The features of the E-cadherin/β-catenin signaling pathway in the placenta and peripheral blood of pregnant women with fetal growth restriction
Krasnyi A.M., Khachaturyan A.A., Tyutyunnik V.L., Sorokina L.E., Kan N.E., Borisova A.G., Krasnova L.D., Sorivko E.R.
It is known that reduced placental size, abnormal development of placental villi and reduced E-cadherin expression are observed in pregnancies with fetal growth restriction.
Objective: To identify the features of the E-cadherin/β-catenin signaling pathway in the placenta and peripheral blood of pregnant women by determining expression of the MMP-9, CCND1 and BIRC5 genes induced by the nuclear β-catenin translocation, as well as WNT2 gene expression, and to analyze the features of apoptosis of the placental cells.
Materials and methods: The study included 82 patients. The main group consisted of 46 women with the postnatal diagnosis of fetal growth restriction without hypertension complications. The control group consisted of 36 patients with normal pregnancy. Investigation of the features of the E-cadherin/β-catenin signaling pathway was carried out by determining expression of the MMP-9, CCND1 and BIRC5 genes induced by the nuclear β-catenin translocation, and WNT2 gene expression in the placental tissue and peripheral blood, as well as analyzing the features of apptosis of placental cells.
Results: It was found that MMP-9 and BIRC5 expression levels in the placenta were significantly higher in fetal growth restriction (p=0.03 and p=0.02, respectively). MMP-9 expression level in the peripheral blood was lower in fetal growth restriction (p=0.001). Increased apoptosis of the placental cells was observed in fetal growth restriction (p=0.03). Excessive apoptosis of trophoblast cells was in fetal growth restriction compared with normal pregnancy (p=0.017).
Conclusion: Lower level of E-cadherin expression in the placenta in fetal growth restriction can be associated with activation of the E-cadherin/β-catenin signaling pathway and development of apoptosis of placental trophoblast cells.
Authors' contributions: Krasnyi A.M., Khachaturyan A.A., Tyutyunnik V.L., Sorokina L.E., Kan N.E., Borisova A.G., Krasnova L.D., Sorivko E.R. – the concept and design of the study, data obtaining for analysis, literature review, processing and analysis of material on the topic of the study, manuscript writing, article editing.
Conflicts of interest: The authors confirm that they have no conflicts of interest to declare.
Funding: The study was conducted without any sponsorship.
Ethical Approval: The study was approved by the local Ethics Committee of the Academician V.I. Kulakov National Medical Research Centre for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia.
Patient Consent for Publication: The patients have signed informed consent for publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Krasnyi A.M., Khachaturyan A.A., Tyutyunnik V.L., Sorokina L.E., Kan N.E., Borisova A.G.,
Krasnova L.D., Sorivko E.R. The features of the E-cadherin/β-catenin signaling pathway in the
placenta and peripheral blood of pregnant women with fetal growth restriction.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (5): 49-56 (in Russian)
https://dx.doi.org/10.18565/aig.2025.43
Keywords
According to the modern concept, fetal growth restriction (FGR) is considered as polyetiologic pathological condition, when the fetus is unable to reach its genetically programmed growth potential and has a high risk of perinatal complications [1]. According to data from varisous sources, FGR complicates up to 10–15% of all pregnancies every year. Despite the heterogeneity of this complication, it is believed that it is of placental origin, that is the most common cause of its development [2]. Placental dysfunction is defined as the pathophysiological basis, and manifests itself in placental dysfunction with preceding cascade of pathological processes at several levels of regulation [3, 4].
