The effect of incretin hormone GLP-1 on the phenotypic profile of mononuclear cells in fetal growth restriction
Krasnyi A.M., Kan N.E., Borisova A.G., Soldatova E.E., Tyutyunnik V.L., Volochaeva M.V.
Relevance: Glucagon-like peptide-1 (GLP-1) is an incretin hormone that regulates insulin secretion, and elevated GLP-1 level is in fetal growth restriction. GLP-1 can bind to its membrane receptors on leukocytes and change their phenotype.
Objective: To study the impact of GLP-1 on the phenotypic profile of peripheral blood mononuclear cells in pregnant women with fetal growth restriction and identify potential diagnostic markers.
Materials and methods: The study was conducted in two stages. In stage one, the impact of the GLP-1 receptor agonist liraglutide on the phenotypic profile of peripheral blood mononuclear cells in 12 pregnant women (6 patients with fetal growth restriction and 6 patients with normal pregnancy) was assessed using flow cytometry. In stage two, 56 pregnant women were enrolled in the study, and divided into two groups. The main group consisted of 32 women with fetal growth restriction. The comparison group consisted of 24 patients with normal pregnancy.
The diagnostic significance of the obtained markers (CD4, CD8, CD86 and CD163) was evaluated by measuring their counts and expression levels in the peripheral blood of pregnant women.
Results: In vitro assessment of the effect of liraglutide on mononuclear cells in the peripheral blood of pregnant women showed statistically significant decrease in CD8+ lymphocyte number (p=0.03), reduction in CD8 expression in lymphocytes (p=0.03) and elevated expression of CD163 on monocytes (p=0.05). In the blood of pregnant women with fetal growth restriction, statistically significant relatively high levels of CD4+ (p=0.02) and CD163+ (p=0.001) monocytes and relatively low levels of CD8+ lymphocytes (p=0.006) were found. At the same time, the levels of CD163 expression in the main group were significantly elevated (p=0.02), while the levels of CD86 expression on monocytes was reduced (p=0.02). The ROC analysis showed the potential diagnostic value of the relative level of CD163+ monocytes in the blood of pregnant women for the diagnosis of fetal growth restriction (AUC=0.83).
Conclusion: The obtained data showed that in fetal growth retardation glucagon-like peptide-1 triggers a specific cellular response, which is manifested by activation of a pronounced anti-inflammatory effect in women’s blood. Studying this signaling pathway may help to understand new mechanisms of fetal growth restriction and identify potential markers that will enable to verify this pregnancy complication at the antenatal stage.
Authors' contributions: Krasnyi A.M., Kan N.E., Borisova A.G., Soldatova E.E., Tyutyunnik A.A., Volochaeva M.V. – the concept and design of the study, data obtaining for analysis, literature review, processing and analysis of the material, writing the text of the manuscript, article editing.
Conflicts of interest: The authors confirm that they have no conflicts of interest to declare.
Funding: The study was conducted without any sponsorship.
Ethical Approval: The study was approved by the Ethics Committee of the Academician V.I. Kulakov National Medical Research Centre of Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia.
Patient Consent for Publication: All patients have signed voluntary informed consent for publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Krasnyi A.M., Kan N.E., Borisova A.G., Soldatova E.E., Tyutyunnik V.L., Volochaeva M.V.
The effect of incretin hormone GLP-1 on the phenotypic profile of mononuclear cells in fetal growth restriction.
Akusherstvo i Gynecologia/Obstetrics and Gynecology. 2024; (10): 74-81 (in Russian)
https://dx.doi.org/10.18565/aig.2024.156
Keywords
Fetal growth restriction (FGR) is still an issue in obstetrics and ranks first among the causes of perinatal morbidity and mortality [1, 2].
Low birth weight may lead to increased morbidity in the perinatal period and increased risk of cardiovascular, neurological and metabolic diseases in adulthood [3]. Exposure to various risk factors during pregnancy has a significant impact on epigenetic processes, which play a key role in fetal programming. According to this concept, adverse intrauterine environment leads to serious changes in immune system metabolism and functioning [3–5].
In our previous study [5], the hormones and adipokines involved in regulation of energy metabolism in FGR and their role in the pathogenesis of this pregnancy complication have been described. Of greatest interest was analysis of glucagon-like peptide-1 (GLP-1). In patients with FGR, the level of GLP-1 remained high throughout pregnancy versus the comparison group, and increased closer to full-term.
