Criteria for assessing fetal neurogenesis dysfunction in early-onset growth restriction using extracellular vesicles
Kan N.E., Leonova A.A., Gusar V.A., Chagovets V.V., Tyutyunnik V.L., Volochaeva M.V., Soldatova E.E., Ryzhova K.O., Serebriakova A.P.
Objective: To evaluate neurogenesis dysfunction during early-onset fetal growth restriction by obtaining fetal neuronal exosomes (FNE) from maternal plasma, assessing the expression of neurotrophin proteins involved in neurogenesis and the regulation of fetal nervous system plasticity, and establishing relationships with clinical and functional data.
Materials and methods: This study included 45 pregnant women. The study group consisted of 20 women with early-onset fetal growth restriction, whereas the control group comprised 25 women with normal pregnancies. The gestational age of the newborns in both groups did not exceed 34 weeks. Extracellular vesicles were isolated from maternal plasma using a commercial kit, followed by immunoprecipitation to obtain FNE. The expression of neurotrophin proteins—nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF)—was assessed using Western blotting.
Results: A decrease in the expression of BDNF (pro-BDNF) and NGF (pro-NGF) precursors was observed in the FNE of the study group. Mature forms of neurotrophin proteins were not detected in FNE. Changes in pro-BDNF levels were noted only in cases of intraventricular hemorrhage (IVH), while pro-NGF levels varied in IVH, cerebral ischemia, and asphyxia in newborns during the neonatal period.
Conclusion: The results demonstrate, for the first time, the potential to detect brain neurodysfunction in fetuses with growth restriction by assessing the expression of neuronal proteins in FNE isolated from maternal blood via immunoprecipitation. Changes in these protein levels may reflect the degree of brain dysfunction and serve as potential prognostic and diagnostic markers of pathological conditions.
Authors' contributions: Kan N.E., Leonova A.A., Gusar V.A., Chagovets V.V., Tyutyunnik V.L., Volochaeva M.V., Soldatova E.E., Ryzhova K.O., Serebriakova A.P. – conception and design of the study, acquisition of data for analysis, review of literature, preparation and analysis of material on the topic, statistical analysis, drafting of the manuscript, editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: The study was conducted within the framework of the initiative project "Epigenetic criteria for diagnosis of fetal growth restriction from the perspective of neurogenesis dysfunction" (research project No. 19-И23 dated 8 December 2022) (registration number in the EGISU NIOKTR system (state accounting) – 123060500032-8).
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Kan N.E., Leonova A.A., Gusar V.A., Chagovets V.V., Tyutyunnik V.L., Volochaeva M.V., Soldatova E.E., Ryzhova K.O., Serebriakova A.P. Criteria for assessing fetal neurogenesis dysfunction
in early-onset growth restriction using extracellular vesicles.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (3): 56-64 (in Russian)
https://dx.doi.org/10.18565/aig.2025.30
Keywords
Fetal growth restriction (FGR) results from altered placental function due to various factors [1]. This condition increases the risk of several complications, including motor and sensory deficits in the development of the nervous system and, in the long term, cognitive dysfunction and learning problems [2]. Generally, FGR is characterized by a reduction in the total brain volume and structural changes in the cortex and dendritic processes, leading to impaired neurogenesis [3–5]. Neurotrophic factors play essential roles in neurogenesis and synapse formation within the central nervous system (CNS). They are critical for the survival, proliferation, migration, and functioning of neurons as well as synaptic plasticity. A decrease in neurotrophic factor activity has been linked to reduced synapse density in model organisms and altered functional connections between brain regions, resulting in cognitive impairments [6, 7]. Recent data suggest that neurotrophin proteins are involved in the regulation of implantation, maternal immunity, angiogenesis [8], and placental maturation and development of the placenta [7, 9, 10].