It is known that the physiology of placentation is based on the balanced differentiation of different types of cells. The canonical Wnt signaling pathway, which regulates embryogenesis, cell differentiation, etc. plays the central role in normal placental development [5–7]. Activation of Wnt signaling leads to a complex cascade of intracellular reactions, resulting in stabilization and migration of β-catenin into the cell nucleus. β-catenin molecule acts as a cofactor of the signaling pathway and regulates the processes of cell proliferation, differentiation, and apoptosis. It is believed that activation of the canonical Wnt signaling occurs with formation of a specific binding interaction between the WNT ligand and the Frizzled receptor (FZD) [8, 9]. At least ten WNT ligands were identified in the placenta. Among them, WNT2 showed the highest levels of expression [10]. The canonical Wnt signaling pathway is a mechanism associated with stabilization of the key intracellular element of this signaling pathway – the cytoplasmic protein β-catenin (Wnt/β-catenin signaling pathway). The transmission of Wnt signals is activated by ligands. Also, one of the regulators of the Wnt signaling pathway is the cytoplasmic protein β-catenin. After binding of the Wnt ligand to the transmembrane receptor Frizzled and co-receptors – lipoprotein receptor-related proteins 5 (LRP5) and 6 (LRP6), the cytoplasmic protein DVL activates and promotes degradation of the APC/Axin/CK1a (casein kinase 1a)/GSK3β (glycogen synthase kinase 3 β) complex leading to stabilization and accumulation of the active form of β-catenin unphosphorylated in the cytoplasm. The accumulated β-catenin migrates to the nucleus. It binds to the TCF/LEF family – a group of transcription factors (T-cell factor/lymphoid enhancer-binding factor), which subsequently activate the transcription of target genes. In the absence of Wnt ligands, when the canonical Wnt pathway is not activated, the APC/Axin/CK1a/GSK3β complex binds to β-catenin and causes phosphorylation, that is inactivation of β-catenin and its proteasomal degradation.
The cell adhesion molecule E-cadherin plays an important role in regulation of the nuclear translocation of β-catenin. It binds to β-catenin in the perimembrane region and forms a strong complex that blocks the intracellular migration of this protein. These data indicate that E-cadherin can act as a regulator of β-catenin-dependent gene transcription [11, 12]. Our previous studies showed that E-cadherin expression level is lower in FGR placental villi, that can be critical in the expression of β-catenin-dependent genes, such as MMP-9, CCND1, and BIRC5 [13].
In addition, the previous studies suggested that decreased E-cadherin levels can be directly associated with increased cellular pro-apoptotic activity [14], that can be due to smaller placental size in FGR.
The objective of the study was to identify the features of the E-cadherin/β-catenin signaling pathway in the placenta and blood of pregnant women by determination of expression of MMP-9, CCND1 and BIRC5 genes induced by nuclear translocation of β-catenin, as well as expression of WNT2 gene and to explore the apoptosis of the placental cells.
Materials and methods
The study was carried out at the Academician V.I. Kulakov National MedicalResearch Centre for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. The study included 82 women, who were divided into 2 groups. The main group consisted of 46 patients with the postnatal diagnosis of fetal growth restriction without hypertension complications. The control group consisted of 36 patients with normal pregnancy.
The diagnosis of fetal growth restriction was made in accordance with the clinical recommendations approved by the Minstry of Health of Russia “Fetal growth restriction, requiring maternal healthcare” (slowing of fetal growth velocity; the estimated fetal weight (EFW) and/or abdominal circumference (AC) <10th percentile in combination with abnormal blood flow according to Doppler ultrasound, or EFW and/or AC <3rd percentile [1].
Inclusion criteria in the study were the following: the age of pregnant women 22–45 years; 35–40 weeks of pregnancy; singleton pregnancy complicated with FGR (the main group); normal pregnancy (the control group); obtaining of informed consent to participate in the study.
Exclusion criteria were ages below 22 years and over 45 years; multiple pregnancy; pregnancy achieved by using assisted reproductive technologies; decompensated extragenital pathology; acute infectious, oncological diseases, and mental disorders; hypertensive complications during pregnancy; antiphospholipid syndrome; fetal malformations.
All women enrolled in the study underwent standard examination, including collection and evaluation of somatic, obstetric and gynecological anamnesis, laboratory and instrumental tests.
