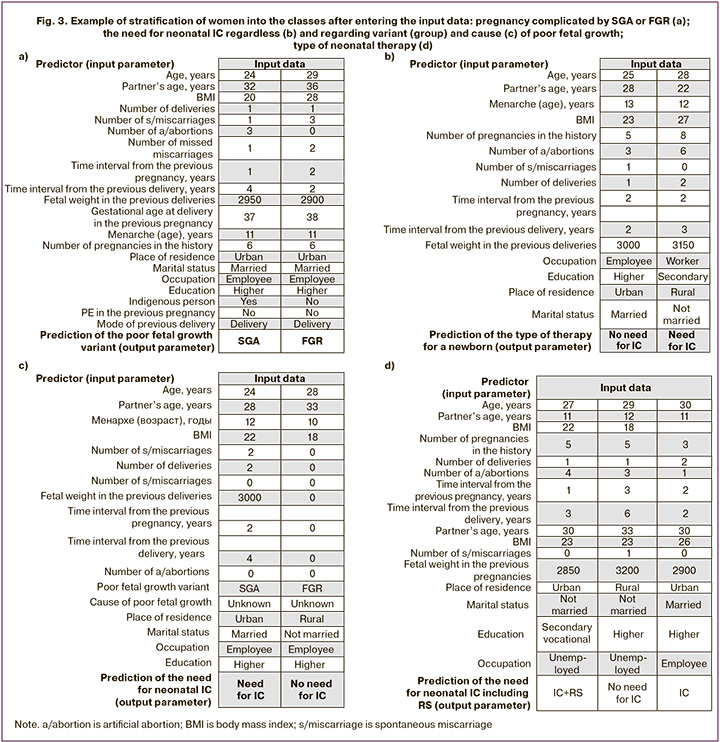
Figure 3a shows that the prediction of FGR, but not SGA, is determined by such parameters as non-indigenous ethnicity, higher BMI, and a history of reproductive loss, though the socio-demographic characteristics are almost identical. The need for neonatal IC (Fig. 3b) is partly determined by occupation, namely being a worker, rural residence, secondary education, and unmarried status. When pregnancy was complicated by SGA but not by FGR and the cause of poor fetal growth was unknown, the need for neonatal IT was associated with the woman’s urban residence, married status, reproductive experience, and younger age of both parents of the newborn (Fig. 3c). The need for RS is associated with the woman’s urban residence, vocational education, unmarried status, lack of employment, and newborn’s birth weight less than 2850g (Fig. 3d).
We assessed the informative value of the proposed models in women (n=50) who received preconception counselling; later they became pregnant and pregnancies were complicated by poor fetal growth. The validation of the predictive models in practice demonstrated their efficacy, as evidenced by the results presented in Table 4. These findings support the recommendation for further practical application.
Discussion
Poor fetal growth is a problem that lies at the crossroads of two disciplines. Neonatologists are the primary providers of neonatal care for severe fetal and neonatal outcomes, especially FGR. But prognosis, screening and differential diagnosis of FGR or SGA, choice of pregnancy management tactics, determining the time and mode of delivery are the exclusively the tasks for obstetricians and gynecologists. Early prediction of poor fetal growth is fundamental because it gives time to perform preventive measures starting with preconception counselling. The prognosis or early differential diagnosis of FGR means that the pregnant woman can be timely referred to a proper hospital with the necessary equipment for accurate diagnosis of the functional state of the uterine-fetal-placental system and high-tech neonatology service in case of early or emergency delivery or severe fetal/newborn condition.
It is now beyond question that modern medicine is profoundly dependent on the use of AI, which enables the adaptation of patient management and treatment tactics to high-probability risk factors (17–19). The present study continued a series of existing developments in obstetrics, gynecology, perinatology and reproductive science to improve methods for assessing fertility, the need for assisted reproductive technologies, the risk of gestational complications (PE, arterial hypertension, DM), miscarriage or prematurity, and the formation of fetal malformations [20–23]. The importance of assessing potential gestational risks prior to pregnancy is supported by studies conducted by reproductive medicine specialists. Machine learning helped to reconsider the risks of habitual miscarriage and to develop models for its prediction at the pregestational stage, taking into account various etiological factors and their combinations [24]. Special attention is paid to such risk factors as medical and family history, age, stress, lifestyle, smoking, obesity, etc.; their absence does not exclude the disease, but their combined presence significantly increases its risk.