The study of specific biomarkers of brain damage in the peripheral blood is undoubtedly of great interest. However, limitations exist concerning the ability to correlate their levels with brain pathology and the uncertainty surrounding their tissue of origin [11]. Over the past decade, significant research has focused on extracellular vesicles (EVs) as specific biomarkers for various pathological conditions, including those related to pregnancy [12]. Exosomes, a type of EVs, play a crucial role in signaling between the mother and fetus and can penetrate the blood-brain barrier bidirectionally [13]. They contain a unique cargo of various proteins, including neurotrophins, which reflect the state of the donor cell and may serve as biomarkers of fetal brain damage [12]. A novel method for isolating exosomes from maternal blood has been proposed, offering an innovative, non-invasive platform for assessing fetal neurological development during early pregnancy [14, 15]. Nonetheless, the mechanisms underlying perinatal brain damage in FGR remain unclear, and opportunities for the early detection of neurodysfunction in utero are virtually nonexistent. Our previous pilot study results indicated changes in the levels of several proteins associated with neurogenesis, pre- and postsynaptic signal transmission, and post-translational modifications such as sumoylation and neddylation [16]. Consequently, this study focused on obtaining fetal neuronal exosomes (FNE) from maternal blood plasma in cases of early-onset FGR to assess the expression of neurotrophins within these exosomes and identify their relationship with fetal brain damage.
The aim of this study was to evaluate neurogenesis dysfunction in early FGR by obtaining EVs from maternal blood plasma to assess the expression of neurotrophin proteins in their composition, involved in neurogenesis and the regulation of fetal nervous system plasticity and to establish a relationship with clinical and functional data.
Materials and methods
The study was conducted at the V.I. Kulakov NMRC for OG&P of the Ministry of Health of the Russian Federation and was approved by the local ethics committee. Informed consent was obtained from all participating pregnant women. A total of 45 pregnant women were selected for the study; the study group included 20 women with early-onset FGR, and the control group consisted of 25 women with physiological pregnancy. The gestational age at the time of blood sampling did not exceed 32 weeks, and all patients in both groups delivered before 34 weeks of pregnancy. Pregnancy management and delivery followed the clinical guidelines of the Ministry of Health of the Russian Federation titled ‘Insufficient fetal growth requiring maternal assistance (fetal growth restriction)’ (2021) [17].
The condition of the newborns, including their body weight and height, was assessed according to established criteria using the INTERGROWTH-21 and Fenton growth tables. The diagnosis of neonatal growth restriction was confirmed based on the Consensus-Based Definition of Growth Restriction in the Newborn. All neonates underwent neurosonographic examinations, and their neurological outcomes were evaluated using the Thompson Score for Hypoxic-Ischemic Encephalopathy at 2 hours of life and the Griffith Mental Development Scale at 28 days of life or at hospital discharge.
Isolation of EV population from pregnant women's blood plasma
Blood samples were collected from the patients in both groups. Sample preparation included stepwise centrifugation, followed by isolation of total maternal exosomes using the ExoQuick Plasma Prep and Exosome Precipitation kit according to the manufacturer's instructions. To isolate the EV population, immunoprecipitation was performed according to the protocol described in detail by Goetzl et al. [14, 15, 18].
Evaluation of EV neurotrophin protein expression by Western blotting
EV proteins were separated by polyacrylamide gel electrophoresis (PAGE) (12.5%), followed by transfer to a 0.45 μm nitrocellulose membrane (Trans-Blot SD), staining, and detection by immunoblotting with antibodies against BDNF and NGF. The exosomal marker CD81 was used to assess the plasma EV levels. Densitometry was performed using Bio-Rad ImageLab 6.0.
Statistical analysis
The normality of the distribution of continuous variables was tested using the Shapiro–Wilk test and described using arithmetic means (M) and standard deviations (SD). The 95% confidence interval (95% CI) was used to measure the representativeness of the mean values. In the absence of a normal distribution, continuous variables were described using the median (Me) and lower and upper quartiles (Q1 and Q3). Categorical data were presented as counts and percentages. The 95% CI for percentages was calculated using the Clopper–Pearson method. Comparison of two groups by continuous variables with normal distribution and equal variances was performed using Student's t-test, and with unequal variances, using the Welch t-test. Comparisons of the two groups by non-normal continuous variables were performed using the Mann–Whitney U-test. Comparisons of percentages in the analysis of 2×2 contingency tables were performed using Fisher's exact test (with expected frequencies <10). The odds ratio (OR) with 95% confidence interval (CI) was calculated as a quantitative measure of the effect of relative indicators. In the case of zero values for the number of observations in the cells of the contingency table, the odds ratio was calculated using the Haldane×Anscombe correction. Comparisons of percentages when analyzing multi-way contingency tables were performed using the Pearson’s chi-square test. Differences were considered statistically significant at p<0.05.
Results
The clinical and anamnestic characteristics of the study participants are shown in Table 1.