Investigation of the features of the E-cadherin/β-catenin signaling pathway in pregnant women was carried out by determining the expression of MMP-9, CCND1 and BIRC5 genes caused by the nuclear β-catenin translocation, expression of the WNT2 gene in the placental tissue and the peripheral blood, as well as by studying the features of the apoptosis of placental cells.
Detection of gene expression in the peripheral blood and the placental tissue
Venous blood samples were collected from the cubital vein in the morning, on an empty stomach, in the Vacuette blood collection tubes with an anticoagulant. The samples were processed within 30 minutes after material collection. Then 200 µl of ExtractRNA lysis buffer (“Evrogen”, Russia) was added to 200 µl of whole blood. The samples were subsequently stored at -80°C.
Placental tissue samples were collected immediately following childbirth. Small pieces of villous placental tissue (0.5×0.5 cm) were collected in the area about 3 cm from the umbilical cord. The fragments of placental tissue were placed in cryo-tube containing RNAlater solution and then stored at -80°C.
Total RNA was extracted from the samples of peripheral blood and placental tissue using phenol extraction. The degree of purification and concentration of the isolated RNA were determined using the DeNovix fluorimeter (“TermoFisher”, USA). Each isolated RNA sample was converted to cDNA using the OT-1 reverse transcription kit (“Syntol”, Russia).
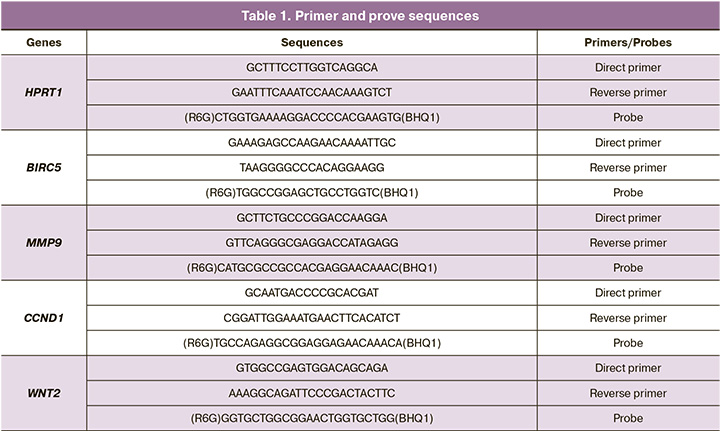
Amplification of RNA for gene expression of MMP-9, CCND1, BIRC5, WNT2 genes and housekeeping gene HPRT1 by real-time polymerase chain reaction (RT-PCR) with specific primers and probes for the analyzed gene regions was performed using the CFX-96 device (“Bio-Rad”, USA). Primer and probe sequences are represented in Table 1.
RT-PCR protocol was the following: 95°С – 5 minutes; 40 cycles: 95°С – 10 sec, 60°С – 25 sec.
Analysis of the features of the apoptosis of placental cells
Placental tissue was fixed for 24 h in 4% paraformaldehyde solution in phosphate-buffered saline (PBS) at 4°C. Then without washing, they were transferred into 30% sucrose for another 24 hours at 4°C. Subsequently, the tissue was frozen in the cryo embedding medium using Tissue-Tek OCT Compound. (“Sakura Finetek”, USA). Sections (12 µm thick) were obtained in the Microm HM525 cryostat (“Thermo Scientific”, Great Britain). The TUNEL reaction was performed using In Situ Cell Death Detection Kit, Fluorescein (“Roche”, Germany) according to the manufacturer’s protocols. The sections were examined using a Leica SP5 confocal microscope (Germany). Each placental sample was examined using 5 fields of view. The number of apoptotic cells was represented as a percentage of the total number.
Conjugated E-cadherin antibodies were used to differentiate trophoblast cells from stromal cells (“R&D systems”, USA).
Apoptosis assessment was performed in all E-cadherin-positive trophoblast cells.