It should be added that today there are five main types of Analytics: Descriptive, Diagnostic, Predictive, Prescriptive and Discovery; moreover, there are three levels of effectiveness and engagement (operational, tactical and strategic), each of them making a distinctive contribution to improving health care [25]. Predictive analytics based on retrospective data and construction of predictive models makes it possible to predetermine the most probable outcome of the event in question (belonging to a particular group, etc.). The present study confirmed that. It turned out that the combination of maternal clinical and anamnestic characteristics as well as socio-demographic parameters (place and permanence of residence, education, occupation, marital status; age, including paternal data), height and weight indicators, characteristics of reproductive function, reproductive and gestational experience, history of complicated pregnancy by PE, delivery and its mode, fetal weight in previous deliveries) predetermine not only the variant of poor fetal growth (Fig. 4a), but also the condition of the newborn, its need for IC (Fig. 4b), including RS (Fig. 4c).
The socio-economic parameters of fetal developmental delay have been known for decades. The focus has been on exposure factors from a public health perspective, with a significant etiological share (attributable risk to the population) [26]. FGR is characterized by large socio-economic disparities.
It is surprising that, even in the context of significant technological advances, certain risk factors associated with FGR, such as female illiteracy, BMI<18.5 kg/m2, family size ≥7, remain prevalent. However, there is growing evidence that enhanced women’s education, better nutrition before and during pregnancy, and regular antenatal care can improve gestational outcomes [27]. Risk factors for FGR include maternal race, lower socio-economic status and living in a developing country, which tend to be strongly associated with poor nutrition, anemia, prenatal care and use of recreational drugs [28]. Short interval between pregnancies is associated with FGR. It is also recognized as an independent risk factor for FGR. Smoking during pregnancy is associated with increased SGA risk, maternal heroin and cocaine addiction is associated with increased neonatal morbidity; fetal alcohol syndrome is usually associated with FGR.
The results of the present study are consistent with the available evidence, but in part. Among the women in the study cohort, smoking was not found to be prognostically significant in the models developed, and alcoholism and drug use were not present at all. Anemia is not shown to be an independent cause of poor fetal growth either. This finding only reflects local characteristics of women and does not reduce their overall risk, as we have previously pointed out [8, 11, 28].
The place of residence and marital status of the woman were found to be two fundamental parameters determining the risk of FGR, which were strongly associated with the outcome of pregnancy and fetal delivery. It is worth noting that not only the place of residence but also the place of birth played an important role in the predictive models.
There were no women in the cohort who could not read or write, but the level of education proved to be a significant factor. In our case study (Figure 4b), higher education was one of the key factors that distinguished women whose pregnancy was complicated by poor fetal growth and whose newborns did not require IC. Predictors of poor fetal growth and the type of therapy for neonates were occupation, marital status, maternal and paternal age, maternal height and weight parameters; all of them are modifiable factors. In the context of the presented data, monitoring of the reproductive function and reproductive experience is also a partial measure to prevent poor fetal growth and severe condition of the newborn. The inclusion of parameters related to previous pregnancies (fetal weight at delivery, gestational experience, PE in the previous pregnancy, history and mode of delivery) in the diagnostic models suggests the existence of a probable personal gestational ‘stereotype’ among women or the acquisition of risks in the previous pregnancy that manifest in the subsequent pregnancies.
In order to emphasize the ‘autonomous’ importance of pregestational factors in the risk of poor fetal growth (FGR or SGA) and the need for neonatal IC, including RS, we deliberately did not include risk factors that developed during pregnancy in the prognostic models. The introduction of parameters such as delivery time as input data in predicting the need for neonatal IC not only reinforced the significance of socio-demographic data, but also validated their primacy: marital status, place of residence and education proved to be primary, followed by gestational age at delivery. The main predictors in prognosing the need for neonatal IC, including RS, taking into account the variant of poor fetal growth (FGR or SGA) and its cause, were place of residence and marital status. Clinical and anamnestic factors (age, including paternal one), height and weight parameters, characteristics of reproductive function, reproductive experience, fetal weight in previous deliveries, gestational experience, PE in previous pregnancy, the history of previous delivery and its mode had lower sensitivity, but together they determined the accuracy of training, testing and validation of neural networks up to 100%. This is where AI has an advantage over other data analysis methods in the prediction of group outcomes/classification. AI enables us to generalize numerous examples and identify a common functional dependence that determines the prognosis for a patient [29]. This is achieved through the process of learning.