GLP-1 is an incretin hormone secreted by small intestinal L cells in response to meal ingestion and regulates glucose homeostasis [6]. According to literature, the GLP-1 receptor (GLP-1R) is expressed on many cell types, including macrophages [7]. However, to date, there is still little research on the significance of signaling and activation of GLP-1/GLP-1R.
Shiraishi D. et al. found that GLP-1 induces signal transducer and activator of transcription 3 (STAT3) activation. As is known, STAT3 in macrophages is involved in regulation of the immune response [8]. STAT3 activation is important for macrophage differentiation macrophages from the M1 phenotype, predominantly producing pro-inflammatory cytokines toward the M2 phenotype, which generate anti-inflammatory factors [9]. Analysis of the effect of GLP-1 on lymphocytes found that GLP-1 can block SDF-1-induced migration of CD4+ lymphocytes [10].
In view of the above, GLP-1 can influence the phenotype of leukocytes by binding to its membrane receptors, that leads to the apparent presence of specific leukocyte phenotype (macrophages and lymphocytes), namely anti-inflammatory. Given the role of GLP-1 in the development of FGR, we decided to study its impact on pro- and anti-inflammatory effects of mononuclear blood cells in pregnant women with FGR.
The objective of the study was to investigate the impact of GLP-1 on the phenotypic profile of peripheral blood mononuclear cells in pregnant women with FGR and identify potential diagnostic markers.
Materials and methods
The study was conducted at the Academician V.I. Kulakov National Medical Research Centre for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia (further named as Kulakov Center) and included 56 pregnant women [5].
The main group (group I) consisted of 32 patients with postnatal diagnosis of fetal growth restriction. The comparison group (group II) consisted of 24 patients without FGR.
In the antenatal period, the diagnosis of FGR was made in accordance with the clinical recommendations “Fetal growth restriction requiring health care for mother” [2], and the Delphi consensus criteria for FGR. After birth, the newborn's weight and length parameters were assessed using the INTERGROWTH-21st centile charts [5]. The diagnosis of small for dates or small for gestational age babies was made when newborns had birth weight below the 10th percentile [11].
The study was approved by the local Ethics Committee of Kulakov Center of the Ministry of Health of Russia. Upon admission of patients to profile departments of the Center, patient selection and blood sample collection was performed using pair selection method according to inclusion and exclusion criteria, after the patients have sighed informed consent to participate in the study.
Inclusion criteria: the patients aged 18–45 years, singleton pregnancy (22–40 weeks) complicated with FGR (the main group), without FGR (the comparison group), patient’s informed consent to participate in the study [12].
Exclusion criteria: multiple pregnancy, preeclampsia during pregnancy, donor egg program for using assisted reproductive technologies, severe external genital pathology, antiphospholipid syndrome, exacerbation of infectious diseases, malformations and hemolytic disease of the fetus [12].
Venous blood samples from pregnant women were collected into tubes with sodium heparin and subsequently delivered to the laboratory within 30 minutes. The study was conducted in two stages.
In the first stage, the impact of GLP-1 receptor agonist (liraglutide) on the phenotypic profile of mononuclear cells obtained from 6 patients with normal pregnancy and 6 patients with FGR was studied to determine the biological effect of GLP-1. For this purpose, peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll–Plaque method, and subsequently cultured in RPMI 1640 medium with 10% fetal bovine serum for 24 hours. After culture, 5 ng of liraglutide was added to 500 μl medium.
Blood samples were analyzed using BD FACSCalibur flow cytometer (USA). Antibodies with fluorescent labels were added to mononuclear cells, and the percentage of cells, as well as expression levels of certain markers was determined using flow cytometry. Relative concentrations of cell surface markers CD4, CD8, CD86 and CD163 were determined by assessment of the relative fluorescence units. Relative concentrations (%) of monocytes and lymphocytes were measured as the number of each cell population to the total number of monocytes and lymphocytes.
In the second stage of the study, evaluation of the diagnostic value of the obtained markers was made by assessment of their concentration and expression level in the peripheral blood of pregnant women with FGR (n=32) and in women with normal pregnancy (n=24) according to the above described method.
