Fetal sex as a risk factor for fetal growth restriction or small for gestational age
Ziyadinov A.A., Novikova V.A., Radzinsky V.E.
Objective: To evaluate the association between fetal sex and insufficient fetal growth (IFG), including fetal growth restriction (FGR) and small for gestational age (SGA).
Materials and methods: A prospective cohort study was conducted at N.A. Semashko Republican Clinical Hospital from 2018 to 2023. A total of 611 women with singleton pregnancies diagnosed with IFG were included in the study, with FGR (n=435) and SGA (n=176).
Results: Fetal sex in patients with IFG was associated with various factors, including parental age, age at menarche, maternal BMI, weight gain, blood pressure, hemoglobin levels, leukocyte counts, ALT and AST levels, blood creatinine, Doppler data of uteroplacental blood flow, CTG results, IFG variant (FGR or SGA), causes of IFG, prematurity, low birth weight (LBW), and the need for intensive care (IC) for the newborn. FGR, compared to SGA, was characterized by (p<0.05–0.001) a younger age of both parents, earlier age at menarche, greater weight gain, lower systolic and diastolic blood pressure in the first trimester, lower CTG indicators, lower hemoglobin levels, but higher creatinine, ALT, and AST levels, and an earlier gestational age at delivery. Female fetuses are more commonly associated with FGR, impaired blood flow in the uterine and middle cerebral arteries, LBW, severe preeclampsia (PE), and gestational arterial hypertension as causes of IFG as well as a higher need for IC in newborns. Male fetuses are more often linked to unknown causes of IFG, chronic arterial hypertension, moderate PE, and prematurity. Decision tree analysis revealed that the IFG variant is associated with fetal sex, parental age, the cause of IFG, LBW, and the need for IC.
Conclusion: Fetal sex is interconnected with the IFG variant and numerous factors that contribute to or result from this condition. The specific nature of this relationship is influenced by the IFG variant (FGR or SGA), parental age, maternal and fetoplacental hemodynamics, and the maternal hemic, hepatic, and renal responses to pregnancy complicated by IFG. It is advisable to implement individualized centile tables adjusted for fetal sex during ultrasound monitoring.
Authors' contributions: Ziyadinov A.A. – conception and design of the study, data analysis, drafting and editing of the manuscript, interpretation of results, approval of the manuscript for submission; Novikova V.A. – conception and design of the study, statistical analysis and interpretation of results; Radzinsky V.E. – conception and design of the study, editing of the manuscript, approval of the manuscript for submission.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the Patrice Lumumba Peoples' Friendship University of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Ziyadinov A.A., Novikova V.A., Radzinsky V.E. Fetal sex as a
risk factor for fetal growth restriction or small for gestational age.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (8): 109-122 (in Russian)
https://dx.doi.org/10.18565/aig.2025.164
Keywords
Insufficient fetal growth (IFG) poses a long-term risk, primarily determined by the presence of fetal growth restriction (FGR) or small for gestational age (SGA). The pathogenesis of IFG is complex and debated, making it a topic of interest among obstetrician-gynecologists, neonatologists, geneticists, microbiologists, cardiologists, endocrinologists, pediatricians, and others. A particular focus is on the potential link between FGR and fetal sex. The longstanding debate between opponents [1] and proponents [2] of this connection has evolved over the years, and most arguments currently support this hypothesis. In the absence of a clear understanding of intrauterine programming mechanisms, many researchers associate FGR more frequently with the female sex [3–5]. Female fetal sex is identified as an independent risk factor for preeclampsia (PE) or FGR in singleton pregnancies, with adjusted odds ratios (OR) of 1.169 and 1.563 (95% confidence interval (CI): 1.036–1.319 and 1.349–1.810, respectively). Additionally, female twins are regarded as an independent risk factor for PE, with an adjusted OR of 1.367 (95% CI: 1.011–1.849), particularly for early PE [6].
Several explanations for these observations have been proposed. Recent studies (2024) indicate that fetal development is mediated through the gut-placenta axis; for instance, maternal galectin-3 deficiency (which binds to the β-galactosidase protein gal-3) leads to dysbiosis of the maternal gut microbiome, resulting in sex-specific (primarily female) FGR [7]. Conversely, prenatal exposure to phthalates is more closely associated with an increased risk of FGR in males [8]. An unexpected interaction has been identified between fetal sex, preexisting diabetes mellitus (DM), and presumed fetal weight. Female sex is linked to a higher risk of perinatal morbidity (OR=1.3, 95% CI: 1.1–1.5), particularly in FGR cases with presumed fetal weights in the 3rd to 9th percentiles (OR=1.6, 95% CI: 1.3–2.1) [9]. Differences in blood pressure reactivity, renal function, and glucose metabolism responses to postnatal high-fat and high-sugar diets have been observed in offspring of both sexes with FGR, suggesting that FGR programs sex-specific cardiorenal and metabolic risks in offspring, with the female sex being considered more vulnerable [10].
The association between SGA and sex-related ante- and neonatal complications and outcomes has been less extensively studied. Fetal sex in SGA infants is more frequently linked to adverse neonatal outcomes [11], developmental features at one year [12], and various other factors [13, 14].
The roles of fetal sex specificity and paternal-maternal factors in SGA risk remain largely unexplored. Fetal sex specificity is thought to be influenced by genetic, epigenetic, or hormonal responses of both parents. Specifically, SGA is associated with paternal height and BMI in male fetuses and maternal height and BMI in female fetuses [15].