The tool we have obtained for stratification of high-risk women for delivery whose pregnancy is complicated by poor fetal growth followed by the need for neonatal IC, including RS, is the key to timely diagnosis of these disorders, prevention, and determining the tactics of pregnancy management and delivery in medical institutions of the appropriate level. It is predictive analytics that helps managers at various levels to justify their decision-making. It can be used to assess possible scenarios for the development of diseases, as well as the burden on medical organizations and the need for equipment and medicines. Clinical predictive analytics supports medical decision-making by analyzing patients’ data, monitoring drug therapies, and optimizing patient care pathways [30]. The models we have developed are new, but they are based on the experience of predictive clinical analytics. These models have proven themselves during the COVID-19 pandemic [23], specifically in the form of calculators for assessing a woman’s risk of developing such a complication of gestation as one of the variants of poor fetal growth, and assistance systems for managing pregnant women at high risk for IC, RS of newborns. It is essential that the results of the prediction provide a reasonable warning to a woman about the personal risks of poor fetal growth at the stage of planning pregnancy and during its course. The availability of predictors is particularly important in resource-limited settings.
Conclusion
On the basis of neural network models that are widely used in daily practice, it is possible to identify women at risk of developing a poor fetal growth variant (FGR or SGA) (model 1); AI also helps determine the need for neonatal IC (model 2) and RS (model 3). This neural network prediction is effectively provided by maternal clinical and anamnestic characteristics, socio-demographic data (place and permanence of residence, education, occupation, marital status; age, including paternal one), height and weight parameters, characteristics of the reproductive function, reproductive experience, fetal weight in previous delivery, gestational experience, PE in previous pregnancy, the history of delivery and its mode. The obtained neural network models demonstrate the possibility of developing tools that provide predictive clinical and management analytics. These tools can be used by clinicians in daily practice and help them choose optimal pregnancy management, screening and diagnosis of disorders, and timely routing of pregnant women at the institutions of the appropriate level.
References
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Недостаточный рост плода, требующий предоставления медицинской помощи матери (задержка роста плода). М.; 2020. 71 с. [Ministry of Health of the Russian Federation. Clinical guidelines. Insufficient growth of the fetus, requiring the provision of medical care to the mother (fetal growth retardation). Moscow; 2020. 71 p. (in Russian)].
- Hokken-Koelega A.C.S., van der Steen M., Boguszewski M.C.S., Cianfarani S., Dahlgren J., Horikawa R. et al. International Consensus Guideline on small for gestational age: etiology and management from infancy to early adulthood. Endocr. Rev. 2023; 44(3): 539-65. https://dx.doi.org/10.1210/endrev/bnad002.
- Lawn J.E., Ohuma E.O., Bradley E., Idueta L.S., Hazel E., Okwaraji Y.B. et al.; Lancet Small Vulnerable Newborn Steering Committee; WHO/UNICEF Preterm Birth Estimates Group; National Vulnerable Newborn Measurement Group; Subnational Vulnerable Newborn Measurement Group. Small babies, big risks: global estimates of prevalence and mortality for vulnerable newborns to accelerate change and improve counting. Lancet. 2023; 401(10389): 1707-19. https://dx.doi.org/10.1016/S0140-6736(23)00522-6.
- Nguyen Van S., Lobo Marques J.A., Biala T.A., Li Y. Identification of latent risk clinical attributes for children born under IUGR condition using machine learning techniques. Comput. Methods Programs Biomed. 2021; 200: 105842. https://dx.doi.org/10.1016/j.cmpb.2020.105842.
- Mutamba A.K., He X., Wang T. Therapeutic advances in overcoming intrauterine growth restriction induced metabolic syndrome. Front. Pediatr. 2023; 10: 1040742. https://dx.doi.org/10.3389/fped.2022.1040742.
- Vasilache I.-A., Scripcariu I.-S., Doroftei B., Bernad R.L., Cărăuleanu A., Socolov D. et al. Prediction of intrauterine growth restriction and preeclampsia using machine learning-based algorithms: a prospective study. Diagnostics. 2024; 14(4): 453. https://dx.doi.org/10.3390/diagnostics14040453.
- Rescinito R., Ratti M., Payedimarri A.B., Panella M. Prediction models for intrauterine growth restriction using artificial intelligence and machine learning: a systematic review and meta-analysis. Healthcare (Basel). 2023; 11(11): 1617. https://dx.doi.org/10.3390/healthcare11111617.
- Papastefanou I., Wright D., Nicolaides K.H. Competing-risks model for prediction of small-for-gestational-age neonate from maternal characteristics and medical history. Ultrasound Obstet. Gynecol. 2020; 56(2): 196-205. https://dx.doi.org/10.1002/uog.22129.