Statistical analysis
Statistical data processing was perfomed using Microsoft Excel, OriginPro 8 и SPSS Statistics 17. The data are represented as median (Мe) and upper and lower quartiles (Q25%; Q75%). The quantitative data were compared using the Mann–Whitney U test. Fisher’s exat test was used for comparison of categorical variables between the groups. The threshold was considered statistically significant at p<0.05.
Results
The clinical characteristics of patiens is represented in Table 2.
There were no differences between the groups of patients in BMI and age. However, impaired uteroplacental blood flow was significantly more often in the group of patients with FGR. Gestational age was significantly less in the main group versus the control group. In the main group, pretern birth was in 11/46 women (23.9%), and term birth was in the others.

Analysis of gene expression in the placental tissue and peripheral bollod found that MMP-9 and BIRC5 expression levels in placental tissue were significantly higher in patients in the main group compared with women in the control group (р=0.03 and р=0.02, respectively). There were no significant differences between the groups in the expression of the CCND1, WNT2 genes (Fig. 1). The comparison of patients with preterm and term birth in the main group found no significant differences in the expression levels of the MMP-9 and BIRC5 genes in the placental tissue; the values for MMP-9 were 2.4 (1.1; 6.9) and 2 (1.4; 7), respectively, р=0.62; and for BIRC5 – 1.6 (1.5;3.2) and 1.5 (1.2; 4.7), respectively, р=0.7.
The comparison of gene expression in the maternal blood flow showed low expression levels of MMP-9 in the main group versus the control group (р=0.001). There were no significant differences between the groups in the expression levels of CCND1 and BIRC5. It should be noted that there was no expression of the WNT2 gene in patients in both groups (Fig.2).
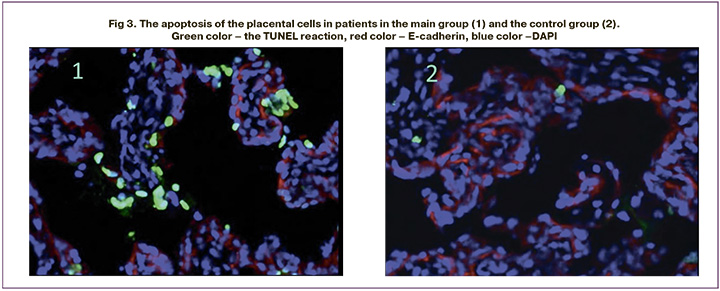
Assessment of the apoptosis in the placental cells showed higher apoptotic activity in patients in the main group compared with the control group; the values were 0.16 (0.08; 0.23)% and 0.09 (0.04; 0.16)%, respectively (р=0.03). At the same time, in the presence of FGR, trophoblast apoptosis increased in the placentas versus stromal cells. The comparative analysis of the apoptotic activity of trophoblast cells showed that in FGR apoptosis was more pronounced compared with normal pregnancy – 0.15 (0.04; 0.24)% and 0.07 (0,04; 0,15)%, respectively (р=0.017) (Fig. 3).
Discussion
In recent decades, the scientists have focused on the effects of the Wnt signaling pathway, which plays a key role in cell proliferation, differentiation and apoptosis during the embryonic development. [5–7].
Previously conducted foreign studies indicate the activation of Wnt signaling in FGR. The study by Sola I.M. et al. [15] showed higher expression of WNT5A protein and β-catenin in the placental tissue of patients with FGR compared with women with normal pregnancy. These results are consistent with the previously published results [16], demonstrating higher Dishevelled family proteins (DVL1-3) expression in IUGR-related placentas. Being Wnt signaling members, these proteins promote accumulation of β-catenin in the cytoplasm [17].