The sex-specific effects of SGA persist up to 12 months of age, particularly in boys [12]. The causes of SGA are multifactorial, including maternal lifestyle and obstetric factors, placental dysfunction, and various fetal (epi)genetic anomalies [16]. Current potential confounders for prenatally diagnosed SGA and neonatal complications include family status (whether the mother lives alone or with a partner), maternal age, geographic origin, smoking status, BMI at the beginning of pregnancy, number of previous births, previous cesarean sections, and previous complications or adverse pregnancy outcomes. Birth weight and male fetal sex are recognized as significant factors [17]. Some researchers have suggested that intrauterine adaptation to environmental changes exhibits important sex-specific patterns, with male fetuses being more vulnerable to adverse intrauterine factors than females [12]. The contentiousness of this issue is further complicated by studies that found no association between fetal sex and SGA compared with normal weight [18]. There are virtually no studies comparing the relationship between fetal sex, FGR, and SGA.
This study aimed to evaluate the association between fetal sex and the risk of FGR and SGA.
Materials and methods
This retrospective cohort study was conducted at N.A. Semashko Republican Clinical Hospital from 2018 to 2023. Of the total cohort of 24,282 patients who delivered during this period, 611 women were included in the study and stratified by the IFG variant (FGR or SGA), with FGR (n=435) and SGA (n=176). The inclusion criteria for the study were singleton pregnancy, presence of IFG (FGR or SGA) confirmed after delivery, a dominant cause of IFG (PE (severe or moderate), DM (gestational DM or type 1 DM), chronic arterial hypertension (CAH), gestational arterial hypertension (GAH), or unknown cause), and live birth. Patients with multiple pregnancies or discrepancies between ante- and postnatal diagnoses of IFG as well as those with two or more competing causes of IFG were excluded from the study.
The clinical outcomes (endpoints) studied were the sex of the newborn and IFG variant. The criteria for assessing FGR and SGA fully corresponded to the national clinical guidelines.
Statistical analysis
Mathematical and statistical analyses were performed using Statistica 12.0 and Microsoft Excel 2016. The number of variations (n), mean (M), and standard deviation (SD) were calculated. The significance of differences (p) was assessed using Student's t-test. The significance of the differences in outcomes influenced by the studied factors was evaluated using the chi-square test (χ²), with Yates' correction applied when n-10. The relationship between the studied factors and the outcome/group was assessed based on odds ratios (OR) with 95% confidence intervals (CI). In the presence of multiple factor variables, the relationship with the outcome variable was assessed using arbitrary contingency tables and analyzed using the χ² test. The Feature Selection module was used to identify highly informative (p<0.05) risk factors for the outcomes.
The data were subjected to mathematical processing to select the optimal set of indicators most likely to be interconnected with FGR or SGA. The prediction of the classification of the studied feature into one of the classes of the categorical dependent variable was based on the construction of decision trees (classification trees). This method was chosen because of its capacity for multidimensional exploratory analysis to solve the classification problem, allowing for the prediction of object classification. Exhaustive chi-squared automatic interaction detection (CHAID) was utilized in the Classification Trees module. Predictors of the studied outcome (categorical response) were identified in the data sample. The resulting classification model was considered qualitative, as indicated by an area under the ROC (Receiver Operating Characteristic) curve (AUC) greater than 0.8.
Using the Feature Selection module of Statistica 12.0, variables closely related to the studied outcome (p<0.05) were selected.
Results and discussion
From the entire dataset, we used the Feature Selection module of Statistica 12.0, to select variables that were closely (p<0.05) related to fetal sex (Table 1).
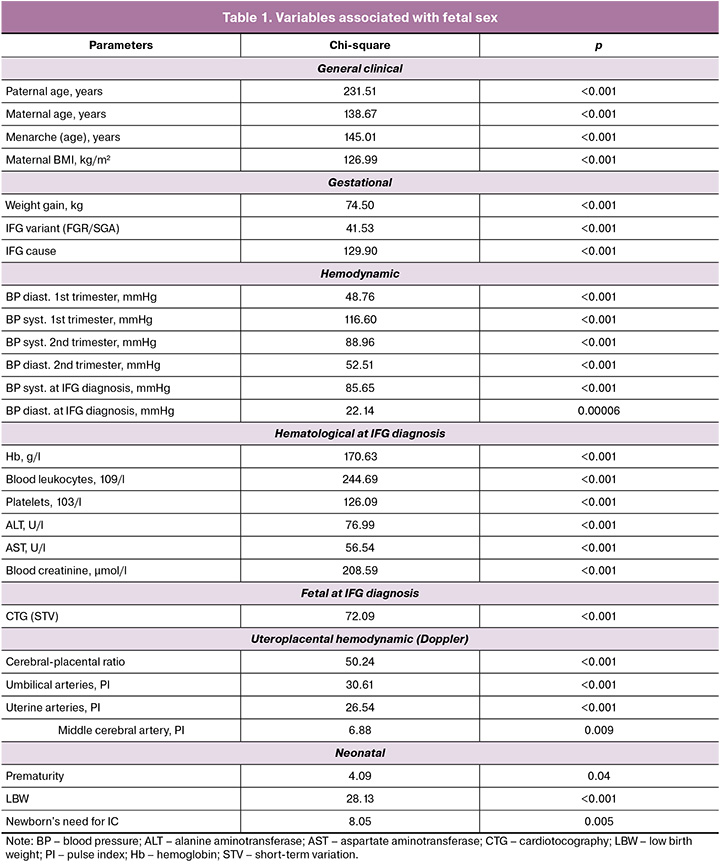
Conditionally, these parameters can be divided into pregestational (general clinical) and gestational, which are formed in the progress of pregnancy, recorded during the diagnosis of IFG and after birth: hemodynamic (maternal – BP; maternal-fetal – uteroplacental blood flow) and hematological (reflecting many functions of the pregnant woman including hemic, hepatic and renal), fetal electrophysiological (cardiotocography (CTG)), the cause of IFG, the outcome of gestation for the newborn (variant of IFG (FGR/SGA), prematurity, LBW, need for intensive care (IC)).
