Neonatal complications and characteristics of postnatal development of infants with fetal growth restriction
Leonova A.A., Kan N.E., Tyutyunnik V.L., Serebriakova A.P., Khachaturyan A.A., Pekareva N.A.
Objective: To investigate neonatal complications and characteristics of postnatal development of infants with fetal growth restriction.
Materials and methods: The retrospective study was carried out in two stages at the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia (Kulakov Center). The first stage included analysis of the clinical and anamnestic data and the features of the course of pregnancy in 620 women (300 women with fetal growth restriction (FGR) in pregnancy and 320 women with normal pregnancy), and analysis of neonatal complications in 620 newborns (300 newborns who had fetal growth restriction, 320 newborns without fetal growth restriction). All newborns (n=620) underwent neurosonographic examination after birth, followed by analysis of brain structural changes, as well as analysis of somatic, neurological and ophthalmological complications in the neonatal period. The second stage included analysis of postneonatal complications (from day 29 of postnatal development till the age of 1 year) in 207 infants of the total sample (207/620; 33.4%), who underwent follow-up at the Scientific Pediatric Consultative Department of Kulakov Center after discharge. All infants were divided into 2 groups. The main group included infants who had fetal growth restriction (n=115). The comparison group included infants with normal anthropometric parameters (n=92).
Results: The study found that the frequency of complications in newborns with fetal growth restriction was significantly increased. In the neonatal period, these infants were significantly more likely to have central nervous system depression (p<0.001), hypoxic ischemic encephalopathy (p<0.001), retinopathy of prematurity (p<0.001), and pulmonary hypertension (p<0.001). It should be noted that necrotizing enterocolitis (p<0.001), skin hemorrhagic syndrome (p<0.001) and disseminated intravascular coagulation (DIC) (p<0.001) occurred exclusively in the main group. In the postneonatal period, there was delay in physical and psychomotor development in infants with FGR, as well as the frequency of visual impairments increased by 2 times (p<0.001).
Conclusion: Fetal growth restriction is associated with adverse neonatal and postneonatal outcomes. This highlights the need for prevention and early diagnosis of this pregnancy complication, as well as the comprehensive step-by-step rehabilitation for infants with fetal growth restriction in the postnatal period.
Authors' contributions: Leonova A.A., Kan N.E., Tyutyunnik V.L., Serebriakova A.P., Khachaturyan A.A., Pekareva N.A. – development of study concept and design, data obtaining for analysis, review of publications, material collection and analysis on the topic of the study, statistical analysis of the obtained data, manuscript writing and editing.
Conflicts of interest: The authors confirm that they have no conflicts of interest to declare.
Funding: The study was carried out within the framework of the initiative scientific research topic No. 19-I23 of December 08, 2022 “Epigenetic criteria for the diagnosis of fetal growth restriction from the perspective of impaired neurogenesis” (registration No. 123060500032-8 in the Unified State Information System for Accounting the Results of Research, Development and Technological Work for Civil Purposes (EGISU NIOKTR).
Ethical Approval: The study was approved by the local Ethics Committee of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia.
Patient Consent for Publication: All patients have signed informed consent for publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Leonova A.A., Kan N.E., Tyutyunnik V.L., Serebriakova A.P., Khachaturyan A.A., Pekareva N.A. Neonatal complications and characteristics of postnatal development of infants with fetal growth restriction.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (7): 67-75 (in Russian)
https://dx.doi.org/10.18565/aig.2025.134
Keywords
Fetal growth restriction remains to be one of the most important issues in perinatal medicine. It has a significant impact on child health not only in the neonatal period [1–3], but also at later stages of child development [2, 4, 5]. Numerous studies demonstrate that implications of fetal growth restriction extend far beyond the perinatal period, and form the basis for chronic diseases and psychomotor development disorders [6–14]. However, the issues of predicting the outcomes in infants with growth restriction, especially taking into account the periods of manifestation and the severity of fetal growth restriction, remain poorly understood [2].