The data obtained in our study does not exclude the results of foreigh researchers. Expression level of the MMP-9 и BIRC5 genes was significantly higher in the placental tissue of patients in the main group, that indicates an increase in the translocation of a portion of β-catenin into the nucleus with subsequent triggering of activation cascade of transcription factors and genes. At the same time the WNT2 gene, the protein product of which is a key player in the canoninc signaling pathway in the placenta [10], did not demonstrate significant differences between the groups in the expression level. The absence of significant differences in expression of WNT2 suggests that the Wnt signaling pathway is not involved in the development of FGR. However, increased expression of β-catenin-dependent genes (MMP-9 and BIRC5) , can be explained by reduced expression of E-cadherin in FGR, as we have previously established [13], and as a consequence, reduced expression of E-cadherin/β-catenin complexes and the appearance of free β-catenin.
Gene expression in the maternal blood flow was assessed to identify noninvasive diagnostic markers. Comparative analysis found no association between gene expression in the placental tissue and systemic circulation. At the same time, significantly low expression level of MMP-9 was identified in women in the main group. According to literature data, MMP-9 I in leukocytes participates in various inflammatory reactions, such as permeability of physical barriers, leukocyte migration cytokine secretion [18]. Therefore, reduced MMP-9 expression in women with FGR may indicate dysregulation of the immune response.
It is known that with the development of placental complications during pregnancy, there is elevated activation of villous trophoblast apoptosis compared with normal pregnancy, that indicates the importance of normal regulation of these processes during trophoblast invasion and subsequent development [19]. The data represented by us is partially consistent with the data in the studies by Smith S.C. et al. [20] and Ishihara N. et al. [21], which also demonstrated increased placental cell apoptosis in the development of FGR. However, their studies showed no differences in the ratio of the apoptosis in the villous trophoblast to placental stroma.
At the same time, the mechanisms of intensification of apoptosis in FGR remain unclear. Han H.J. et al.[14] noted that reduced levels of E-cadherin can be directly associated with increased cellular pro-apoptotic activity. Taking into account previously identified decreased levels of transmembrane and soluble E-cadherin in patients with FGR, there are reasons to believe that this mechanism underlies the increased number of trophoblastic cells with the signs of apoptosis in women with this pregnancy pathology. Our study expanded the knowledge of the pathophysiological mechanisms of placental complications during pregnancy. In particular, it was found that E-cadherin is a regulator of the WNT signaling pathway. However, regulatory mechanisms cadherin expression are not fully understood, that indicates the prospects for further research in this area.
Conclusion
Thus, the identified placental MMP-9 and BIRC5 overexpression caused by the nuclear β-catenin translocation, as well as development of apoptosis of placental trophoblast cells in patients with FGR can be associated with reduced expression of E-cadherin/β-catenin complexes. Reduced MMP-9 expression in the peripheral blood of women with FGR may indicate dysregulation of the immune response. In view of the above, it seems appropriate to conduct additional in-depth studies to expand understanding of the pathophysiological mechanisms of FGR at the molecular level.
References
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Недостаточный рост плода, требующий предоставления медицинской помощи матери (задержка роста плода). 2022. [Ministry of Health of the Russian Federation. Clinical guidelines. Insufficient growth of the fetus, requiring the provision of medical care to the mother (fetal growth retardation). 2022. (in Russian)].