When assessing the causes of IFG, it was noted that in 45.5% (278/611) it was unknown, in 22.59% (138/611) it was caused by severe PE, in 2.62% (16/611) – moderate, in 12.77 (78/611) – GDM, in 8.67% (53/611) – GAH, in 7.86% (48/611) – CAH. Preterm delivery occurred with a frequency of 24.22% (148/611), a fetus with low body weight – 46.97% (287/611). IC was needed in 52.21% of newborns, with respiratory support in 20.95% (128/611). The previously mentioned association between fetal sex and categorical parameters was confirmed. Fetal sex was interconnected not only with the IFG variant (FGR or SGA) but also with the cause of IFG, prematurity, low birth weight, and the need for IC (Fig. 1).
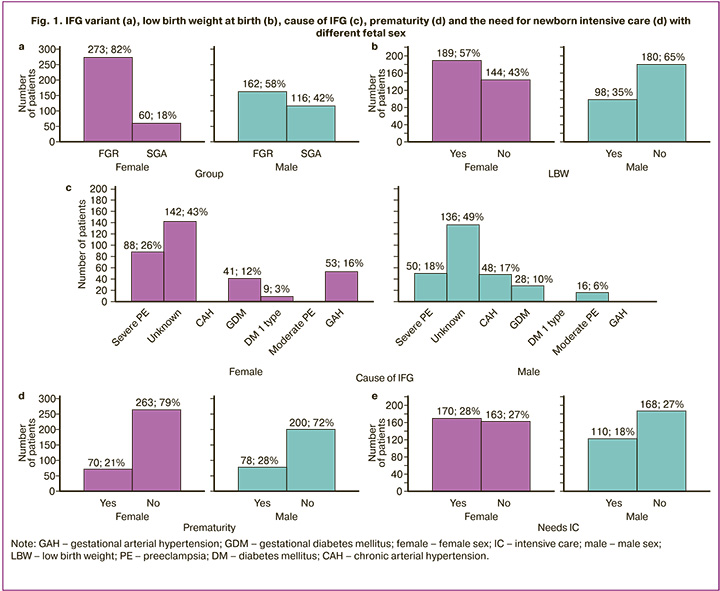
FGR was more characteristic for females (273/333 versus 162/278, OR=3.26, 95% CI 2.26–4.70), LBW (189/333 versus 98/278, OR=2.41, 95% CI 1.74–3.35), severe PE (88/333 versus 50/278, OR=1.64, 95% CI 1.11–2.42), GAH (53/333 versus 0/278, χ2=46.46, p<0.001) as the cause of IFG, the need for IC of the newborn (170/333 versus 110/278, OR=1.59, 95% CI 1.15–2.20); for males – CAH (48/278 versus 0/333, χ2=41.52, p<0.001) or moderate PE (16/278 versus 0/333, χ2=11.90, p<0.001), prematurity (78/278 versus 70/333, OR=1.47, 95% CI 1.01–2.12). A trend of greater association of males with an unknown cause of IFG was revealed (136/278 versus 142/333, OR=1.29, 95% CI 0.94–1.77).
Fetal sex was reflected in the ultrasound Doppler of uteroplacental blood flow (Fig. 2), a method that has been established for the differential diagnosis of FGR and SGA [20].
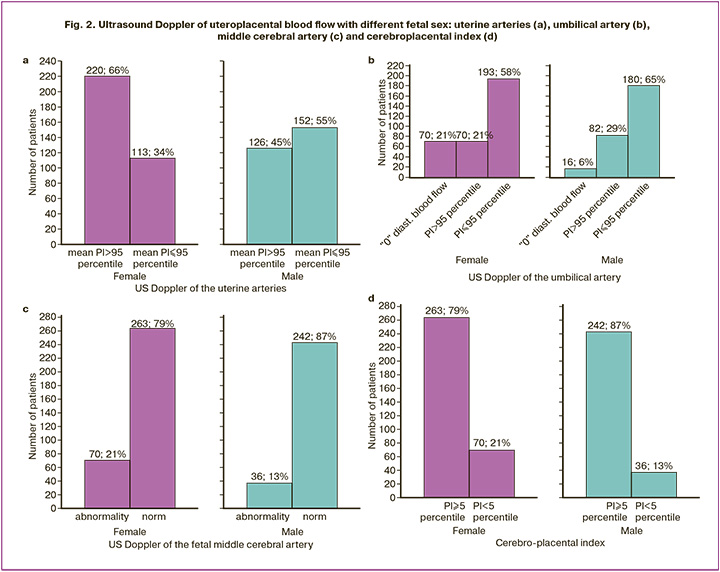
More specific for female fetuses was Doppler uterine abnormalities (mean PI>95th percentile) (220/333 versus 126/278, OR=2.35, 95% CI 1.69–3.26) and middle cerebral arteries ((PI<5th percentile) (70/333 versus 36/278, OR=1.79, 95% CI 1.15–2.77), as well as the cerebroplacental index (70/333 versus 36/278, OR=1.79, 95% CI 1.15–2.77); for males – Doppler violation of the umbilical artery (mean PI>95th percentile) (82/278 versus 70/333, OR=1.57, 95% CI 1.09–2.27).
The relationship between fetal sex, IFG variants, and numerical parameters is presented in Table 2.