A comprehensive assessment of the relationship between the characteristics of intrauterine growth in the antenatal period, the features of neonatal adaptation and long-term consequences for child health is of particular importance. At the same time, special attention should be paid to exploring not only the somatic status, but also neuropsychological development of children born with fetal growth restriction.
This study was aimed to analyze the long-term implications of fetal growth restriction and assess physical and psychomotor development of children at different ages. The obtained results will help to improve the algorithms for dynamic monitoring of this cohort of patients and develop personalized rehabilitation programs, that will ultimately contribute to improvement of their quality of life.
The objective of the study was to investigate neonatal complications and characteristics of postnatal development of infants with fetal growth restriction.
Materials and methods
The study was carried out in two stages. The first stage included analysis of the clinical and anamnestic data, and the features of the course of pregnancy in 620 women, who were followed-up and gave birth at the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia (Kulakov Center). The main group included pregnant women with fetal growth restriction (FGR) (n=300). The comparison group included women with normal pregnancy (n=320). Inclusion criteria were the following: singleton pregnancy, age of women 18–45 years, absence of severe extragenital pathology, chromosomal abnormalities and fetal congenital anomalies. The diagnosis of fetal growth restriction was made in accordance with the Delphi definition for FGR [15] and clinical recommendations of the Ministry of Health of Russia [16].
All newborns (n=620) in this study underwent neurosonographic examination after birth, followed by analysis of brain structural changes. In addition to neurosonographic assessment, somatic, neurological and ophthalmological complications in the neonatal period were evaluated including anthropometric measurements of 620 newborns in accordance with the INTERGROWTH-21st fetal growth standards [17]. The main group included 300 newborns, who had fetal growth restriction. The comparison group included 320 infants without fetal growth restriction.
The second stage included analysis of postneonatal complications (from day 29 of postnatal development till the age of 1 year) in infants, who underwent follow-up at the Scientific Pediatric Consultative Department of Kulakov Center after discharge (n=207). The main group included infants with fetal growth restriction (n=115). The comparison group included infants with normal anthropometric parameters (n=92). All neonates underwent a comprehensive assessment of health status for admission to neonatal departments and after discharge from hospital.
The study was carried out in compliance with ethical standards. The approval from the local Ethics Committee was obtained prior to conducting the study. The women were included in the study after signing informed voluntary consent.
Statistical analysis
Modern methods of data analysis were used for statistical data processing taking into account the characteristics of distribution of the explored indicators. The quantitative variables were tested for normality of distribution using the Shapiro–Wilk test for the samples of <50 observations, and the Kholmogorov–Smirnov test for larger sample sizes. Non-normal distribution of quantitative variables is represented as median (Me) and interquartile range (Q1; Q3).
The qualitative data were analyzed using absolute values and percentage distribution in the general sample. The non-parametric Mann–Whitney U-test was used for comparison of quantitative variables with non-normal distribution between the groups to determine statistical significance of the results for heterogeneous data.
The qualitative variables were assessed using Pearson's chi-square test for a sufficient sample size, and Fisher’s exact test was used for a small sample size. The effect size for qualitative variables included calculation of relative risk (RR) with 95% confidence interval (CI). When there were zero values in contingency tables, the Haldane–Anscombe correction was used for the accuracy of statistical estimates.
The level of statistical significance was set at p<0.05, which is a standard in evidence-base medicine.
Results
The results of a comprehensive analysis of somatic and gynecological anamnesis, and the features of the course of pregnancy are represented in Table 1.