- Firatligil F.B., Sucu S.T., Tuncdemir S., Saglam E., Dereli M.L., Ozkan S. et al. Evaluation of systemic immune-inflammation index for predicting late-onset fetal growth restriction. Arch. Gynecol. Obstet. 2024; 310: 433-9. https://dx.doi.org/10.1007/s00404-024-07453-x.
- Mohammad N., Sohaila A., Rabbani U., Ahmed S., Ahmed S., Ali S.R. Maternal predictors of intrauterine growth retardation. J. Coll. Physicians Surg. Pak. 2018; 28(9): 681-5. https://dx.doi.org/10.29271/jcpsp.2018.09.681.
- Yang L., Feng L., Huang L., Li X., Qiu W., Yang K. et al. Maternal factors for intrauterine growth retardation: systematic review and meta-analysis of observational studies. Reprod. Sci. 2023; 30(6): 1737-45. https://dx.doi.org/10.1007/s43032-021-00756-3.
- Sufriyana H., Amani F.Z., Al Hajiri A.Z.Z., Wu Y.W., Su E.C. Prognosticating fetal growth restriction and small for gestational age by medical history. Stud. Health Technol. Inform. 2024; 310: 740-4. https://dx.doi.org/10.3233/SHTI231063.
- Wang Y., Shi Y., Zhang C., Su K., Hu Y., Chen L. et al. Fetal weight estimation based on deep neural network: a retrospective observational study. BMC Pregnancy Childbirth. 2023; 23(1): 560. https://dx.doi.org/10.1186/s12884-023-05819-8.
- Taeidi E., Ranjbar A., Montazeri F., Mehrnoush V., Darsareh F. Machine learning-based approach to predict intrauterine growth restriction. Cureus. 2023; 15(7): e41448. https://dx.doi.org/10.7759/cureus.41448.
- Magboo V.P.C., Magboo M.S.A. Prediction of late intrauterine growth restriction using machine learning models. Procedia Computer Science. 2022; 207(2): 1427-36. https://dx.doi.org/10.1016/j.procs.2022.09.199.
- Dapkekar P., Bhalerao A., Kawathalkar A., Vijay N. Risk factors associated with intrauterine growth restriction: a case-control study. Cureus. 2023; 15(6): e40178. https://dx.doi.org/10.7759/cureus.40178.
- Jhee J.H., Lee S., Park Y., Lee S.E., Kim Y.A., Kang S.W. et al. Prediction model development of late-onset preeclampsia using machine learning-based methods. PLoS One. 2019; 14(8): e0221202. https://dx.doi.org/10.1371/journal.pone.0221202.
- Benner M., Feyaerts D., Lopez-Rincon A., van der Heijden O.W.H., van der Hoorn M.L., Joosten I. et al. A combination of immune cell types identified through ensemble machine learning strategy detects altered profile in recurrent pregnancy loss: a pilot study. F S Sci. 2022; 3(2): 166-73. https://dx.doi.org/10.1016/j.xfss.2022.02.002.
- Hoffman M.K., Ma N., Roberts A. A machine learning algorithm for predicting maternal readmission for hypertensive disorders of pregnancy. Am. J. Obstet. Gynecol. MFM. 2021; 3(1): 100250. https://dx.doi.org/10.1016/j.ajogmf.2020.100250.
- Жуков О.Б. Черных В.Б. Искусственный интеллект в репродуктивной медицине. Андрология и генитальная хирургия. 2022; 23(4): 00-00. [Zhukov O.B., Chernykh V.B. Artificial intelligence in reproductive medicine. Andrology and Genital Surgery 2022; 23(4): 00-00. (in Russian)]. https://dx.doi.org/10.17650/2070-9781-2022-23-4-00-00.
- Ившин А.А., Багаудин Т.З., Гусев А.В. Искусственный интеллект на страже репродуктивного здоровья. Акушерство и гинекология. 2021; 5: 17-24. [Ivshin A.A., Bagaudin T.Z., Gusev A.V. Artificial intelligence on guard of reproductive health. Obstetrics and Gynecology. 2021; (5): 17-24. (in Russian)]. https://dx.doi.org/10.18565/aig.2021.5.17-24.
- Буянова С.Н., Щукина Н.А., Темляков А.Ю., Глебов Т.А. Искусственный интеллект в прогнозировании наступления беременности. Российский вестник акушера-гинеколога. 2023; 23(2): 83‑7. [Buyanova S.N., Schukina N.A., Temlyakov A.Yu., Glebov T.A. Artificial intelligence in pregnancy prediction. Russian Bulletin of Obstetrician-Gynecologist. 2023; 23(2): 83‑7. (in Russian)]. https://dx.doi.org/10.17116/rosakush20232302183.