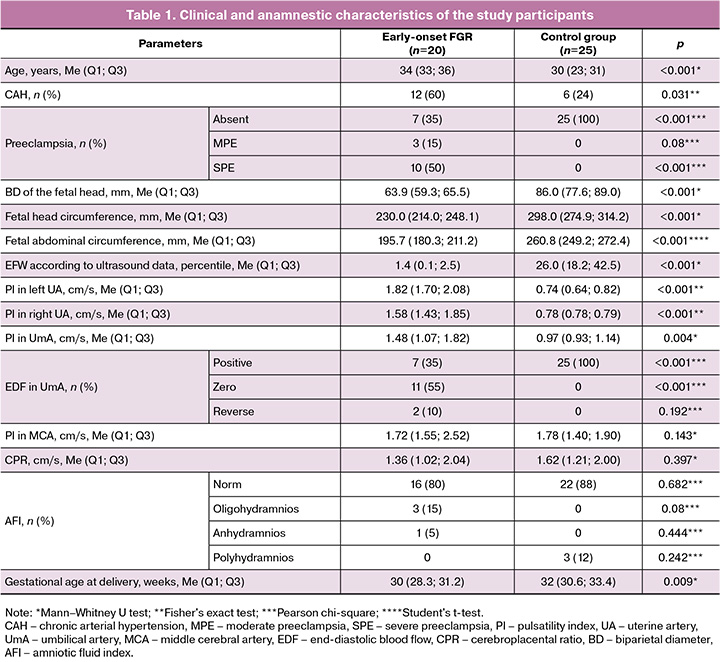
The study group was dominated by women of older reproductive age (median age 34 years). The median age of the control group was 30 years (p<0.001).
Chronic arterial hypertension in the study group was 4.75 times more common than in the control group (OR=0.021; 95% CI 0.058–0.758) – 60% and 24% (by group, respectively). In the study group, preeclampsia was detected in 65% (13/20) of the patients (p<0.001). When comparing the incidence of moderate and severe preeclampsia, the incidence of moderate preeclampsia was 77% (10/13). When analyzing the ultrasound and Doppler data, statistically significant differences were found relative to the control group in the following parameters: biparietal diameter (BD) (p<0.001), fetal head circumference (HC) (p<0.001), fetal abdominal circumference (AC) (p<0.001), percentile of estimated fetal weight (p<0.001), pulsatility index (PI) values in the left uterine artery (UA) (p<0.001), PI in the right UA (p<0.001), PI values in the umbilical artery (p=0.004), and type of end-diastolic blood flow in the umbilical artery (p<0.001). Statistically significant differences were found in gestational age at delivery (p=0.009): the median in the study group was 30 weeks and in the control group, 32 weeks. The clinical and anamnestic characteristics of the newborns and neonatal outcomes are presented in Table 2.
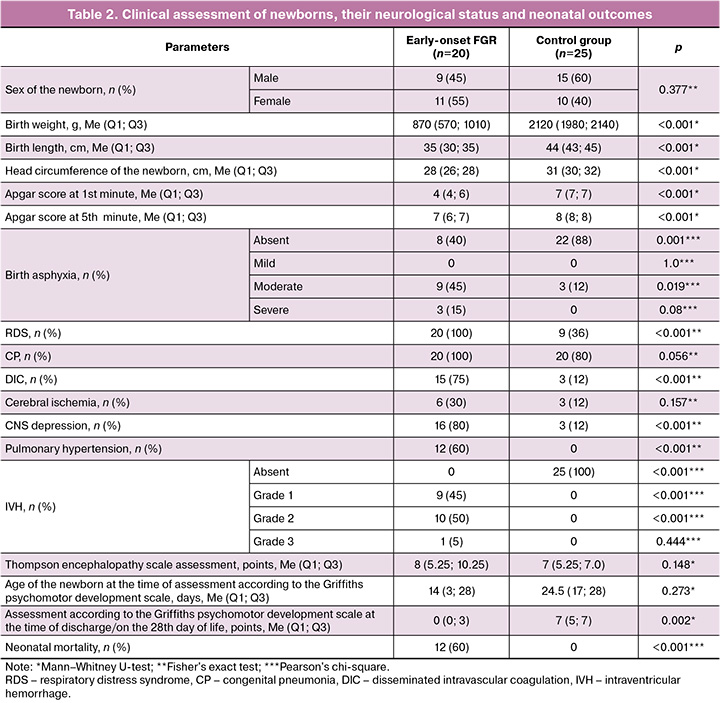
The birth length and height of newborns (newborn weight, length, and head circumference) differed significantly between the study groups (p<0.001, p<0.001, and p<0.001, respectively). There were also differences in the control of the assessments of the newborns' condition according to the Apgar score at the 1st minute (p<0.001) and the 5th minute (p<0.001) between the study and control groups.