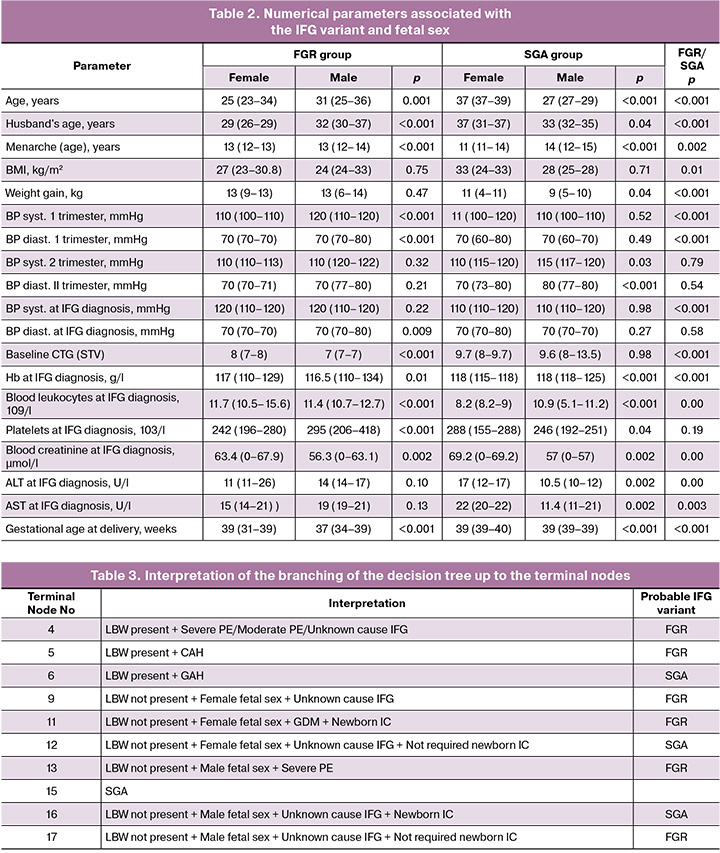
Differences in the sex of the newborn determined most of these parameters. In FGR, the female sex of the newborn differed from that of the male: younger age of the mother and father of the child, earlier menarche, lower systolic and diastolic BP in the first trimester of pregnancy, and diastolic BP during the diagnosis of IFG; higher CTG (STV) indicators during the diagnosis of IFG; higher hemoglobin, leukocyte, creatinine, and lower platelets in the blood during the diagnosis of IFG; and later gestational age at delivery.
In SGA, the female sex of the newborn differed from that of the male: older age of the mother and father of the child, earlier menarche, greater weight gain, lower systolic and diastolic BP in the second trimester of pregnancy; in the blood during the diagnosis of IFG, lower Hb, leukocyte, and higher - platelets, creatinine, ALT, and AST; and later gestational age at delivery.
The method of exploratory data mining, decision trees, allowed us to reveal a probable relationship between the IFG variant, sex of the newborn, and other factors presented earlier, as shown in Figure 3.
There are 13 terminal nodes in this tree, each of which demonstrates the most likely IFG variant associated with the age of the mother and father of the fetus, cause of IFG, fetal sex, presence of LBW at birth, and need for IC of the newborn. The interpretation of the branching of the decision tree up to the terminal nodes and the choice of the probable IFG variant are presented in Table 3.
The association of the risk of one of the IFG variants with sex was confirmed in the assessment of the parental factor – the age of the father and mother of the fetus or newborn. In the decision tree presented below, the age of either the mother or father determined the formation of terminal nodes – the most likely IFG variant – FGR or SGA (Fig. 4).
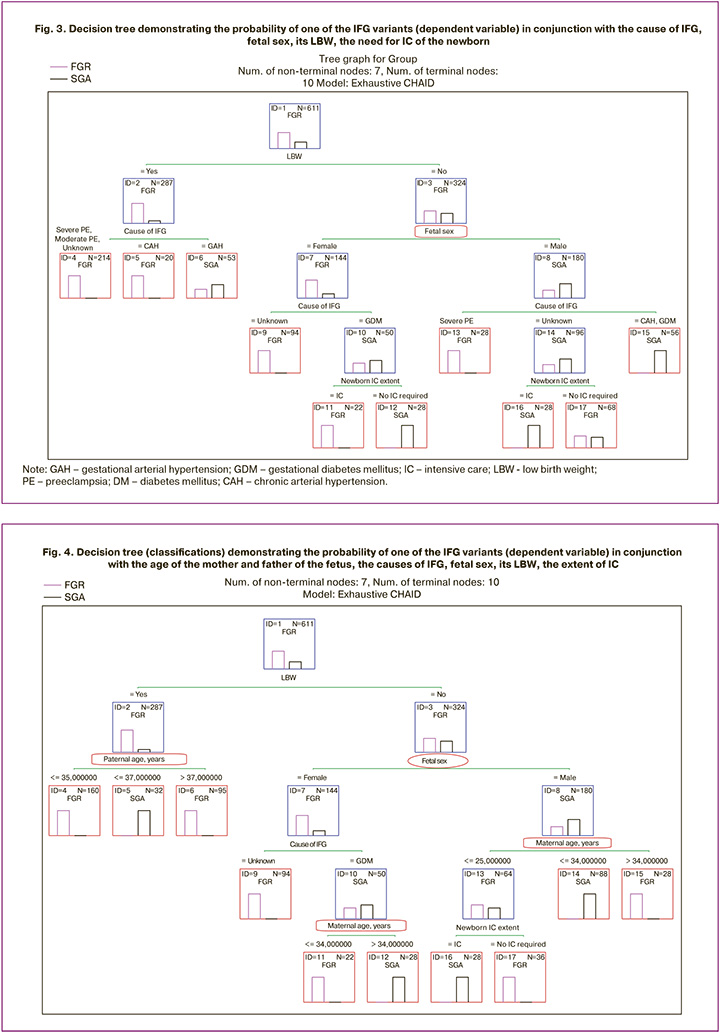
There were ten terminal nodes in the tree. The interpretation of the branching of this decision tree in search of the most probable IFG variant is presented in Table 4.