Statistical analysis
Statistical analysis of the results of the study was performed using R statistical software, Version 4.0.2 (Vienna, Austria), OriginPro 8.5 (USA) and Microsoft Excel. The Shapiro–Wilk test was used to test the hypothesis of normal distribution (< 50 observations). The Mann–Whitney U test was used to compare the quantitative parameters in two groups, that had distribution different from normal. The data are represented as median (Me), the lower and upper quartiles (Q1; Q3). Comparison of qualitative parameters was done using Fisher's exact test. The qualitative characteristics are presented as absolute values and the percent proportion (the number of samples to the total number of samples, %) [5]. The Wilcoxon signed-rank test was used to analyze related samples. The diagnostic value of the obtained markers was assessed using ROC analysis, as well as by calculation of sensitivity and specificity at threshold [12]. The differences were considered to be statistically significant at p<0.05.
Results and discussion
The clinical and anamnestic characteristics of patients enrolled in the study were comparable (Table 1.).
Assessment of the body weight and height of patients enrolled in the study, as well as analysis of somatic and obstetric and gynecological anamnesis found no statistically significant differences between the groups (р>0.05). At the same time, analysis of the newborns’ body weight and length showed that the weight in newborns with fetal growth restriction was 1940 (1518; 2287) g, and 3160 (2960; 3527) g in term newborns. According to the INTERGROWTH-21st centile chart, babies’ weight after birth was 2.0 (0,9; 3,0) and 69.5 (47,2; 84,5) percentiles in the groups, respectively, р<0.001. Apgar scores ranged from 5 to 9 [5].
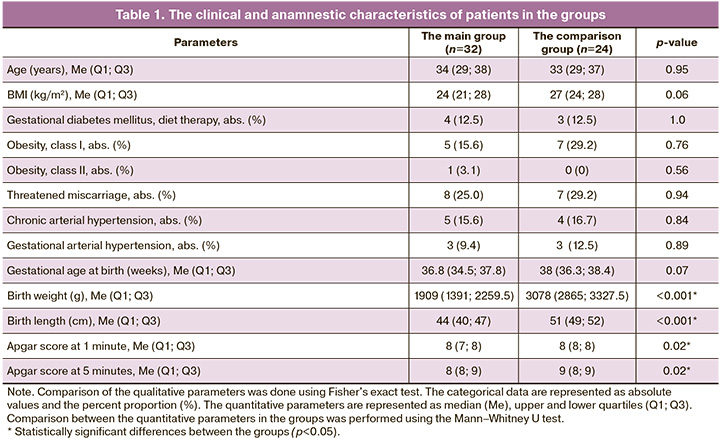
Analysis of the effect of liraglutide, a synthetic analog of GLP-1 on lymphocytes and monocytes in the blood of healthy pregnant women (n=6) and women with FGR (n=6) in in vitro experiment showed that liraglutide has a multidirectional effect on relative concentrations of CD4+ and CD8+ lymphocytes, CD163+ and CD86+ monocytes and expression level in the peripheral blood of pregnant women (Fig. 1, Table 2) [13, 14].
The experiment showed that activation of GLP-1 receptors on the surface of PBMCs can alter the phenotype of peripheral blood monocytes and lymphocytes. [13, 15], that is consistent with literature data. [8]. In our previous study, we analyzed concentration of factors of energy metabolism in plasma from pregnant women with FGR [5]. It was found that GLP-1 plasma concentrations were significantly higher in pregnant women with FGR (p=0.003).
According to literature data, GLP-1 has anti-inflammatory properties [15–17]. The study by Shiraishi D. et al. showed that GLP-1R activation in vitro can inhibit inflammation and modulate immune response [8, 18]. Synthetic GLP-1 analogues (liraglutide, exendin and exenatide) have also the capacity to suppress the macrophage secretion of different inflammatory cytokines in vitro [8, 14]. The authors have shown that exenatide shifts macrophage phenotype toward anti-inflammatory phenotype by significantly increasing the level of the anti-inflammatory cytokine interleukin-10 (IL-10) and reducing the levels of both tumor necrosis factor (TNF)-α and IL-1β in cultured human monocytes/macrophages [8]. It has also been reported that GLP-1 receptor agonists reduce monocyte/macrophage accumulation within the arterial wall by inhibiting inflammatory response of macrophages. Moreover, human monocyte-derived macrophages cultured with GLP-1 increased CD163 and CD204 surface expression and IL-10 secretion, and activated the STAT3 signaling pathway, while STAT1 was inhibited [8]. Based on these data, it was suggested that GLP-1 is involved in activation and polarization of macrophages toward the anti-inflammatory M2 phenotype, that provides the first evidence that GLP-1/GLP-1R signaling correlates with STAT3 activation and macrophage polarization.