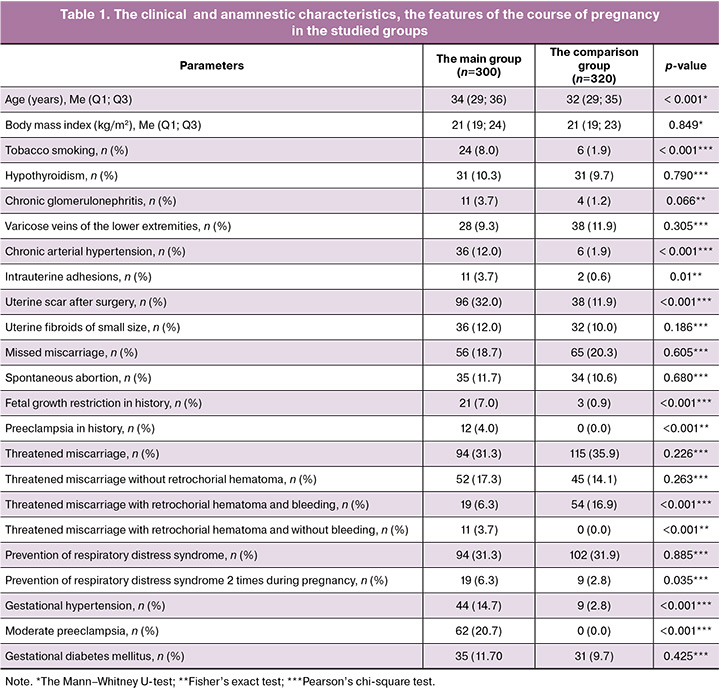
The median age of women was 34 (29; 36) years in the main group, and 32 (29; 35) years in the comparison group (p<0.001). Also, tobacco smoking (p<0.001), chronic arterial hypertension (p<0.001), intrauterine adhesions (p=0,01), uterine scars after surgery (p<0,001), fetal growth restriction (p<0,001) and preeclampsia (p<0.001) in history were found significantly more often in the main group. Analysis of the features of the course of pregnancy showed that in patients with fetal growth restriction gestational hypertension (p<0.001) and moderate preeclampsia (p<0,001) developed significantly more often, while the frequency of threatened miscarriage with retrochorial hematoma and bleeding was significantly lower versus the comparison group (p<0,001). Antenatal care for prevention of respiratory distress syndrome was taken two times for 6.3% (19/300) of pregnant women with fetal growth restriction (p=0,035). At the same time, there was no statistically significant difference in the frequency of prevention (one or two times) between the groups (p=0.885).
According to analysis, the gestational age at birth was comparable both in the main group and in the comparison group: in the group with fetal growth restriction the median gestational age at birth was 36.0 (34.0; 38.0) weeks, and 36.2 (34.5; 38.0) weeks in the comparison group (p=0,124). The percentage of newborns with gestational age less than 37 weeks was 41.3% (n=124) in the main group and 47.5% (n=152) in the comparison group.
Analysis showed that there were statistically significant differences between the groups in the anthropometric parameters of newborns at birth. The median body weight percentile was 2.0 (0.9; 4.6) in the main group, and 53.7 (31.1; 70.3) in the comparison group (p<0,001). The median growth percentile in newborns with fetal growth restriction was 10.0 (2,1; 32,0), and 79.5 (56.2; 89.4) in the comparison group (p<0.001). The median head circumference percentile was 20.1 (7.3; 49.1) in the main group and 74.2 (56.3; 89.1) in the comparison group (p<0,001). All newborns with fetal growth restriction met the criteria for small (weight and/or length <the 10th percentile) and low birth weight (<2500 g) for gestational age; In the comparison group, only 35.9% of newborns (n=115) were born with a birthweight below 2500 g.
In general, high neonatal morbidity was among newborns in the main group (Table 2).
