Anatomical pathology of the umbical cord in cases of fetal congenital heart disease
Yarygina T.A., Gasanova R.M., Marzoeva O.V., Sypchenko E.V., Leonova E.I., Lyapin V.M., Shchegolev A.I., Gus A.I.
Objective: The purpose of the study was to determine the pathological features of the umbilical cord structure associated with live born small for gestational age and premature baby with prenatally diagnosed congenital heart disease (CHD).
Materials and methods: A retrospective analysis of the results of macroscopic examination of the umbilical cords was carried out in antenatally formed in the cohort with 115 cases of fetal CHD. A group of cases was divided into subgroups depending on the gestational age at delivery and baby weight at birth. Subroup 1 included 15 cases with premature and/or SGA infants; subgroup 2 consisted of 100 full-term infants with birth weight ≥ 10th percentile.
Results: Short (58.2% of cases) and lean (43.4% of cases) umbilical cord, abnormal attachment to the placenta (35.6% of cases), hypo-and hypercoiled cord (4.3% and 19.1% of cases) were most commonly observed, respectively. Pathological variants and their combinations in one umbilical cord were found in 74% and 34% of cases.
There were differences between the subgroups in the frequency of occurrence of hypercoiled and lean umbilical cord, and combined pathology, that was 46.6% versus 15%, 73.3% versus 39%, 60% versus 30% in subgroups 1 and 2, respectively, (р<0,05). At the same time, the odds ratio for the birth of premature and/or small for gestational age baby with CHD was 4.96 (95% CI 1.56–15.7), 4.3 (95% CI 1.28–14.45) and 3.5
(95% CI 1.14–10.8), respectively.
Conclusion: Postnatal assessment showed that umbilical cord pathology was in 74% of cases in babies born with congenital heart disease. Hypercoiled, lean umbilical cord and combination of umbilical cord abnormalities were significantly associated with preterm births and small for gestational age newborns. The obtained results indicate the need to expand the protocol for ultrasound assessment of the umbilical cord in cases of fetal cardiac pathology.
Authors' contributions: Yarygina T.A., Gasanova R.M., Shchegolev A.I., Gus A.I. – the concept and design of the study; Yarygina T.A., Marzoeva O.V., Sypchenko E.V., Leonova E.I., Lyapin V.M. – manuscript writing; Yarygina T.A., Leonova E.I., Lyapin V.M., Shchegolev A.I. – material collection and processing; Gasanova R.M., Shchegolev A.I., Gus A.I. – manuscript editing.
Conflicts of interest: The authors confirm that they have no conflicts of interest to declare.
Funding: The study was conducted within the framework of applied scientific research topic No. 123020300017-1.
Ethical Approval: The study was approved by the local Ethics Committee of A.N. Bakulev National Medical Research Center for Cardiovascular Surgery, Ministry of Health of Russia.
Patient Consent for Publication: The patients have signed informed consent for participation in the study and publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Yarygina T.A., Gasanova R.M., Marzoeva O.V., Sypchenko E.V., Leonova E.I., Lyapin V.M.,
Shchegolev A.I., Gus A.I. Anatomical pathology of the umbical cord in cases of fetal congenital heart disease.
Akusherstvo i Gynecologia/Obstetrics and Gynecology. 2024; (8): 48-57 (in Russian)
https://dx.doi.org/10.18565/aig.2024.133
Keywords
Over the past decades, significant efforts of healthcare system have been directed towards identification of risk factors and improvement of antenatal diagnostics of fetal developmental abnormalities, as well as treatment and medical and social rehabilitation of patients with congenital pathology [1].
From 2010 to 2022, infant mortality rates due to congenital anomalies (malformations), deformations and chromosomal abnormalities decreased by almost 2 times – from 18.2 to 9.4 per 10,000 live births in the Russian Federation [2]. However, until the present time, among all congenital malformations, cardiovascular anomalies remain one of the main causes of neonatal deaths [3]. Thus, according to the Federal State Statistics Service (Rosstat), congenital malformations of circulatory system ranked second among all congenital malformations as the cause of early neonatal deaths in general in the Russian Federation. At the same time, congenital heart defects (CHD) account for more than 80% of congenital malformations of the circulatory system and were the cause of early neonatal deaths in 32.0% of all deaths due to congenital anomalies [4]. In addition, it should be noted that during COVID-19 pandemic (2020) the rate of CHD as the primary cause of stillbirths increased from 13.9% to 16.2% among all cases of death due to congenital anomalies compared with the period before the pandemic (2019) [5] and, on the contrary, among all congenital malformations, the rate of CHD as the cause of early neonatal deaths decreased from 28.3% to 24.6% [6].