- Gęca T., Stupak A., Nawrot R., Goździcka-Józefiak A., Kwaśniewska A., Kwaśniewski W. Placental proteome in late‑onset of fetal growth restriction. Mol. Med. Rep. 2022; 26(6): 356. https://dx.doi.org/10.3892/mmr.2022.12872
- Malhotra A., Allison B.J., Castillo-Melendez M., Jenkin G., Polglase G.R., Miller S.L. Neonatal morbidities of fetal growth restriction: pathophysiology and impact. Front. Endocrinol. (Lausanne). 2019; 10: 55. https://dx.doi.org/10.3389/fendo.2019.00055
- Закурина А.Н., Павлова Н.Г. Внутриплацентарный кровоток в III триместре беременности, осложненной плацентарной недостаточностью. Журнал акушерства и женских болезней. 2014; 63(5): 51-7. [Zakurina A.N., Pavlova N.G. Intraplacental blood flow in third trimester of placental insufficiency pregnancy. Journal of Obstetrics and Women's Diseases. 2014; 63(5): 51-7. (in Russian)]. https://dx.doi.org/10.17816/JOWD63551-57
- Knöfler M, Pollheimer J. Human placental trophoblast invasion and differentiation: A particular focus on Wnt signaling. Front. Genet. 2013; 4: 190. https://dx.doi.org/10.3389/fgene.2013.00190
- Matsuura K., Jigami T., Taniue K., Morishita Y., Adachi S., Senda T. et al. Identification of a link between Wnt/β-catenin signalling and the cell fusion pathway. Nat. Commun. 2011; 2: 548. https://dx.doi.org/10.1038/ncomms1551
- Aoki M., Mieda M., Ikeda T., Hamada Y., Nakamura H., Okamoto H. R-spondin3 is required for mouse placental development. Dev. Biol. 2007; 301(1): 218-26. https://dx.doi.org/10.1016/j.ydbio.2006.08.018
- Clevers H., Nusse R. Wnt/b-catenin signaling and disease. Cell. 2012; 149(6): 1192-205. https://dx.doi.org/10.1016/j.cell.2012.05.012
- Liu J., Xiao Q., Xiao J., Niu C., Li Y., Zhang X. et al. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target Ther. 2022; 7(1): 3. https://dx.doi.org/10.1038/s41392-021-00762-6
- Sonderegger S., Husslein H., Leisser C., Knöfler M. Complex expression pattern of Wnt ligands and frizzled receptors in human placenta and its trophoblast subtypes. Placenta. 2007; 28 Suppl A(Suppl A): S97-S102. https://dx.doi.org/10.1016/j.placenta.2006.11.003
- Du W., Liu X., Fan G., Zhao X., Sun Y., Wang T. et al. From cell membrane to the nucleus: an emerging role of E-cadherin in gene transcriptional regulation. J. Cell. Mol. Med. 2014; 18(9): 1712-9. https://dx.doi.org/10.1111/jcmm.12340
- Xu W., Kimelman D. Mechanistic insights from structural studies of β-catenin and its binding partners. J. Cell Sci. 2007; 120(Pt 19): 3337-44. https://dx.doi.org/10.1242/jcs.013771
- Красный А.М., Хачатурян А.А., Кан Н.Е., Хачатрян З.В., Тютюнник В.Л., Волгина Н.Е., Ганичкина М.Б., Мантрова Д.А., Садекова А.А. Роль Е-кадгерина в формировании задержки роста плода. Акушерство и гинекология. 2018; 6: 38-43. [Krasnyi A.M., Khachaturyan A.A., Kan N.E., Khachatryan Z.V., Tyutyunnik V.L., Volgina N.E., Ganichkina M.B., Mantrova D.A., Sadekova A.A. The role of E-kadherin in the formation of intrauterine growth restriction. Obstetrics and Gynecology. 2018; (6): 38-43. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.6.38-43
- Han H.J., Kwon H.Y., Sohn E.J., Ko H., Kim B., Jung K. et al. Suppression of E-cadherin mediates gallotannin induced apoptosis in Hep G2 hepatocelluar carcinoma cells. Int. J. Biol. Sci. 2014; 10(5): 490-9. https://dx.doi.org/10.7150/ijbs.7495
- Sola I.M., Karin-Kujundzic V., Paic F., Lijovic L., Glibo M., Serman N. et al. WNT5A, β‑catenin and SUFU expression patterns, and the significance of microRNA deregulation in placentas with intrauterine growth restriction. Mol. Med. Rep. 2023; 27(2): 28. https://dx.doi.org/10.3892/mmr.2022.12914