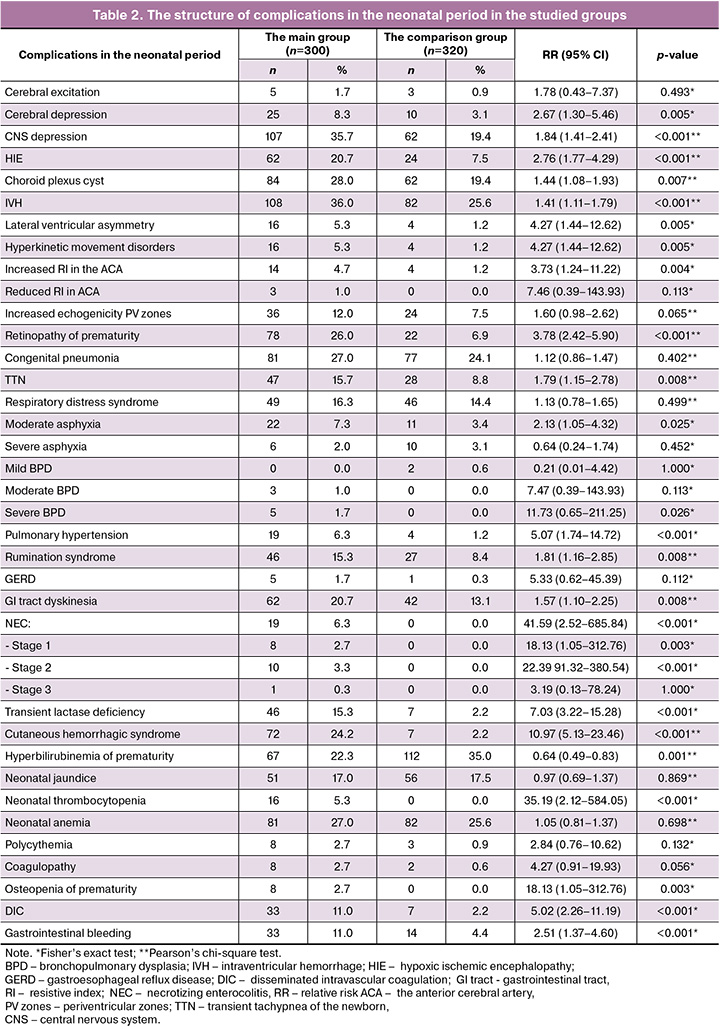
As is shown is Table 2, the signs of cerebral depression (p=0.005) and CNS depression (p<0.001) occurred significantly more often in the main group. The frequency of occurrence of hypoxic ischemic encephalopathy was 2.8 times higher in the main group versus the comparison group (p<0,001). Hyperkinetic movement disorders were found 4.2 times (p=0.005) more often in the main group compared with the comparison group. In neurosonographic assessment, choroid plexus cysts (p=0.007), intraventricular hemorrhages (p<0.001), lateral ventricular asymmetry (p=0.005), and increased resistive index in the anterior cerebral artery (p=0.004) were diagnosed significantly more often.
Retinopathy of prematurity occurred 3.8 times more often than in comparison group (p<0.001).
Analysis of respiratory disorders showed significantly high incidence of moderate asphyxia (p=0.025), transient tachypnea of the newborn (p=0.008), severe bronchopulmonary dysplasia (p=0,026), as well as pulmonary hypertension (p<0.001) in the main group.
Among gastrointestinal diseases, the frequency of gastrointestinal tract dyskinesia was 1.6 times higher (p=0.008), rumination syndrome was 1.8 times higher (p=0.008), and transient lactase deficiency was 7 times higher (p<0.001) in the main group versus the comparison group. It is important to note, that cases of necrotizing enterocolitis were found exclusively in the main group (p<0.001).
Cutaneous hemorrhagic syndrome occurred 11 times more often (p<0.001), thrombocytopenia – 35 times more often (p<0.001), disseminated intravascular coagulation 5 times more often (p<0.001), and gastrointestinal bleeding – 2.5 times more often (p<0.001) in the main group versus the comparison group.
Osteopenia of prematurity occurred exclusively in the main group (p=0.003).
Comparative analysis of postneonatal complications in the studied groups is represented in Table 3.
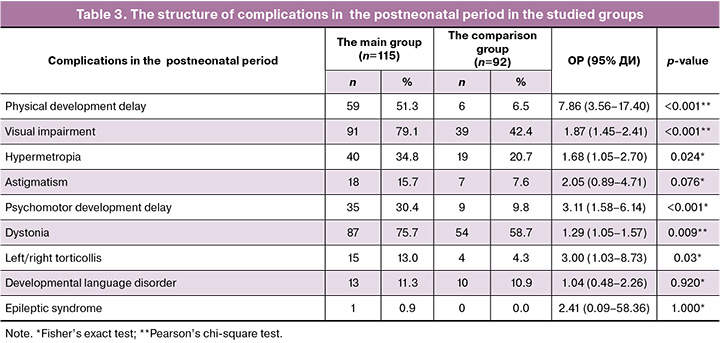
There were significant differences in the frequency of physical development delay between the groups. It was 8 times more often (p<0001) and visual impairment was 2 times more often (p<0,001) in the main group versus the comparison group.