Analysis of neonatal outcomes revealed significant differences between the two study groups in complications, such as birth asphyxia (p<0.001), respiratory distress syndrome (RDS) (p<0.001), and disseminated intravascular coagulation (DIC) syndrome (p<0.001). Birth asphyxia was significantly more common in the study group than in the control group (p=0.001). Moderate birth asphyxia was six times more common in the study group than in the control group (p=0.019; OR =0.167, 95% CI 0.037–0.742). Pulmonary hypertension was observed only in the study group (p<0.001). The presence of intraventricular hemorrhage (IVH) in neonates with growth restriction according to neurosonography data was significantly different from that in the control group (p<0.001), while grade 2 IVH was statistically more often observed in 50% of the observations (10/20) (p<0.001). When assessing the neurological status of neonates in the study group, CNS depression was recorded 29 times more often (p<0.001; OR=0.034; 95% CI, 0.007–0.174).
We did not find any significant differences in the Thompson Scale score at 2 h of life in newborns in either group. The score on the R. Griffiths’ psychomotor development scale score at the time of discharge from the hospital or on the 28th day of life was significantly lower in the study group than in the control group (p=0.002). In addition, the children in the study group had a longer hospital stay. Neonatal mortality was only recorded in the study group (p<0.001).
Evaluation of EV neurotrophin protein expression
Comparative analysis revealed a significant decrease in the expression of BDNF (-0.7 (-1.13; 0.22), p≤0.04) and NGF (-0.72 (-1.1; 0.03), p≤0.02) in early-onset FGR relative to the control group – BDNF (0.36 (-0.09; 1.12), p≤0.04) and NGF (0.72 (0.06; 1.08), p≤0.02) (Fig. 1). It is interesting to note that the sizes of BDNF and NGF fragments corresponded to ~28–30 kDa, which are not the mature form, but precursors of pro-BDNF and pro-NGF. All samples demonstrated positive results for CD81.
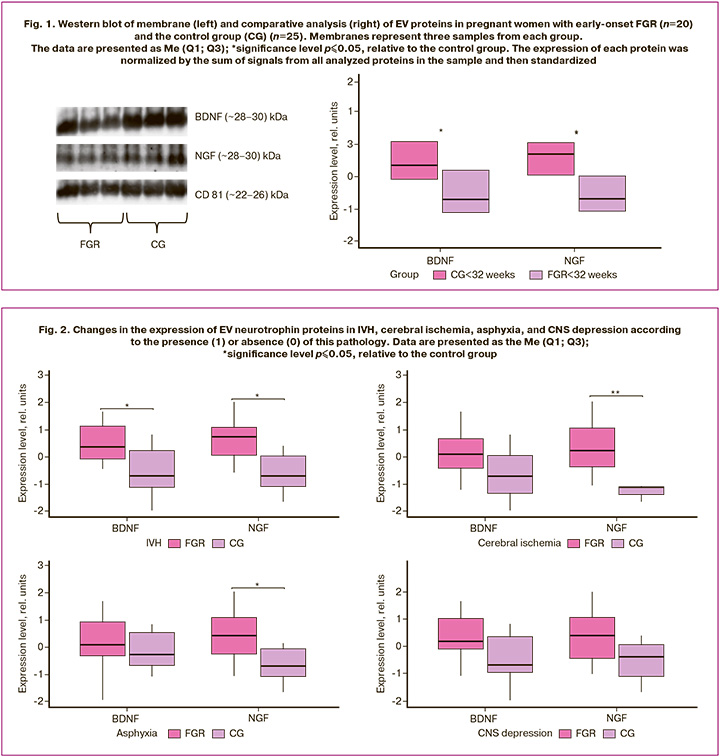
Evaluation of EV neurotrophin protein expression according to the presence or absence of neonatal complications
Considering the significant changes identified in the clinical and anamnestic data of newborns, it was interesting to evaluate the differences in the expression of neurotrophin proteins in EV, depending on the presence of neonatal complications (Fig. 2).