In addition to hemodynamic impairments caused by heart defects, other factors leading to perinatal hypoxia also make a negative impact on condition of babies with cardiac pathology [7, 8], and the most important factors are structural abnormalities and disorders of placenta and umbilical cord. [8, 9]. Thus, the analysis of literature data [10] found that umbilical cord disorders are considered to be the cause of about 25% of all late complications of pregnancy, including adverse (lethal) perinatal outcomes.
According to Rosstat data on stillbirth in 2012, umbilical cord pathology was the cause of fetal death in the intranatal period by 2 times more often versus antenatal death in the Russian Federation [11].
Observations of early neonatal death showed that in 2010, placental pathology in general and umbilical cord pathology in particular, were the conditions that caused neonatal death in 17.2% and 1.6% of cases, respectively [12]. In 2019, all pathological changes in placenta in general and umbilical cord in particular lead to neonatal death in 23.9% and 1.2% of cases, respectively [6]. In turn, during COVID-19 pandemic in 2020 in the Russian Federation, the rate of stillbirth due to placental pathology increased by 5.6% versus 2019 [13].
Unfortunately, according to the most authoritative modern international classification of placental lesions by Amsterdam Placental Workshop Group, umbilical cord pathology is included in the group “Other disorders of |placenta” and is represented only by abnormal placental attachments [14]. Marginal and velamentous cord insertions are considered as abnormal (pathologic) placental attachments. At the same time velamentous cord insertion occurs significantly more often in multiple pregnancy compared with singleton pregnancy (9% versus 1%) [15].
In addition, the causes of blood flow disturbances in the umbilical cord vessels may be due to a knot, compression, stricture, prolapse and hypercoiling, as well as thrombosis and aneurysms [16, 17].
Impaired gas exchange and nutrient transport through blood vessels in the umbilical cord reduce growth velocity in the antenatal period, and occur 1.4–2.0 times more often in fetuses with CHD compared with the general population [18].
This fact indicates that placental research in cases with fetal congenital heart disease is of particular relevance, since prematurity, low gestational weight, and birth asphyxia significantly increase the risk of subsequent disability and the death of a child [19].
The results obtained by the group of authors in our previous study showed significant pathomorphological changes in placentas in cases of prenatally identified fetal CHD [20]. Determination of the impact of umbilical cord pathology on the pathogenesis of developmental defects in this group of fetuses may be one of the key focuses of subsequent prevention of adverse perinatal outcomes.
However, until present time, only few research devoted to identification of umbilical cord pathology in cases of different fetal structural malformations, has been conducted in the Russian Federation with very limited sample size [21, 22] not analyzing the condition of newborns with cardiac pathology.
All of the above justifies the relevance of this study aimed to determine the detection rate and structure of umbilical cord pathology in cases with prenatally diagnosed congenital heart disease in fetuses.
Materials and methods
A multicenter retrospective study was carried out at the Perinatal Cardiology Center of A.N. Bakulev National Medical Research Center for Cardiovascular Surgery, Ministry of Health of Russia and Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia in 2023. The first step of the study was observational analysis of the results of postnatal examination of umbilical cords in the cohort with cases of prenatally diagnosed fetal CHD. The second step was “case-control” comparisons of the incidence rate of umbilical cord abnormalities between the subgroups of the general cohort, which were formed taking into account the weight of newborns and the gestational age at the time of delivery, as well as the hemodynamic characteristics of CHD.
The general inclusion criteria in the study were the cases of live birth and prenatally diagnosed and postnatally confirmed CHD excluding isolated ventricular septal defects (VSDs), reported results of pathologic examination of the umbilical cord, patient’s informed consent to participate in the study.
The general non-inclusion criteria were prenatally non-diagnosed CHD, cases of VSDs, multiple pregnancy, stillbirth, absence of the results of examination of the umbilical cord, patient’s non-consent to participate in the study.
Inclusion criteria in subgroup 1 were birth cases of preterm babies born at gestational age <37 weeks and/or SGA infants with birth weight < the 10th percentile [23] and prenatally diagnosed CHD.
Inclusion criteria in subgroup 2 were birth cases of term babies with prenatally diagnosed CHD and birth weight ≥ the 10th percentile for gestational age.
Additionally, given the hemodynamic characteristics of the defects, the overall cohort was divided into 4 subtypes: with depleted systemic blood flow, hypervolemia in pulmonary circulation, hypovolemia in pulmonary circulation, as well as the cases with vascular rings around the trachea and esophagus without impaired systemic or pulmonary circulation.