It is important to note that specific refractive error, such as hypermetropia was also significantly more often diagnosed in infants in the main group (p=0,024).
Psychomotor development delay was significantly more often in the main group (p<0.001) versus the comparison group.
Dystonia and left/right torticollis were also significantly more often found in the main group versus the comparison group (p=0.009 and p=0.03, respectively).
Discussion
Our study found significant differences in neonatal outcomes and long-term adverse health effects in infants, who had fetal growth restriction compared with the group without FGR. The data obtained in the study confirm that fetal growth restriction is a significant risk factor for both early and late complications, that is consistent with the results in the studies over the last few years [1, 3, 4, 8, 16, 18].
Pulmonary hypertension in fetal growth restriction can occur due to chronic intrauterine hypoxia, that leads to increased pulmonary vascular resistance [19].
The occurrence of disseminated intravascular coagulation in newborns with FGR is associated with immaturity of hemostatic system, aggravated by a combination of factors, such as intranatal asphyxia and preeclampsia during pregnancy of the mother, while skin hemorrhagic syndrome develops due to a combination of thrombocytopenia and vascular endothelial dysfunction. Numerous studies confirm that hypoxic ischemic central nervous system disorders caused by perinatal asphyxia significantly increase the risk of coagulation disorders
The identified neurological complications, including cerebral depression, CNS depression, hypoxic ischemic encephalopathy and intraventricular hemorrhages occur due to chronic intrauterine hypoxia in FGR leading to neuronal energy deficiency and impaired neurogenesis [6, 7, 21–24]. Additionally, oxidative stress damages neuronal structures, especially precursor cells, and reduces the regenerative potential of the brain [25].
Retinopathy of prematurity is associated with immaturity of the vascular network of the retina, increased sensitivity to oxidative stress and impaired neurogenesis of the visual pathway function against the background of placental insufficiency [26–28].
The occurrence of necrotizing enterocolitis in newborns with FGR can be explained by intestinal ischemia due blood flow redistribution during chronic hypoxia [3].
In the postneonatal period, delay in physical and psychomotor development in infants in the main group was signigicantly more often. These results are consistent with the data reported by Sacchi C. et al. [4]. They showed persistent cognitive disorders in children, who had fetal growth restriction. High frequency of delay in psychomotor and physical development, as well as dystonia in infants with FGR by the first year of life is also due to compound effect of chronic intrauterine hypoxia on the developing structures of the CNS [4, 18]. The pathogenetic basis is the hypoxic ischemic damage to the neurons of basal ganglia and motor cortex, impaired myelination and lower synaptic density in the sensor and motor areas [29]. Energy imbalance due to the deficiency of glucose and amino acids leads to a impaired synthesis of neurotrophins [23, 30, 31], aggravating the neurologic deficit.
This study has some limitations. Our study is a retrospective study, that can influence the completeness of data collection, as well as long-term outcome assessment could be done not for all patients, that potentially limits generalization of the study results. Another methodological limitation is a lack of standardization for assessment of neurological status using validated tools (for example, Bely-III scales – Bayley Scales of Infant Development (BSID-III)).
Conclusion
Fetal growth restriction is associated with adverse neonatal and postneonatal outcomes. This highlights the need for prevention and early diagnosis of this pregnancy complication, as well as the importance of broad screening, including ophthalmological observation, long-term catamnestic observation of neuropsychological development, as well as comprehensive step-by-step rehabilitation for infants with fetal growth restriction in the postnatal period.