In the group with combined manifestations of cerebral ischemia, IVH, and asphyxia, a change in the expression of neurotrophin proteins was observed. The expression of pro-NGF was significantly reduced in IVH (-0.72 (-1.1; 0.03), p≤0.02), asphyxia (-0.72 (-1.11; -0.09), p≤0.03), and cerebral ischemia (-1.13 (-1.4; -1.11), p≤0.003) in the study group compared to the control group – (0.72 (0.06; 1.08), p≤0.02), (0.4 (-0.28; 1.06), p≤0.03) and (0.23 (-0.39; 1.06), p≤0.003), respectively. At the same time, a statistically significant change in the expression of another neurotrophin protein, pro-BDNF, was observed only in neonates with IVH (-0.7 (-1.13; 0.22), p≤0.04) in the study group, whereas pro-BDNF levels in the control group were (0.36 (-0.09; 1.12), p≤0.04). No statistically significant difference in neurotrophin protein expression was observed in neonates with CNS suppression.
Discussion
The results of the clinical data analysis, namely the median age of 35 years and the significantly higher prevalence of hypertensive disorders, including preeclampsia, among the pregnant women in the study group, are in accordance with the risk factors for the development of FGR specified in Russian and international clinical guidelines [1, 17]. The ultrasound parameters of the fetuses in the study group, such as the estimated fetal weight percentile, BD, HC) and AC, also met the diagnostic criteria for confirming FGR at the antenatal stage [1, 17]. Elevated Doppler values of PI in UA, PI in the umbilical artery, and the presence of zero end-diastolic blood flow in the umbilical artery were consistent with the diagnostic criteria for early-onset FGR set forth by the international Delphi consensus [1, 17, 19]. RDS and pulmonary hypertension, which were statistically significant in fetuses with growth restriction, may result from chronic intrauterine hypoxia and the presence of moderate to severe birth asphyxia, both of which increase pulmonary vascular resistance [20]. The development of DIC syndrome in preterm infants with growth restriction can be attributed to an underdeveloped blood coagulation system, further complicated by prematurity, birth asphyxia, and maternal preeclampsia [21]. Previous studies have indicated that newborns with hypoxic-ischemic brain damage resulting from perinatal asphyxia are at risk for hemostasis disorders, which have a prevalence of 18–69% [21]. The observed impairment in feto- and uteroplacental blood flow may serve as an indicator of the severe condition of neonates with growth restriction, corroborated by significant differences in their assessment according to the Apgar scale and psychomotor development as measured by R. Griffiths scale. In this regard, it is obvious that in such children, we observed changes in the nervous system, particularly IVH, cerebral ischemia, asphyxia, and CNS depression. It should be noted that CNS lesions dominate among the causes of early morbidity, disability and mortality in neonates. IVH, which occurs as a result of rupture of the embryonic matrix through the ependyma into the lateral ventricle, is the most severe and widespread type of CNS lesion, especially in premature infants (approximately 60-90% of cases) [2]. In addition, the most severe course of IVH in neonates with growth restriction may be due to the presence of DIC syndrome [21]. Accumulated data on neurotrophic factors indicate that BDNF mediates synaptic formation. During normal brain development, these proteins are highly expressed in the hippocampus and the cortex. In addition to these brain regions, NGF is also present in high concentrations in the pituitary gland [7], whereas BDNF can be expressed in the cerebellum, thalamus, amygdala, and spinal cord [22]. It has been previously shown that neurotrophic factors, having a powerful ability to enhance neurogenesis, can have a therapeutic effect on the restoration of the ischemic brain [7]. Therefore, the changes in their levels reflect the degree of damage. However, despite a significant number of studies identifying biomarkers of neuronal origin, the relationship between their levels in the brain and peripheral fluids remains unclear.