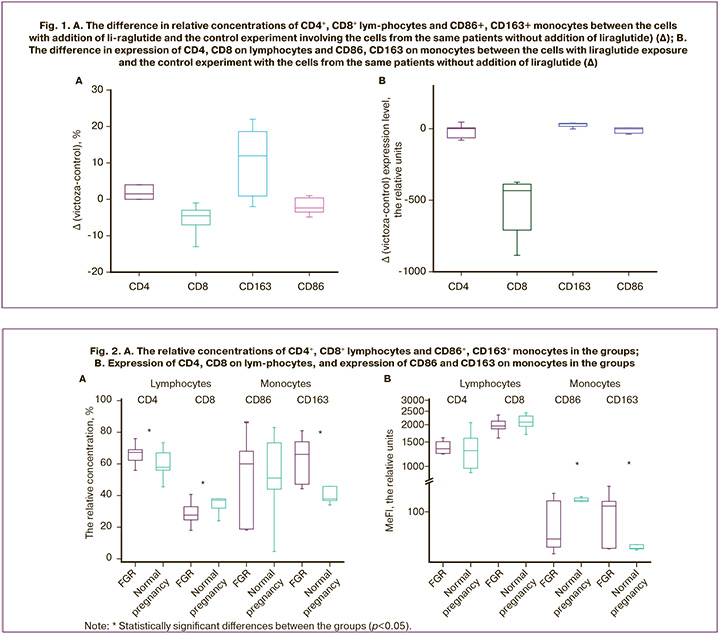
Since plasma GLP-1 concentration is elevated in FGR, it can be expected that the PBMC phenotype in women with FGR will differ from the phenotype of women with normal pregnancy. To test this assumption, it was decided to analyze relative concentrations (Fig. 2A) of CD4+ and CD8+ lymphocytes, CD86+ and CD163+ monocytes and expression level in the blood of women with FGR (n=32) and women with normal pregnancy (n=24). (Fig. 2B).
The relative concentration of CD4+ monocytes (p=0.02) was significantly high, and the relative concentration of CD8+ lymphocytes (p=0.006) was low in the peripheral blood of women with FGR – 67.25 (62.77; 68.63) % and 27.60 (24.65; 31.93) %, respectively. At the same time in women with normal pregnancy, the levels of concentration were 57.85 (56.00; 67.00)% and 37.05 (32.00; 38.00)%. However, expression of CD4 and CD8 on lymphocytes did not show statistically significant differences between the groups. So, CD4 expression was 1340.50 (1246.25; 1493.50) relative units in the main group, and 1299.00 (969.00; 1596.00) relative units (р=0.82) in the comparison group. CD8 expression was 1956.0 (1872.0; 2086.4) relative units in woman with normal pregnancy, and 2086.0 (1945.0; 2308.0) relative units (р=0.07) in women with FGR.
Expression of CD86 on monocytes was significantly low in the main group – 35.9 (26.4; 151.0) relative units, and 153.95 (147.00; 168.00) relative units (p=0.02) in the comparison group. However, relative concentrations of CD86+ monocytes did not show statistically significant differences between the groups (p=1.00). So, concentration of CD86+ was 60.0 (18.8; 68.0) % in the main group and 51.1 (44.0; 73.3)% in the comparison group.
Analysis of relative concentrations of CD163+ monocytes and expression levels showed statistically significant differences between the groups. Significantly increased level of CD163 expression was in the main group – 124.0 (25.2; 148.0) relative units, and 25.2 (24.8; 29.0) relative units in the comparison group (р=0.02). The similar tendency was observed in analysis of the relative concentration of CD163+ monocytes in the blood of pregnant women. The relative concentration of CD163+ was 66.0 (47.1; 74.0)% in the main group and 37.8 (36.8; 45.8)% in the comparison group (р=0.001).