References
- Fernandez-Rodriguez B., de Alba C., Galindo A., Recio D., Villalain C., Pallas C.R. et al. Obstetric and pediatric growth charts for the detection of late-onset fetal growth restriction and neonatal adverse outcomes. J. Perinat. Med. 2020; 49(2): 216-24. https://dx.doi.org/10.1515/jpm-2020-0210
- Melekoglu R., Yilmaz E., Yasar S., Hatipoglu I., Kahveci B., Sucu M. The ability of various cerebroplacental ratio thresholds to predict adverse neonatal outcomes in term fetuses exhibiting late-onset fetal growth restriction. J. Perinat. Med. 2020; 49(2): 209-215. https://dx.doi.org/10.1515/jpm-2020-0244
- Bahia M.L.R., Velarde G.C., Silva F.C.D., Araujo Júnior E., Sá R.A.M. Adverse perinatal outcomes in fetuses with severe late-onset fetal growth restriction. J. Matern. Fetal Neonatal Med. 2022; 35(25): 8666-72. https://dx.doi.org/10.1080/14767058.2021.1995858
- Sacchi C., Marino C., Nosarti C., Vieno A., Visentin S., Simonelli A. Association of intrauterine growth restriction and small for gestational age status with childhood cognitive outcomes: a systematic review and meta-analysis. JAMA Pediatr. 2020; 174(8): 772-81. https://dx.doi.org/10.1001/jamapediatrics.2020.1097
- Muniz C.S., Dias B.F., Motoyama P.V.P., Almeida C.T.C., Feitosa F.E.L., Araujo Júnior E., Alves J.A.G. Doppler abnormalities and perinatal outcomes in pregnant women with early-onset fetal growth restriction. J. Matern. Fetal Neonatal Med. 2022; 35(25): 7276-9. https://dx.doi.org/10.1080/14767058.2021.1946786
- Chawla D. Fetal growth restriction and neurodevelopmental outcome. Indian J. Pediatr. 2021; 88(6): 538-9. https://dx.doi.org/10.1007/s12098-021-03789-3
- Dudink I., Hüppi P.S., Sizonenko S.V., Castillo-Melendez M., Sutherland A.E., Allison B.J. et al. Altered trajectory of neurodevelopment associated with fetal growth restriction. Exp. Neurol. 2022; 347: 113885. https://dx.doi.org/10.1016/j.expneurol.2021.113885
- Sacchi C., O'Muircheartaigh J., Batalle D., Counsell S.J., Simonelli A., Cesano M. et al. Neurodevelopmental outcomes following intrauterine growth restriction and very preterm birth. J. Pediatr. 2021; 238: 135-144.e10. https://dx.doi.org/10.1016/j.jpeds.2021.07.002
- Halevy J., Peretz R., Ziv-Baran T., Katorza E. Fetal brain volumes and neurodevelopmental outcome of intrauterine growth restricted fetuses. Eur. J. Radiol. 2023; 168: 111143. https://dx.doi.org/10.1016/j.ejrad.2023.111143
- Benítez-Marín M.J., Marín-Clavijo J., Blanco-Elena J.A., Jiménez-López J., González-Mesa E. Brain sparing effect on neurodevelopment in children with intrauterine growth restriction: a systematic review. Children (Basel). 2021; 8(9): 745. https://dx.doi.org/10.3390/children8090745
- Olsen E.M., Nilsson K.K., Wright C.M., Michaelsen K.F., Skovgaard A.M. Infancy weight faltering and childhood neurodevelopmental disorders: a general population birth-cohort study. Eur. Child. Adolesc. Psychiatry. 2023; 32(7): 1179-88. https://dx.doi.org/10.1007/s00787-021-01915-2
- Wang B., Zeng H., Liu J., Sun M. Effects of prenatal hypoxia on nervous system development and related diseases. Front. Neurosci. 2021; 15: 755554. https://dx.doi.org/10.3389/fnins.2021.755554
- Richter A.E., Salavati S., Kooi E.M.W., Heijer A.E. den, Foreman A.B., Schoots M.H. et al. Fetal brain-sparing, postnatal cerebral oxygenation, and neurodevelopment at 4 years of age following fetal growth restriction. Front. Pediatr. 2020; 8: 225. https://dx.doi.org/10.3389/fped.2020.00225
- Gardella B., Dominoni M., Caporali C., Cesari S., Fiandrino G., Longo S. et al. Placental features of fetal vascular malperfusion and infant neurodevelopmental outcomes at 2 years of age in severe fetal growth restriction. Am. J. Obstet. Gynecol. 2021; 225(4): 413.e1-413.e11. https://dx.doi.org/10.1016/j.ajog.2021.03.037
- Gordijn S.J., Beune I.M., Thilaganathan B., Papageorghiou A., Baschat A.A., Baker P.N. et al. Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet. Gynecol. 2016; 48(3): 333-9. https://dx.doi.org/10.1002/uog.15884
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Недостаточный рост плода, требующий предоставления медицинской помощи матери (задержка роста плода). М.; 2022. 71 с. [Ministry of Health of the Russian Federation. Clinical guidelines. Insufficient growth of the fetus, requiring the provision of medical care to the mother (fetal growth retardation). Moscow; 2022. 71 p. (in Russian)].