In this regard, we used the technology of isolating fetal neuronal exosomes from maternal plasma to assess the levels of neurotrophins BDNF and NGF, which were significantly reduced in fetuses with growth restriction [3, 7, 23]. As shown previously, the source of EVs isolated from maternal blood is the fetal brain, not the placenta [12, 24]. In addition, we noted an interesting feature: EVs did not contain mature forms of BDNF and NGF, but their precursors, pro-BDNF (~28–30 kDa) and pro-NGF (~28–30 kDa), the expression of which was reduced. The BDNF protein is synthesized as a precursor of pro-BDNF with a molecular weight of ~31–35 kDa, which is cleaved to the mature form of 14 kDa. A significant decrease in pro-BDNF levels has been observed in Alzheimer's disease [25]. It has been shown that pro-BDNF is packaged into secretory vesicles and, once released from the cell, its functions depend on the type of target receptor it binds to [26]. NGF supports the development, survival, and function of neurons in peripheral (sympathetic and sensory) and central (cholinergic) nervous systems. It has been previously shown that the mature form of NGF is completely absent from the human brain [8], whereas pro-NGF is secreted by many cells, including neurons and astrocytes, and is released via the constitutive secretory pathway, unlike pro-BDNF [13]. Given the dual nature of neurotrophins, as well as the fact that the physiological consequences of changes in the levels of their precursors are not fully understood, we propose that the expression of pro-BDNF and pro-NGF in EVs promotes the activation of specific signaling pathways, which leads to opposite effects and pathogenesis [27]. In general, decreased levels of neurotrophin precursors in growth-restricted fetuses are associated with neuronal and synaptic dysfunction [26, 28].
Considering the long-term consequences of neurogenesis dysfunction in growth-restricted neonates, we evaluated whether changes in the levels of the studied proteins were linked to processes of fetal brain damage. Our findings revealed that the expression pattern of neurotrophin proteins is associated with the manifestations of ischemia, IVH, and asphyxia. Notably, changes in pro-BDNF levels were observed only in the presence of IVH, whereas pro-NGF levels varied in response to cerebral ischemia and asphyxia. Our data suggest that alterations in BDNF levels may specifically reflect the degree of brain dysfunction and severity of IVH. This hypothesis is supported by previous in vivo experiments using model organisms [22].
Conclusion
The results of this study demonstrate, for the first time, the feasibility of isolating EVs from the maternal blood. These EVs are an integral part of the placental-mediated mechanisms of perinatal brain injury in fetuses experiencing growth restriction. Altered levels of neurotrophin proteins can both reflect the degree of brain dysfunction and serve as potential prognostic and diagnostic markers of pathological conditions. These findings are promising for translational medicine.
References
- American College of Obstetricians and Gynecologists' Committee on Practice Bulletins—Obstetrics and the Society for Maternal-Fetal Medicin. ACOG Practice Bulletin No. 204: Fetal Growth Restriction. Obstet. Gynecol. 2019; 133(2): e97-e109. https://dx.doi.org/10.1097/AOG.0000000000003070.
- Wan L., Luo K., Chen P. Mechanisms underlying neurologic injury in intrauterine growth restriction. J. Child Neurol. 2021; 36(9): 776-84. https://dx.doi.org/10.1177/0883073821999896.
- Giouleka S., Tsakiridis I., Mamopoulos A., Kalogiannidis I., Athanasiadis A., Dagklis T. Fetal growth restriction: A comprehensive review of major guidelines. Obstet. Gynecol. Surv. 2023; 78(11): 690-708. https://dx.doi.org/10.1097/OGX.0000000000001203.
- Polat O.A., Kirlangic M.M., Sahin E., Madendag Y., Evereklioglu C., Horozoglu F., Karaca C. Role of the brain-sparing effect on retinopathy of prematurity in newborns with fetal growth restriction. Curr. Med. Res. Opin. 2024; 40(4): 629-34. https://dx.doi.org/10.1080/03007995.2024.2320289.
- Dudink I., Hüppi P.S., Sizonenko S.V., Castillo-Melendez M., Sutherland M.E., Allison B.J., Miller S.L. Altered trajectory of neurodevelopment associated with fetal growth restriction. Exp. Neurol. 2022; 347: 113885. https://dx.doi.org/10.1016/j.expneurol.2021.113885.
- Milyutina Y.P., Arutjunyan A.V., Korenevsky A.V., Selkov S.A., Kogan I.Y. Neurotrophins: are they involved in i..mmune tolerance .in pregnancy? Am. J. Reprod. Immunol. 2023; 89(4): e13694. https://dx.doi.org/10.1111/aji.13694.
- Sahay A., Kale A., Joshi S. Role of neurotrophins in pregnancy and offspring brain development. Neuropeptides. 2020; 83: 102075. https://dx.doi.org/10.1016/j.npep.2020.102075.