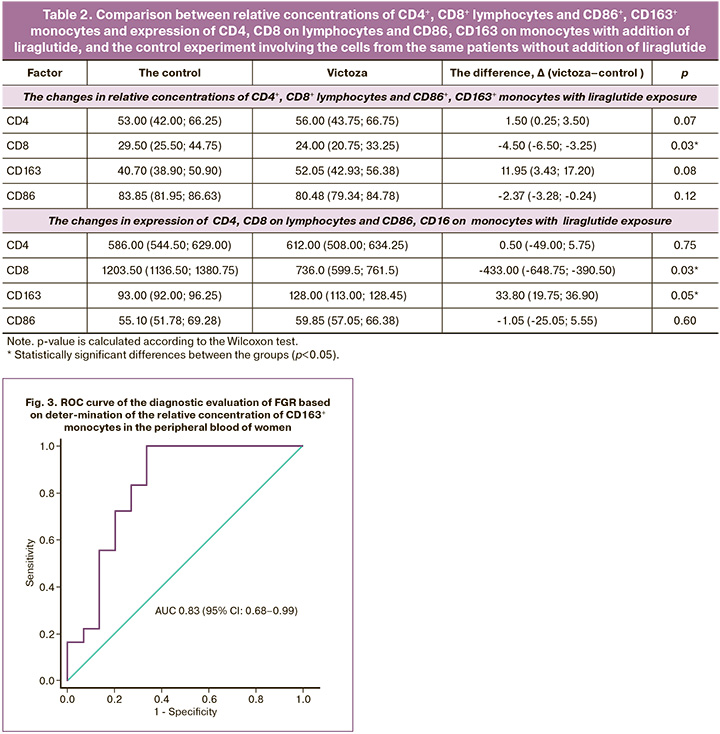
Since statistically, the most significant difference between the groups was found in the relative concentration of CD163+ monocytes in the peripheral blood, this factor was considered as a potential marker of FGR. ROC analysis was performed to assess the diagnostic value of the relative concentration of CD163+ monocytes in the peripheral blood (Fig. 3).
It was found that the area under the ROC curve showing the relative concentration of CD163+ monocytes was 0.83 (95% CI 0.68–0.99) with a threshold value of 40.7%, sensitivity 100% and specificity 77%. The prognostic value of the positive result was 78.3% (95% CI 61.4–95.1) and of the negative result – 100.0% (95% CI 100–100). According to expert scale, detection of the relative concentration of CD163+ monocytes in the peripheral blood of women with FGR has a very good diagnostic value, that makes it possible to consider the relative concentration of CD163+ monocytes as a potential marker for the diagnosis of FGR at the antenatal stage.
Thus, the obtained results confirm the above described experiment in vitro with liraglutide and are consistent with literature data. It seems that increased GLP-1 concentrations in the peripheral blood of pregnant women with FGR, changes the phenotype of monocytes and lymphocytes in the blood by activation of GLP-1 receptors on their surface, thereby exerting an anti-inflammatory effect due to increased relative concentration of CD4+ lymphocytes and CD163+ monocytes and decreased relative concentration of CD8+ lymphocytes in the peripheral blood of pregnant women. It may indicate the possibility of their participation in one signaling pathway, and GLP-1 is a signaling molecule, which induces specific cellular response in terms of activation of the pronounced anti-inflammatory effect due to STAT3 [19]. Activation of STAT3 induced by GLP-1 function in immune cells, that was described by different authors, may directly lead to activation of the Wnt signaling pathway, and is known to be crosstalk [20].
Studying the signaling pathways involving hormones, incretins and adipokines is a promising area, and may help to identify potential markers for the diagnosis and treatment of FGT, and, therefore, requires further research.
Conclusion
Identification of factors involved in regulation of energy metabolism, and their influence on the phenotypic profile of mononuclear cells in the peripheral blood in FGR helps to understand pathogenetic mechanisms of this complication of pregnancy.
Analysis of the phenotypic profile of mononuclear cells in the peripheral blood of women with FGR found changes in relative concentrations of CD4+ and CD8+ lymphocytes, CD86+ and CD163+ monocytes and expression levels in the peripheral blood of pregnant women, that indicates their possible involvement in one signaling pathway. At the same time the relative concentration of CD163+ monocytes may become a potential marker for the diagnosis of FGR (AUC=0.83).
The results of our study showed that in this signaling pathway, GLP-1 is a signaling molecule, which induces specific cellular response in terms of activation of the pronounced anti-inflammatory effect characterized by increased relative concentrations of CD4+ lymphocytes and CD163+ monocytes, and decreased relative concentration of CD8+ lymphocytes in the blood of women with FGR.
Studying this signaling pathway may help to understand new mechanisms of this complication of pregnancy and establish potential markers, which will make it possible to verify FGR at the antenatal stage.