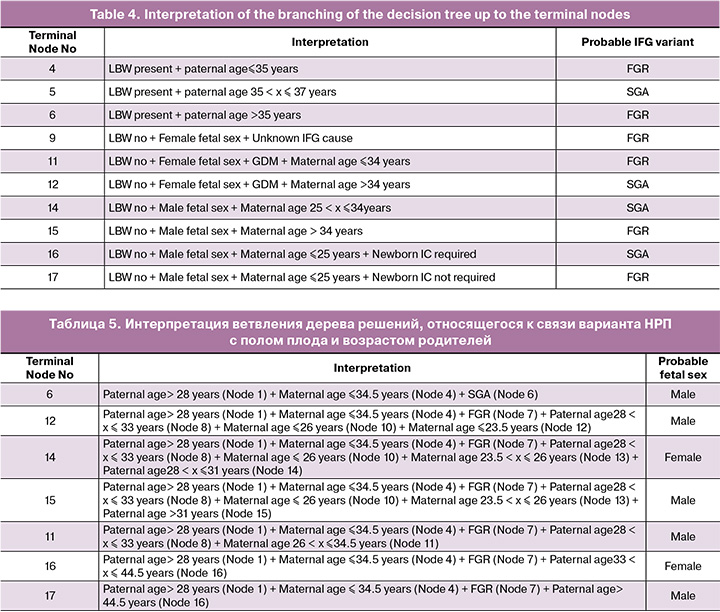
It is noteworthy that, in the presence of low body weight, the father's age was the only factor that determined the difference in IFG (nodes No. 4–6). With normal weight characteristics of the newborn, the IFG variant was determined by the maternal age, but in conjunction with such weighty factors as the cause of IFG and the need for IC of the newborn.
To confirm the connection between the sex factor, including the parental factor, and the IFG variant, we present the following classification tree (Fig. 5).
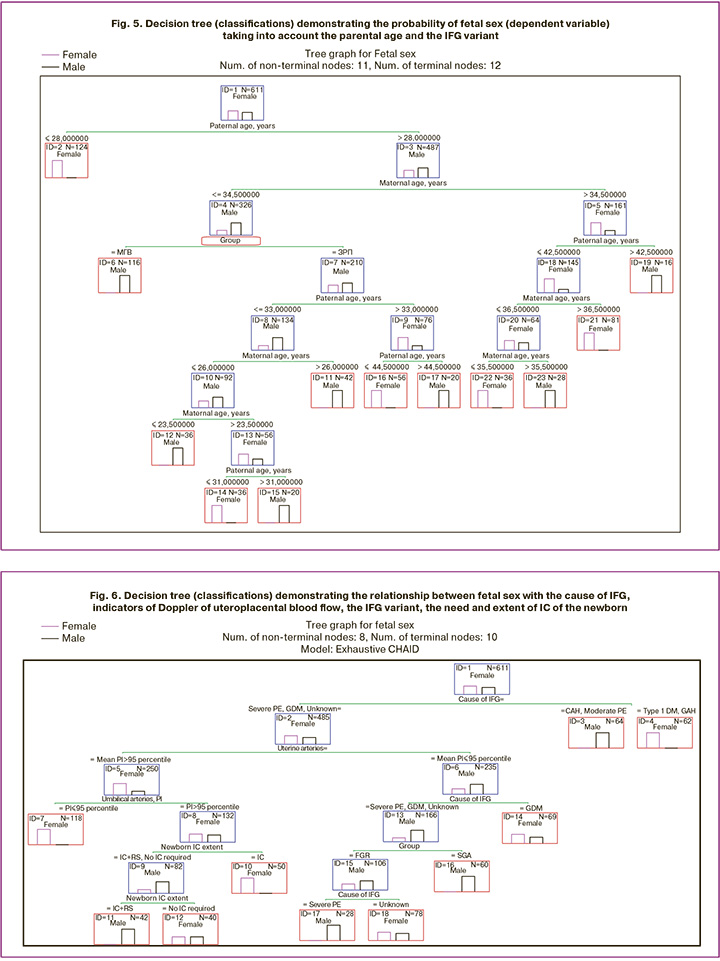
It is obvious that in this classification tree, the first decisive rule was the father's age, and the window of opportunity to predict fetal sex, considering the IFG variant (FGR or SGA), opened when the father's age was more than 28 years and the maternal age was ≤34.5 years. The interpretation of the branching of the decision tree relating to the relationship between the IFG variant, fetal sex, and the age of the parents is presented in Table 5.
Female sex showed an invariable connection with FGR at the age of the mother 23.5 < x ≤ 26 years and the age of the father 28 < x ≤ 31 years (Node 14) and at the age of the mother ≤ 34.5 years and the age of the father 33 < x ≤ 44.5 years (Node 16).