- Ellero N., Lanci A., Baldassarro V.A., Alastra G., Mariella J., Cescatti M. et al. Study on NGF and VEGF dur.ing the equine perinatal Period-Part 1: Healthy foals born from normal pregnancy and parturition. Vet. Sci. 2022; 9(9): 451. https://dx.doi.org/10.3390/vetsci9090451.
- Rozanska O., Uruska A., Zozulinska-Ziolkiewicz D. Brain-derived neurotrophic factor and diabetes. Int. J Mol. Sci. 2020; 21(3): 841. https://dx.doi.org/10.3390/ijms21030841.
- Pathare-Ingawale P., Chavan-Gautam P. The balance between cell survival and death in the placenta: Do neurotrophins have a role? Syst. Biol. Reprod. Med. 2022; 68(1): 3-12. https://dx.doi.org/10.1080/19396368.2021.1980132.
- Huo L., Du X., Li X., Liu S., Xu Y. The emerging role of neural cell-derived exosomes in intercellular communication in health and neurodegeneratcve diseases. Front. Neurosci. 2021; 15: 738442. https://dx.doi.org/10.3389/fnins.2021.738442.
- Afzal A., Khan M., Gul Z., Asif R., Shahzaman S., Parveen A. et al. Extracellular vesicles: the next erontier in pregnancy research. Reprod. Sci. 2024; 31(5):1204-14. https://dx.doi.org/10.1n07/s43032-023-01434-2.
- Upadhya R., Zingg W., Shetty S., Shetty A.K. Astrocyte-derived extracellular vesicles: Neuroreparative propert.ie.s andole in the pathogenesis of neurodegenerative disorders. J. Control. Release. 2020; 323: 225-39. https://dx.doi.org/0.1016/j.jconrel.2020.04.017.
- Goetzl L., Darbinian N., Goetzl E.J. Novel window on early human neurodevelopment via fetal exos.omes in maternal blood. Ann. Clin. Transl. Neurol. 2016; 3(5): 381-5. https://dx.doi.org/10.1002/acn3.29.
- Goetzl L., Darbinian N., Merabova N. Noninvasive assessment of fetal central nervous system insult: Potential application to prenatal diagnosis. Prenat. Diagn. 2019; 39(8): 609-15. https://dx.doi.org/10.1002/pd.5474.
- Gusar V., Kan N., Leonova A., Chagovets V., Tyutyunnik V., Khachatryan Z. et al. Non-invasive assessment of neurogenesis dysfunction in fetuses with early-onset growth restriction using fetal neuronal exosomes isolating from maternal blood: A pilot study. Int. J. Mol. Sci. 2025; 26(4): 1497. https://dx.doi.org/10.3390/ijms26041497.
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Недостаточный рост плода, требующий предоставления медицинской помощи матери (задержка роста плода). М.; 2022. 71 с. [Ministry of Health of the Russian Federation. Clinical guidelines. Insufficient growth of the fetus, requiring the provision of medical care to the mother (fetal growth retardation). Moscow; 2022. 71 p. (in Russian)].
- Goetzl E.J., Mustapic M., Kapogiannis D., Eitan E., Lobach I.V., Goetzl L. et al. Cargo proteins of plasma as.trocyte-derived exosomes in Alzheimer's disease. FASEB J. 2016; 30(11): 3853-9. https://dx.doi.org/10.1096/fj.201600756R.
- Beune I.M., Damhuis S.E., Ganzevoort W., Hutchinson J.C., Khong T.Y., Mooney E.E. et al. Consensus definiti.on of fetal gro. th restriction: a Delphi procedure. Ultrasound Obstet. Gynecol. 2016; 48(3): 333-9. https://dx.doi.org/10.1002/uog.15884.
- Abbas G., Shah S., Hanif M., Shah A., Rehman A.U., Tahir S. et al. The frequency of pulmonary hypertension in newborn with intrauterine growth restriction. Sci. Rep. 2020; 10(1): 8064. https://dx.doi.org/10.1038/s41598-020-65065-2.
- Go H., Ohto H., Nollet KE., Kashiwabara N., Ogasawara K., Chishiki M. et al. Risk factors. and treatmentr disseminated intravascular coagulation in neonates. Ital. J. Pediatr. 2020; 46(1): 54. https://dx.doi.org/10.1186/s13052-020-081.5-7.