References
- Nardozza L.M., Caetano A.C., Zamarian A.C., Mazzola J.B., Silva C.P., Marçal V.M. et al. Fetal growth restriction: current knowledge. Arch. Gynecol. Obstet. 2017; 295(5): 1061-77. https://dx.doi.org/10.1007/s00404-017-4341-9.
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Недостаточный рост плода, требующий предоставления медицинской помощи матери (задержка роста плода). 2022. 76с. [Ministry of Health of the Russian Federation. Clinical guidelines. Insufficient fetal growth requiring maternal medical care (fetal growth restriction). Moscow; 2022. 76p. (in Russian)].
- Oke S.L., Hardy D.B. The role of cellular stress in intrauterine growth restriction and postnatal dysmetabolism. Int. J. Mol. Sci. 2021; 22(13): 6986. https://dx.doi.org/10.3390/ijms22136986.
- Hales C.N., Barker D.J. The thrifty phenotype hypothesis. Br. Med. Bull. 2001; 60: 5-20. https://dx.doi.org/10.1093/bmb/60.1.5.
- Кан Н.Е., Солдатова Е.Е., Тютюнник В.Л., Борисова А.Г., Тезиков Ю.В., Липатов И.С., Садекова А.А., Алексеев А.А., Красный А.М. Факторы энергетического метаболизма при задержке роста плода. Акушерство и гинекология. 2024; 5: 44-52. [Kan N.E., Soldatova E.E., Tyutyunnik V.L., Borisova A.G., Tezikov Yu.V., Lipatov I.S., Sadekova A.A., Alekseev A.A., Krasnyi A.M. Factors of energy metabolism in fetal growth retardation. Obstetrics and Gynecology. 2024; (5): 44-52. (in Russian)]. https://dx.doi.org/10.18565/aig.2024.9.
- Baggio L.L., Drucker D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007; 132(6): 2131-57. https://dx.doi.org/10.1053/j.gastro.2007.03.054.
- Smith N.K., Hackett T.A., Galli A., Flynn C.R. GLP-1: molecular mechanisms and outcomes of a complex signaling system. Neurochem. Int. 2019; 128: 94-105. https://dx.doi.org/10.1016/j.neuint.2019.04.010.
- Shiraishi D., Fujiwara Y., Komohara Y., Mizuta H., Takeya M. Glucagon-like peptide-1 (GLP-1) induces M2 polarization of human macrophages via STAT3 activation. Biochem. Biophys. Res. Commun. 2012; 425(2): 304-8. https://dx.doi.org/10.1016/j.bbrc.2012.07.086.
- Porcheray F., Viaud S., Rimaniol A.C., Léone C., Samah B., Dereuddre-Bosquet N. et al. Macrophage activation switching: an asset for the resolution of inflammation. Clin. Exp. Immunol. 2005; 142(3): 481-9. https://dx.doi.org/10.1111/j.1365-2249.2005.02934.x.
- Marx N., Husain M., Lehrke M., Verma S., Sattar N. GLP-1 receptor agonists for the reduction of atherosclerotic cardiovascular risk in patients with type 2 diabetes. Circulation. 2022; 146(24): 1882-94. https://dx.doi.org/10.1161/circulationaha.122.059595.
- Рюмина И.И., Байбарина Е.Н., Нароган М.В., Маркелова М.М., Орловская И.В., Зубков В.В., Дегтярев Д.Н. Использование международных стандартов роста для оценки физического развития новорожденных и недоношенных детей. Неонатология: новости, мнения, обучение. 2023; 11(2): 48-52. [Ryumina I.I., Baibarina E.N., Narogan M.V., Markelova M.M., Orlovskaya I.V., Zubkov V.V., Degtyarev D.N. The usage of the international growth standards to assess the physical development of newborn and premature children. Neonatology: News, Opinions, Training. 2023; 11(2): 48-52. (in Russian)]. https://dx.doi.org/10.33029/2308-2402-2023-11-2-48-52.
- Кан Н.Е., Солдатова Е.Е., Тютюнник В.Л., Волочаева М.В., Садекова А.А., Красный А.М. Диагностическая значимость определения экспрессии генов энергетического метаболизма при задержке роста плода. Акушерство и гинекология. 2023; 8: 48-55. [Kan N.E., Soldatova E.E., Tyutyunnik V.L., Volochaeva M.V., Sadekova A.A., Krasnyi A.M. Diagnostic significance of determining the expression of energy metabolism genes in fetal growth retardation. Obstetrics and Gynecology. 2023; (8): 48-55 (in Russian)]. https://dx.doi.org/10.18565/aig.2023.93.