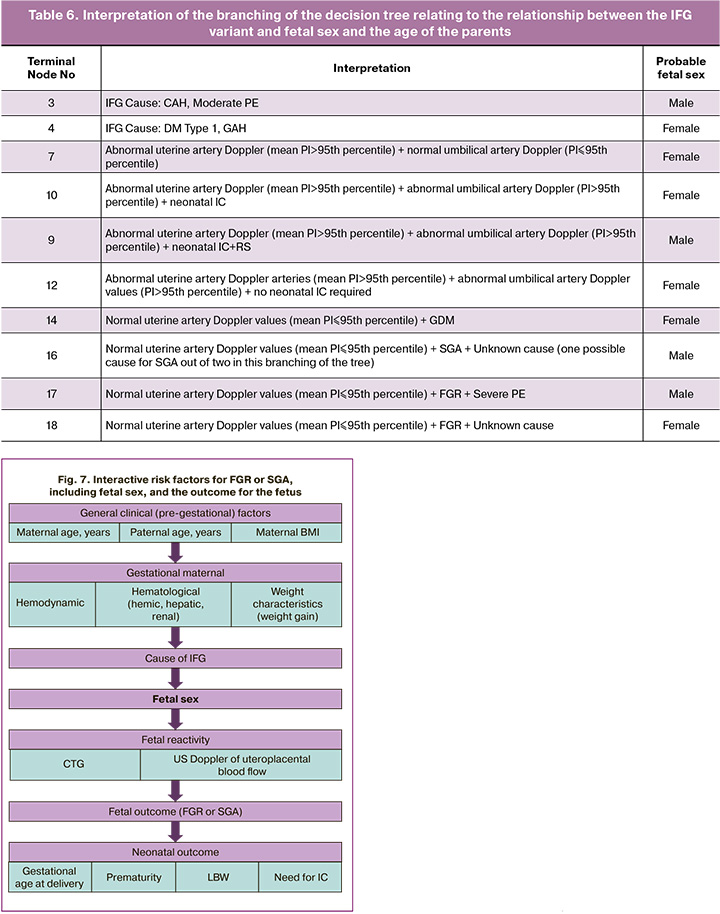
Fetal sex showed a link between the cause of IFG, Doppler of uteroplacental blood flow, IFG variant, and the need and extent of IC of the newborn (Fig. 6).
In the classification tree presented above, the first decisive rule was the cause of the IFG. It is obvious that CAH and moderate PE are more likely for a male fetus (Node 3), and type 1 DM and GAH – for a female (Node 4). The subsequent branching of the classification tree justifies only three reasons: severe PE, GDM and an unknown reason (Node 2). With normal indicators of Doppler of the uterine arteries (mean PI≤95th percentile) (Node 6) and GDM, the female sex of the newborn was more likely (Node 14), regardless of the IFG variant. With severe PE and unknown causes of IFG, the association of fetal sex with the IFG variant is visible; for FGR, male sex is characteristic of severe PE, female sex with an unknown cause (Node 18); for SGA and unknown causes (severe PE was absent in SGA), male sex (Node 17).
With Doppler abnormalities of the uterine arteries (mean PI>95th percentile) (Node 5) but a normal PI of the umbilical artery (PI≤95th percentile), regardless of the cause of IFG, female sex was more likely (Node 7). With a similar violation of Doppler of the uterine arteries (mean PI>95th percentile) (Node 5) and a violation of the PI of the umbilical artery (PI>95th percentile) (Node 8), female sex is associated with both the need for IC (Node 10) and the absence of it (Node 12), and male - with the need for IC and respiratory support (RS) (Node 11).
The interpretation of the branching of the decision tree relating to the relationship between the IFG variant, fetal sex, and the age of the parents is presented in Table 6.
Thus, we managed to provide arguments in favor of the fact that fetal sex is interconnected with the IFG variant and many factors that determine or result from this disorder.
The data partially echoed the global trend of a more frequent combination of FGR with female fetal sex. Indeed, the cohort was dominated by female newborns (54.50%), but unlike most literature data, not only with FGR but also with SGA. It is interesting that the scales for assessing SGA take into account fetal sex [21]. We showed that both FGR and SGA are associated with fetal sex but in different ways. It is obvious that there is a relationship between parental risk factors, the characteristics of pregnancy progress, and the specifics of complications leading to FGR or SGA. Fetal sex is associated with this chain of events as a risk factor (Fig. 7), which, of course, cannot be influenced but cannot be ignored when predicting, screening, diagnosing, treating, and choosing the timing of delivery.
The formation of the maternal reproductive function (age at menarche), the maternal body weight characteristics – pregestational (BMI) and formed during pregnancy (weight gain), and age of both parents with the potential accumulation of epigenetic risk factors were associated not only with the IFG variant, but also with fetal sex. The design of this study did not allow us to convincingly assert the pathogenesis of this interaction and its probable influence. But it should be noted that the reactivity of the maternal hemodynamics (BP systolic and diastolic), starting from the I trimester of pregnancy, the fetal heart rate (CTG), uteroplacental blood flow, blood functions (Hb, leukocyte, platelet counts), liver (ALT and AST), kidneys (creatinine) with IFG of one of the variants, the need for earlier delivery, despite LBW or prematurity, the need for IC of the newborn, overlaps with fetal sex. A connection with fetal sex was found, even in the absence of a clear cause of IFG [22].
The hierarchy of this relationship, the primacy of IFG or hemodynamic (maternal and fetal), hemic, renal, and hepatic characteristics is not clear, as is the involvement of fetal sex, which requires further study.
Taking fetal sex into account showed opposite directions of differences in risk factors for FGR and SGA (Table 7).
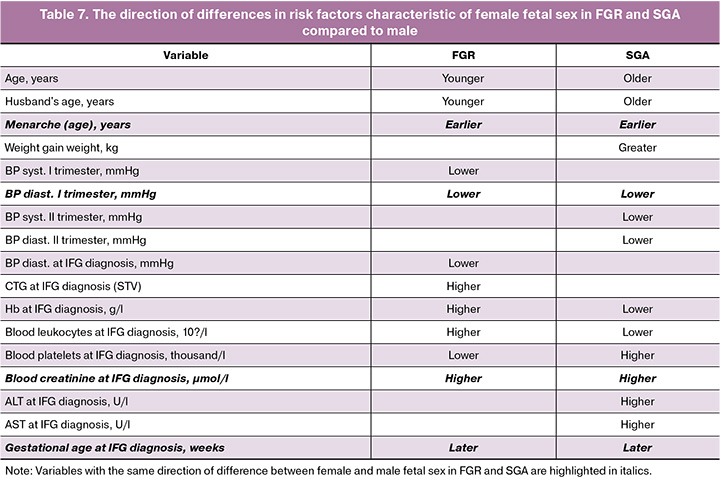
The opposite direction of differences between female and male newborns in FGR compared to SGA was influenced by parental age, leukocyte count, hemoglobin level, and platelet count in diagnosing IFG. Weight gain and CTG (STV) indicators in the diagnosis of IFG, along with systolic BP in the first trimester and both systolic and diastolic BP in the second trimester, as well as blood ALT and AST, distinguished fetuses of different sexes in only one of the two IFG scenarios. The direction of differences in menarche age, diastolic BP in the first trimester, blood creatinine level, and gestational age in the diagnosis of IFG were identical. This finding sheds light on the differences in the pathogenesis of FGR and SGA, highlighting the significance of fetal sex and parental age as markers of epigenetic potential.