- Ahn S.Y., Sung D.K., Kim Y.E., Sung S,. Chang Y.S., Park W.S. Brain-derived neurotropic factor mediates neuroprotection of mesenchymal stem cell-derived extracellular vesicles against severe intraventricular hemorrhage in newborn rats. Stem Cells Transl. Med. 2021; 10 (3): 374-384. https://dx.doi.org/10.1002/sctm.20-0301.
- Gall A.R., Amoah S., Kitase Y., Jantzie L.L. Placental mediated mechanisms of perinatal brain injury: Evolving inflammation and exosomes. Exp. Neurol. 2022; 347: 113914. https://dx.doi.org/10.1016/j.expneurol.2021.113914.
- Reiter C.R., Bongarzone E.R. The role of vesicle trafficking and release in oligodendrocyte biology. Neurochem. Res. 2020; 45(3): 620-9. https://dx.doi.org/10.1007/s11064-019-02913-2.
- Gatti M., Zavatti M., Beretti F., Giuliani D., Vandini E., Ottani A. et al. Oxidative stress in Alzheimer's disease: in vitro therapeutic effect of amniotic fluid stem cells extracellular vesicles. Oxid. Med. Cell. Longev. 2020; 2020: 2785343. https://dx.doi.org/110.1155/2020/2785343.
- Antoniou A., Auderset L., Kaurani L., Sebastian E., Zeng Y., Allahham M. et al. Neuronal extracellular vesicles and associated microRNAs induce circuit connectivity downstream BDNF Cell. Rep. 2023; 42(2): 112063. https://dx.doi.org/10.1016/j.celrep.2023.112063.
- Yakovlev A.A. Neuronal exosomes as a new signaling system. Biochemistry (Mosc.). 2023; 88(4): 457-65. https://dx.doi.org/10.1134/S0006297923040028.
- Sarnat H.B. Sequences of synaptogenesis in the human fetal and neonatal brain by synaptophysin immunocytochemistry. Front. Cell. Neurosci. 2023; 17: 1105183. https://dx.doi.org/10.3389/fncel.2023.1105183.
Received 11.02.2025.
Accepted 28.02.2025
About the Authors
Natalia E. Kan, Professor, Dr. Med. Sci., Merited Scholar of the Russian Federation, Deputy Director for Research – Director of the Institute of Obstetrics,Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow,
Ac. Oparin str., 4, kan-med@mail.ru, Researcher ID: B-2370-2015, SPIN: 5378-8437, Authors ID: 624900, Scopus Author ID: 57008835600,
https://orcid.org/0000-0001-5087-5946
Anastasia A. Leonova, PhD student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, +7(937)453-54-27, nastena27-03@mail.ru, https://orcid.org/0000-0001-6707-3464
Vladislava E. Gusar, PhD, Senior Researcher at the Laboratory of Transcriptomic, Department of Systems Biology in Reproduction, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, v_gusar@oparina4.ru,
https://orcid.org/0000-0003-3990-6224
Vitaliy V. Chagovets, PhD, Senior Researcher at the Laboratory of Transcriptomic, Department of Systems Biology in Reproduction, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, vvchagovets@gmail.com,
https://orcid.org/0000-0002-5120-376X
Victor L. Tyutyunnik, Professor, Dr. Med. Sci., Leading Researcher at the Center for Scientific and Clinical Research, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, tioutiounnik@mail.ru,
Researcher ID: B-2364-2015, SPIN: 1963-1359, Authors ID: 213217, Scopus Author ID: 56190621500, https://orcid.org/0000-0002-5830-5099
Maria V. Volochaeva, PhD, Senior Researcher at the Department of Regional Cooperation and Integration, Physician at 1 Maternity Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4,
m_volochaeva@oparina4.ru, https://orcid.org/0000-0001-8953-7952
Ekaterina E. Soldatova, Researcher at the Obstetric Department of the Institute of Obstetrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, katerina.soldatova95@bk.ru, https://orcid.org/0000-0001-6463-3403
Kristina O. Ryzhova, Resident, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia,
117997, Russia, Moscow, Ac. Oparin str., 4, cr.yanina@gmail.com, https://orcid.org/0009-0007-8318-435X
Anna P. Serebriakova, obstetrician-gynecologist of the Day Hospital Department, Primorsky Regional Perinatal Center, 690042, Russia, Vladivostok, Mozhayskaya str., 1B, serebriakovanna@gmail.com, https://orcid.org/0000-0001-7014-2627
Corresponding author: Anastasia A. Leonova, nastena27-03@mail.ru