- Драпкина Ю.С., Макарова Н.П., Васильев Р.А., Амелин В.В., Калинина Е.А. Сравнение прогностических моделей, построенных с помощью разных методов машинного обучения, на примере прогнозирования результатов лечения бесплодия методом вспомогательных репродуктивных технологий. Акушерство и гинекология. 2024; 2: 97-105. [Drapkina Yu.S., Makarova N.P., Vasiliev R.A., Amelin V.V., Kalinina E.A. Comparison of predictive models built with different machine learning techniques using the example of predicting the outcome of assisted reproductive technologies. Obstetrics and Gynecology. 2024; (2): 97-105. (in Russian)]. https://dx.doi.org/10.18565/aig.2023.263.
- Bruno V., D'Orazio M., Ticconi C., Abundo P., Riccio S., Martinelli E. et al. Machine Learning (ML) based-method applied in recurrent pregnancy loss (RPL) patients diagnostic work-up: a potential innovation in common clinical practice. Sci. Rep. 2020; 10(1): 7970. https://dx.doi.org/10.1038/s41598-020-64512-4.
- Khalifa M. Health analytics types, functions and levels: a review of literature. Stud. Health Technol. Inform. 2018; 251: 137-40.
- Kramer M.S. Socioeconomic determinants of intrauterine growth retardation. Eur. J. Clin. Nutr. 1998; 52 Suppl 1: S29-32; discussion S32-3.
- Tesfa D., Tadege M., Digssie A., Abebaw S. Intrauterine growth restriction and its associated factors in South Gondar zone hospitals, Northwest Ethiopia, 2019. Arch. Public Health. 2020; 78: 89. https://dx.doi.org/10.1186/s13690-020-00475-2.
- Suhag A., Berghella V. Intrauterine Growth Restriction (IUGR): etiology and diagnosis. Curr. Obstet. Gynecol. Rep. 2013; 2: 102-11. https://dx.doi.org/10.1007/s13669-013-0041-z.
- Успенский Ю. П., Иванов С. В., Фоминых Ю. А., Наркевич А. Н., Сегаль А.М., Гржибовский А.М. Прогнозирование развития жизнеугрожающих осложнений воспалительных заболеваний кишечника с использованием нейронных сетей: инструменты для практического здравоохранения. Экспериментальная и клиническая гастроэнтерология. 2023; 217(9): 20-33. [Uspenskiy Yu.P., Ivanov S.V., Fominykh Yu.A., Narkevich A.N., Grjibovski A.M., Segal’ A.M. Prediction of life-threatening complications of infl ammatory bowel disease using neural networks: a practical tool for health care professionals. Experimental and Clinical Gastroenterology. 2023; 217(9): 20-33. (in Russian)]. https://dx.doi.org/10.31146/1682-8658-ecg-217-9-20-33.
- Гусев А.В., Новицкий Р.Э. Технологии прогнозной аналитики в борьбе с пандемией COVID-19. Врач и информационные технологии. 2020; 4: 24-33. [Gusev A.V., Novitsky R.E. Predictive analytics technologies in the management of the COVID-19 pandemic. Medical Doctor and IT. 2020; (4): 24-33. (in Russian)]. https://dx.doi.org/10.37690/1811-0193-2020-4-24-33.
Received 22.05.2024
Accepted 22.10.2024
About the Authors
Arsen A. Ziyadinov, PhD, Associate Professor at the Department of Obstetrics, Gynecology and Perinatology No. 1 of S.I. Georgievsky Medical Institute, V.I. Vernadsky Crimean Federal University; Obstetrician-Gynecologist at the Perinatal Center of N.A. Semashko Republican Clinical Hospital, 295017, Russia, Republic of Crimea, Simferopol, Semashko str., 8, ars-en@yandex.ruVladislava A. Novikova, Dr. Med. Sci., Professor of the Department of Obstetrics and Gynecology with the course of Perinatology, Medical Institute of Peoples’ Friendship University of Russia, 117198, Russia, Moscow, Miklukho-Maklaya str., 6, kafedra-aig@mail.ru
Victor E. Radzinsky, Dr. Med. Sci., Professor, Corresponding Member of the RAS, Head of the Department of Obstetrics and Gynecology with the course of Perinatology, Medical Institute of Peoples’ Friendship University of Russia, 117198, Russia, Moscow, Miklukho-Maklaya str., 6, kafedra-aig@mail.ru