- Рюмина И.И., Байбарина Е.Н., Нароган М.В., Маркелова М.М., Орловская И.В., Зубков В.В., Дегтярев Д.Н. Использование международных стандартов роста для оценки физического развития новорожденных и недоношенных детей. Неонатология: новости, мнения, обучение. 2023; 11(2): 48-52. [Ryumina I.I., Baybarina E.N., Narogan M.V., Markelova M.M., Orlovskaya I.V., Zubkov V.V., Degtyarev D.N. The usage of the international growth standards to assess the physical development of newborn and premature infants. Neonatology: News, Opinions, Training. 2023; 11(2): 48-52. (in Russian)]. https://dx.doi.org/10.33029/2308-2402-2023-11-2-48-52
- Kim H.S., Kim E.K., Park H.K., Ahn D.H., Kim M.J., Lee H.J. Cognitive outcomes of children with very low birth weight at 3 to 5 years of age. J. Korean Med. Sci. 2020; 35(1): e4. https://dx.doi.org/10.3346/jkms.2020.35.e4
- Abbas G., Shah S., Hanif M., Shah A., Rehman A.U., Tahir S. et al. The frequency of pulmonary hypertension in newborn with intrauterine growth restriction. Sci. Rep. 2020; 10(1): 8064. https://dx.doi.org/10.1038/s41598-020-65065-2
- Go H., Ohto H., Nollet K.E., Kashiwabara N., Ogasawara K., Chishiki M. et al. Risk factors and treatments for disseminated intravascular coagulation in neonates. Ital. J. Pediatr. 2020; 46(1): 54. https://dx.doi.org/10.1186/s13052-020-0815-7
- Кан Н.Е., Леонова А.А., Тютюнник В.Л., Хачатрян З.В. Особенности нейрогенеза при задержке роста плода. Акушерство и гинекология. 2022; 11: 24-30. [Kan N.E., Leonova A.A., Tyutyunnik V.L., Khachatryan Z.V. Features of neurogenesis in fetal growth restriction. Obstetrics and Gynecology. 2022; (11): 24-30 (in Russian)]. https://dx.doi.org/10.18565/aig.2022.11.24-30
- Gusar V., Kan N., Leonova A., Chagovets V., Tyutyunnik V., Khachatryan Z. et al. Non-invasive assessment of neurogenesis dysfunction in fetuses with early-onset growth restriction using fetal neuronal exosomes isolating from maternal blood: a pilot study. Int. J. Mol. Sci. 2025; 26(4): 1497. https://dx.doi.org/10.3390/ijms26041497
- Ahn S.Y., Sung D.K., Kim Y.E., Sung S., Chang Y.S., Park W.S. Brain-derived neurotropic factor mediates neuroprotection of mesenchymal stem cell-derived extracellular vesicles against severe intraventricular hemorrhage in newborn rats. Stem Cells Transl. Med. 2021; 10(3): 374-84. https://dx.doi.org/10.1002/sctm.20-0301
- Волочаева М.В., Кан Н.Е., Тютюнник В.Л., Леонова А.А., Солдатова Е.Е., Рыжова К.О. Особенности постнатального развития детей с задержкой роста (катамнестическое исследование). Obstetrics and Gynecology. 2025; 3: 65-71. [Volochaeva M.V., Kan N.E., Tyutyunnik V.L., Leonova A.A., Soldatova E.E., Ryzhova K.O. Postnatal development in children with growth restriction (follow-up study). Obstetrics and Gynecology. 2025; (3): 65-71 (in Russian)]. https://dx.doi.org/10.18565/aig.2025.32
- Schoots M.H., Bourgonje M.F., Bourgonje A.R., Prins J.R., van Hoorn E.G.M., Abdulle A.E. et al. Oxidative stress biomarkers in fetal growth restriction with and without preeclampsia. Placenta. 2021; 115: 87-96. https://dx.doi.org/10.1016/j.placenta.2021.09.013