This study confirmed that female fetuses are more susceptible to a more complicated variant of IFG–FGR. It was found that the female sex is associated with impaired blood flow in the uterine and middle cerebral arteries, a reduced cerebroplacental index, and a higher susceptibility to severe complications such as low body weight, severe PE, GAH, and the need for IC. Male fetal sex was more closely associated with impaired blood flow in the umbilical artery, undetermined causes of IFG and CAH, moderate PE, and, critically, with prematurity.
The decision tree method proposed possible scenarios for the development of IFG into one of its variants, considering maternal and paternal factors, as well as fetal sex. This approach suggests the need for IC for newborns, reflecting the severity of their condition at birth. Although these results do not resolve all issues related to the diagnosis and prevention of IFG and its variants, the regional specificity of this study limits the generalizability of the findings to other populations. Nevertheless, implementing the proposed decision tree models along with individualized centile tables adjusted for fetal sex during ultrasound monitoring, increased BP control, Doppler assessment of uterine arteries, and biochemical monitoring (creatinine, AST/ALT) in pregnant women can increase the risk of potential pregnancy complications and improve delivery outcomes. This approach aids in the timely differential diagnosis of SGA and FGR, as well as in choosing the appropriate timing, method, and location for delivery.
Conclusion
IFG is closely linked to fetal sex, and its variant (FGR or SGA) determines its specificity. It is advisable to assess both SGA and FGR while considering fetal sex. This relationship is influenced by parental age as a marker of epigenetic factors and reflects maternal and fetal hemodynamics as well as maternal hemic, hepatic, and renal responses to the progression of pregnancy complicated by IFG. The solutions proposed in this study serve as guides for stratifying pregnant women by IFG variants, taking into account fetal sex and associated risk factors. We recommend the introduction of individualized centile tables adjusted for fetal sex during ultrasound monitoring. For pregnant women carrying a female fetus at risk of FGR, increased BP control, Doppler assessment of uterine arteries, and biochemical monitoring (creatinine, AST/ALT) are essential.
References
- Quiñones J.N., Stamilio D.M., Coassolo K.M., Macones G.A., Odibo A.O. Is fetal gender associated with adverse perinatal outcome in intrauterine growth restriction (FGR)? Am. J. Obstet. Gynecol. 2005; 193(3 Pt 2): 1233-7. https://dx.doi.org/10.1016/j.ajog.2005.05.053
- James W.H. Fetal sex and intrauterine growth restriction. Am. J. Obstet. Gynecol. 2006; 195(5): 1498-9. https://dx.doi.org/10.1016/j.ajog.2006.01.087
- Radulescu L., Ferechide D., Popa F. The importance of fetal gender in intrauterine growth restriction. J. Med. Life. 2013; 6(1): 38-9.
- Tesfa D., Tadege M., Digssie A., Abebaw S. Intrauterine growth restriction and its associateed factors in South Gondar zone hospitals, Northwest Ethiopia, 2019. Arch. Public Health. 2020; 78: 89. https://dx.doi.org/10.1186/s13690-020-00475-2
- Monier I., Blondel B., Ego A., Kaminski M., Goffinet F., Zeitlin J. Does the presence of risk factors for fetal growth restriction increase the probability of antenatal detection? A French national study. Paediatr. Perinat. Epidemiol. 2016; 30(1): 46-55. https://dx.doi.org/10.1111/ppe.12251
- Bi S., Zhang L., Wang Z., Tang J., Xie S., Gong J. et al. Association of an increased risk of pre-eclampsia and fetal growth restriction in singleton and twin pregnancies with female fetuses. Matern. Fetal Med. 2021; 3(1): 18-23. https://dx.doi.org/10.1097/FM9.0000000000000069
- Xie Y., Zhao F., Wang Y., Borowski S., Freitag N., Tirado-Gonzalez I. et al. Fetal growth restriction induced by maternal gal-3 deficiency is associated with altered gut-placenta axis. Cell Death Dis. 2024; 15(8): 575. https://dx.doi.org/10.1038/s41419-024-06962-6
- Zhao Y., Chen L., Li L.X., Xie C.M., Li D., Shi H.J. et al. Gender-specific relationship between prenatal exposure to phthalates and intrauterine growth restriction. Pediatr. Res. 2014; 76(4): 401-8. https://dx.doi.org/10.1038/pr.2014.103
- Zimmerman R.M., Hernandez E.J., Yandell M., Tristani-Firouzi M., Silver R.M., Grobman W. et al. AI-based analysis of fetal growth restriction in a prospective obstetric cohort quantifies compound risks for perinatal morbidity and mortality and identifies previously unrecognized high risk clinical scenarios. BMC Pregnancy Childbirth. 2025; 25(1): 80. https://dx.doi.org/10.1186/s12884-024-07095-6
- Intapad S., Dasinger J.H., Johnson J.M., Brown A.D., Ojeda N.B., Alexander B.T. Male and female intrauterine growth-restricted offspring differ in blood pressure, renal function, and glucose homeostasis responses to a postnatal diet high in fat and sugar. Hypertension. 2019; 73(3): 620-9. https://dx.doi.org/10.1161/HYPERTENSIONAHA.118.12134