- Bendotti G., Montefusco L., Lunati M.E., Usuelli V., Pastore I., Lazzaroni E. et al. The anti-inflammatory and immunological properties of GLP-1 receptor agonists. Pharmacol. Res. 2022; 182: 106320. https://dx.doi.org/10.1016/j.phrs.2022.106320.
- Lee Y.S., Jun H.S. Anti-inflammatory effects of GLP-1-based therapies beyond glucose control. Mediators Inflamm. 2016; 2016: 3094642. https://dx.doi.org/10.1155/2016/3094642.
- Insuela D.B.R., Carvalho V.F. Glucagon and glucagon-like peptide-1 as novel anti-inflammatory and immunomodulatory compounds. Eur. J. Pharmacol. 2017; 812: 64-72. https://dx.doi.org/10.1016/j.ejphar.2017.07.015.
- Mehdi S.F., Pusapati S., Anwar M.S., Lohana D., Kumar P., Nandula S.A. et al. Glucagon-like peptide-1: a multi-faceted anti-inflammatory agent. Front. Immunol. 2023; 14: 1148209. https://dx.doi.org/10.3389/fimmu.2023.1148209.
- Li Y., Glotfelty E.J., Karlsson T., Fortuno L.V, Harvey B.K., Greig N.H. The metabolite GLP-1 (9-36) is neuroprotective and anti-inflammatory in cellular models of neurodegeneration. J. Neurochem. 2021; 159(5): 867-86. https://dx.doi.org/10.1111/jnc.15521.
- Kim Chung le T., Hosaka T., Yoshida M., Harada N., Sakaue H., Sakai T. et al. Exendin-4, a GLP-1 receptor agonist, directly induces adiponectin expression through protein kinase A pathway and prevents inflammatory adipokine expression. Biochem. Biophys. Res. Commun. 2009; 390(3): 613-8. https://dx.doi.org/10.1016/j.bbrc.2009.10.015.
- Borg A.J., Yong H.E., Lappas M., Degrelle S.A., Keogh R.J., Da Silva-Costa F. et al. Decreased STAT3 in human idiopathic fetal growth restriction contributes to trophoblast dysfunction. Reproduction. 2015; 149(5): 523-32. https://dx.doi.org/10.1530/REP-14-0622.
- Yang J., Wang Y., Yang D., Ma J., Wu S., Cai Q. et al. Wnt/β-catenin signaling regulates lipopolysaccharide-altered polarizations of RAW264.7 cells and alveolar macrophages in mouse lungs. Eur. J. Inflamm. 2021; 19: 205873922110593. https://dx.doi.org/10.1177/20587392211059362.
Received 05.07.2024
Accepted 09.10.2024
About the Authors
Aleksey M. Krasnyi, PhD, Head of the Cytology Laboratory, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology,Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, +7(495)438-22-72, alexred@list.ru, https://orcid.org/0000-0001-7883-2702
Natalia E. Kan, Professor, Dr. Med. Sci., Deputy Director of Science, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, +7(926)220-86-55, kan-med@mail.ru, Researcher ID: B-2370-2015,
SPIN-code: 5378-8437, Authors ID: 624900, Scopus Author ID: 57008835600, https://orcid.org/0000-0001-5087-5946
Anastasia G. Borisova, PhD student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, +7(968)735-40-81, vvv92@list.ru
Ekaterina E. Soldatova, PhD student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, +7(906)110-51-13, katerina.soldatova95@bk.ru, https://orcid.org/0000-0001-6463-3403
Victor L. Tyutyunnik, Professor, Dr. Med. Sci., Leading Researcher at Center of Scientific and Clinical Researches, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, +7(903)969-50-41, tioutiounnik@mail.ru,
Researcher ID: B-2364-2015, SPIN-code: 1963-1359, Authors ID: 213217, Scopus Author ID: 56190621500, https://orcid.org/0000-0002-5830-5099
Maria V. Volochaeva, PhD, Senior Researcher at the Department of Regional Cooperation and Integration, Physician at 1 Maternity Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4,
+7(919)968-72-98, m_volochaeva@oparina4.ru, https://orcid.org/0000-0001-8953-7952
Corresponding author: Ekaterina E. Soldatova, katerina.soldatova95@bk.ru