- Sola I.M., Serman A., Karin-Kujundzic V., Paic F., Skrtic A., Slatina P. et al. Dishevelled family proteins (DVL1-3) expression in intrauterine growth restriction (IUGR) placentas. Bosn. J. Basic Med. Sci. 2021; 21(4): 447-53. https://dx.doi.org/10.17305/bjbms.2020.5422
- Gan X.Q., Wang J.Y., Xi Y., Wu Z.L., Li Y.P., Li L. Nuclear Dvl, c-Jun, beta-catenin, and TCF form a complex leading to stabilization of beta-catenin-TCF interaction. J. Cell Biol. 2008; 180(6): 1087-100. https://dx.doi.org/10.1083/jcb.200710050
- Manicone A.M., McGuire J.K. Matrix metalloproteinases as modulators of inflammation. Semin. Cell Dev. Biol. 2008; 19(1): 34-41. https://dx.doi.rg/10.1016/j.semcdb.2007.07.003
- Crocker I.P., Cooper S., Ong S.C., Baker P.N. Differences in apoptotic susceptibility of cytotrophoblasts and syncytiotrophoblasts in normal pregnancy to those complicated with preeclampsia and intrauterine growth restriction. Am. J. Pathol. 2003; 162(2): 637-43. https://dx.doi.org/10.1016/S0002-9440(10)63857-6
- Smith S.C., Baker P.N., Symonds E.M. Increased placental apoptosis in intrauterine growth restriction. Am. J. Obstet. Gynecol. 1997; 177(6): 1395-401. https://dx.doi.org/10.1016/s0002-9378(97)70081-4
- Ishihara N., Matsuo H., Murakoshi H., Laoag-Fernandez J.B., Samoto T., Maruo T. Increased apoptosis in the syncytiotrophoblast in human term placentas complicated by either preeclampsia or intrauterine growth retardation. Am. J. Obstet. Gynecol. 2002; 186(1): 158-66. https://dx.doi.org/10.1067/mob.2002.119176
Received 24.02.2025
Accepted 17.04.2025
About the Authors
Aleksey M. Krasnyi, PhD, Head of the Cytology Laboratory, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology,Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, alexred@list.ru, https://orcid.org/0000-0001-7883-2702
Anuta A. Khachaturyan, PhD student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, x.anyt37@mail.ru, https://orcid.org/0009-0007-3767-9343
Victor L. Tyutyunnik, Professor, Dr. Med. Sci., Leading Researcher at the Center of Scientific and Clinical Researches, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, tioutiounnik@mail.ru,
Researcher ID: B-2364-2015, SPIN: 1963-1359, Authors ID: 213217, Scopus Author ID: 56190621500, https://orcid.org/0000-0002-5830-5099
Leia E. Sorokina, Junior Researcher at the Cytology Laboratory, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, leya.sorokina@mail.ru, https://orcid.org/0000-0002-1862-6816
Natalia E. Kan, Professor, Dr. Med. Sci., Honored Scientist of the Russian Federation, Deputy Director of Science, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, kan-med@mail.ru, Researcher ID: B-2370-2015, SPIN: 5378-8437, Authors ID: 624900, Scopus Author ID: 57008835600, https://orcid.org/0000-0001-5087-5946
Anastasia G. Borisova, PhD student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4 +7(968)735-40-81, vvv92@list.ru, https://orcid.org/0009-0004-5234-1584
Lidia D. Krasnova, 5th year student of the Institute of Clinical Medicine, majoring in General Medicine, N.I. Pirogov Russian National Research Medical University,
Ministry of Health of Russia, 117513, Russia, Moscow, Ostrovityanova str., 1, bld. 6, li.kr.2402@gmail.com, https://orcid.org/0009-0009-2718-3672
Evgeny R. Sorivko, obstetrician-gynecologist, Melitopol Regional Perinatal Center, 272301, Russia, Melitopol, Kiziyarskaya str., 37, awwgxtf@gmail.com
Corresponding author: Aleksey M. Krasnyi, alexred@list.ru