- van der Vlugt E.R., Verburg P.E., Leemaqz S.Y., McCowan L.M.E., Poston L., Kenny L.C. et al. Sex- and growth-specific characteristics of small for gestational age infants: a prospective cohort study. Biol. Sex Differ. 2020; 11(1): 25. https://dx.doi.org/10.1186/s13293-020-00300-z
- Fink G., Andrews K.G., Brentani H., Grisi S., Scoleze Ferrer A.P., Brentani A. Overall and sex-specific associations between fetal adversity and child development at age 1 year: evidence from Brazil. Am. J. Epidemiol. 2018; 187(11): 2324-31. https://dx.doi.org/10.1093/aje/kwy141
- Hall J., Jaekel J., Wolke D. Gender distinctive impacts of prematurity and small for gestational age (SGA) on age-6 attention problems. Child Adolesc. Ment. Health. 2012; 17(4): 238-45. https://dx.doi.org/10.1111/j.1475-3588.2012.00649.x
- Díez López I., Cernada M., Galán L., Boix H., Ibañez L., Couce M.L. Small for gestational age: concept, diagnosis and neonatal characterization, follow-up and recommendations. An. Pediatr. (Engl. Ed). 2024; 101(2): 124-31.https://dx.doi.org/10.1016/j.anpede.2024.07.012
- Tian M.Y., Wen S.W., Retnakaran R., Wang H.R. , Ma S.J., Chen M.S. et al. Novel sex-specific influence of parental factors on small-for-gestational-age newborns. Sci. Rep. 2020; 10(1): 19226. https://dx.doi.org/10.1038/s41598-020-76196-x
- Finken M.J.J., van der Steen M., Smeets C.C.J., Walenkamp M.J.E., de Bruin C., Hokken-Koelega A.C.S. et al. Children born small for gestational age: differential diagnosis, molecular genetic evaluation, and implications. Endocr. Rev. 2018; 39(6): 851-94. https://dx.doi.org/10.1210/er.2018-00083
- Nohuz E., Rivière O., Coste K., Vendittelli F. Prenatal identification of small-for-gestational age and risk of neonatal morbidity and stillbirth. Ultrasound Obstet. Gynecol. 2020; 55(5): 621-8. https://dx.doi.org/10.1002/uog.20282
- Pierdant G., Ittermann T., Freyer-Adam J., Siewert-Markus U., Grabe H.J., Dörr M. et al. Maternal socioeconomic and lifestyle factors and life dissatisfaction associated with a small for gestational age infant. The Survey of Neonates in Pomerania (SNiP). Arch. Gynecol. Obstet. 2023; 307(4): 1243-54. https://dx.doi.org/10.1007/s00404-022-06598-x
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Недостаточный рост плода, требующий предоставления медицинской помощи матери (задержка роста плода). М.; 2022. 47 с. [Ministry of Health of the Russian Federation. Clinical guidelines. Insufficient fetal growth that requires medical assistance for the mother (fetal growth restriction). Moscow; 2022. 47 p. (in Russian)].
- Волочаева М.В., Тимофеева А.В., Федоров И.С., Кан Н.Е., Тютюнник В.Л., Рыжова К.О., Гасымова Ш.Р. Модель диагностики задержки роста плода с использованием функциональных методов исследования. Акушерство и гинекология. 2025; 2: 31-9. [Volochaeva M.V., Timofeeva A.V., Fedorov I.S., Kan N.E., Tyutyunnik V.L., Ryzhova K.O., Gasymova Sh.R. A model for diagnosing fetal growth restriction using functional diagnostic methods. Obstetrics and Gynecology. 2025; (2): 31-9 (in Russian)]. https://dx.doi.org/10.18565/aig.2025.15
- Morris R.K., Johnstone E., Lees C., Morton V., Smith G. Investigation and care of a small-for-gestational-age fetus and a growth restricted fetus (Green-top guideline No. 31). BJOG. 2024; 131(9): e31-e80. https://dx.doi.org/10.1111/1471-0528.17814
- Yunis K.A., Beydoun H., Tamim H., Nassif Y., Khogali M. Risk factors for term or near-term fetal growth restriction in the absence of maternal complications. Am. J. Perinatol. 2004; 21(4): 227-34. https://dx.doi.org/10.1055/s-2004-828606
Received 20.06.2025
Accepted 12.08.2025
About the Authors
Arsen A. Ziyadinov, PhD, Associate Professor at the Department of Obstetrics, Gynecology and Perinatology No. 1, S.I. Georgievsky Medical Institute of V.I. Vernadsky Crimean Federal University; Obstetrician-Gynecologist, Perinatal Center of N.A. Semashko Republican Clinical Hospital, 295017, Russia, Republic of Crimea, Simferopol, Semashko str., 8; Doctoral student at the Department of Obstetrics and Gynecology with the Course of Perinatology, Medical Institute of Patrice Lumumba Peoples’ Friendship University of Russia, ars-en@yandex.ruVladislava A. Novikova, Dr. Med. Sci., Professor at the Department of Obstetrics and Gynecology with the Course of Perinatology, Medical Institute of Patrice Lumumba Peoples’ Friendship University of Russia, 117198, Russia, Moscow, Miklukho-Maklaya str., 6, vladislavan@mail.ru
Victor E. Radzinsky, Dr. Med. Sci., Professor, Corresponding Member of the RAS, Head of the Department of Obstetrics and Gynecology with the Course of Perinatology, Medical Institute of Patrice Lumumba Peoples’ Friendship University of Russia, 117198, Russia, Moscow, Miklukho-Maklaya str., 6, kafedra-aig@mail.ru