- Chu A., Dhindsa Y., Sim M.S., Altendahl M., Tsui I. Prenatal intrauterine growth restriction and risk of retinopathy of prematurity. Sci. Rep. 2020; 10(1): 17591. https://dx.doi.org/10.1038/s41598-020-74600-0
- Polat O.A., Kirlangic M.M., Sahin E., Madendag Y., Evereklioglu C., Horozoglu F. et al. Role of the brain-sparing effect on retinopathy of prematurity in newborns with fetal growth restriction. Curr. Med. Res. Opin. 2024; 40(4): 629-34. https://dx.doi.org/10.1080/03007995.2024.2320289
- Gilchrist C.P., Cumberland A.L., Kondos-Devcic D., Hill R.A., Khore M., Quezada S. et al. Hippocampal neurogenesis and memory in adolescence following intrauterine growth restriction. Hippocampus. 2021; 31(3): 321-34. https://dx.doi.org/10.1002/hipo.23291
- Wan L., Luo K., Chen P. Mechanisms underlying neurologic injury in intrauterine growth restriction. J. Child. Neurol. 2021; 36(9): 776-84. https://dx.doi.org/10.1177/0883073821999896
- Sahay A., Kale A., Joshi S. Role of neurotrophins in pregnancy and offspring brain development. Neuropeptides. 2020; 83: 102075. https://dx.doi.org/10.1016/j.npep.2020.102075
- Dudink I., Sutherland A.E., Castillo-Melendez M., Ahmadzadeh E., White T.A., Malhotra A. et al. Fetal growth restriction adversely impacts trajectory of hippocampal neurodevelopment and function. Brain Pathol. 2025; 35(4): e13330. https://dx.doi.org/10.1111/bpa.13330
Received 19.05.2025
Accepted 28.05.2025
About the Authors
Anastasia A. Leonova, PhD student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, +7(937)453-54-27, nastena27-03@mail.ru, https://orcid.org/0000-0001-6707-3464Natalia E. Kan, Professor, Dr. Med. Sci., Honored Scientist of the Russian Federation, Deputy Director for Research – Director of the Institute of Obstetrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4,
kan-med@mail.ru, Researcher ID: B-2370-2015, SPIN: 5378-8437, Authors ID: 624900, Scopus Author ID: 57008835600, https://orcid.org/0000-0001-5087-5946
Victor L. Tyutyunnik, Professor, Dr. Med. Sci., Leading Researcher at the Center for Scientific and Clinical Research, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str. 4, tioutiounnik@mail.ru,
Researcher ID: B-2364-2015, SPIN: 1963-1359, Authors ID: 213217, Scopus Author ID: 56190621500, https://orcid.org/0000-0002-5830-5099
Anna P. Serebriakova, obstetrician-gynecologist of the day hospital department for examination of pregnant women, State Healthcare Institution Primorsky Krai Perinatal Center, 690042, Russia, Vladivostok, Mozhayskaya str., 1B, serebriakovanna@gmail.com, https://orcid.org/0000-0001-7014-2627
Anuta A. Khachaturyan, PhD student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str. 4, x.anyt37@mail.ru, https://orcid.org/0009-0007-3767-9343
Natalia A. Pekareva, Dr. Med. Sci., Head of the Scientific Advisory Pediatric Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str. 4, n_pekareva@oparina4.ru, https://orcid.org/0000-0002-2710-864Х
Corresponding author: Anastasia A. Leonova, nastena27-03@mail.ru