Pathological macroscopic examination of the umbilical cord specimens after birth assessed the length, diameter, coiling index, type of attachment to the placenta and the number of vessels in the umbilical cord [24]. Abnormal types of umbilical cord attachment were marginal and velamentous cord insertion. Length anomalies included umbilical cords longer than 70 cm, and short umbilical cords less than 40 cm long, among which absolutely short cords were less than 25 cm long [25].
The value of less than 1.3 cm indicating abnormally reduced umbilical cord circumference was considered as thin cord (also known as “lean” umbilical cord) [26].
Umbilical coiling index (UCI) was determined as the ratio of the total number of coils of 3600 of the umbilical vessels divided by total length of the cord in centimeters. Umbilical cords were classified as hyper- or hypocoiled, when UCI was more than 0.30 coils/cm or less than 0.07 coils/cm, respectively. Non-coiled umbilical cords were classified as hypocoiled. Rightward coiling or mixed pattern of cord coiling were assessed as abnormal umbilical cord coiling [27]. In addition, pathological conditions included true knots and aplasia of the umbilical cord – a single umbilical artery (SUA) in the cord.
Statistical analysis
Statistical data processing was conducted with Python V.3.11 data visualization libraries Numpy, Pandas, Matplotlib, Seaborn. The qualitative characteristics are represented as absolute numbers (n) and the percentage of the total number (%). The quantitative data are represented as arithmetic mean (M) with standard deviation (SD) and medians (Me) with interquartile range Q1–Q3. The Shapiro–Wilk test was used to test normality of data distribution.
The results were statistically processed using Student’s t-test. Mann–Whitney U test was used in cases of non-normally distributed quantitative data or unequal variances. Fisher’s exact test or the chi square (χ2) test was used to analyze the differences in the studied parameters between the subgroups. The odds ratio (IR) with 95% confidence interval (CI) was calculated to estimate the probability of having a premature and/or small for gestational age infant with CHD in the presence of umbilical cord pathology. For statistical hypothesis testing type I error was set at 0.05. The null hypothesis (no differences) was rejected, when probability (p) did not exceed type I error.
Results
Short (58.2% of cases) and lean (43.4% of cases) umbilical cord, abnormal attachment to the placenta (35.6% of cases), hypo-and hypercoiled cord (4.3% and 19.1% of cases) were most commonly observed, respectively. Pathological variants and their combinations in one umbilical cord were found in 74% and 34% of cases.
There were differences between the subgroups in the frequency of occurrence of hypercoiled and lean umbilical cords, and combined pathology, that was 46.6% versus 15%, 73.3% versus 39%, 60% versus 30% in subgroups 1 and 2, respectively, (р<0,05). At the same time, the odds ratio for the birth of premature and/or small for gestational age baby with CHD was 4.96 (95% CI 1.56–15.7), 4.3 (95% CI 1.28–14.45) and 3.5 (95% CI 1.14–10.8), respectively.
The general cohort included 115 cases, among which 39.1% of newborn babies were the girls, and 60.9% were the boys. The median weight at birth was 3240 g and corresponded to the 52nd percentile with the interquartile range 2848g – 3606 g or the 27th – 86th percentile, respectively [23]. Operative delivery was in 37 (32.1%) cases. Preterm births were in 9/115 (7.8%) cases. Birth weight <10th percentile was in 10/115 (8.7%) newborns and was in 4/9 (44.4%) of the total number of preterm babies, and in 6/106 (5.7%) of the total number of full-term babies.
Group 1 included 15/115 cases (13% of the cohort) including 9 preterm infants born at 33+6–36+6 weeks of gestation. Of them, 4 babies were small for gestational age, as well as 6 full-term SGA babies. Group 2 included the remaining 100/115 cases (87% of the cohort).
Analysis of clinical and anamnestic characteristics of mothers has been presented in detail in our previous study [20], where no statistically significant differences were found between the groups.
In groups 1 and 2, operative delivery was in 9/15 (60%) and 28/100 (28%) cases (p=0.019). Babies’ weight at birth was 2380 g (Q1–Q3: 2080–2530 g) and 3412.1 (410) g (р<0.00001, Mann–Whitney U test), respectively. There were no significant differences in Apgar scores between the groups: <7 points in the first minute of life had 4/15 (26.6%) and 8/100 (8%) infants (р=0.05), and 1/15 (6.6%) and 2/100 (2%) infants in the 5th minute (р=0.35, Fisher’s exact test), respectively.
Assessment of umbilical cord parameters in the general cohort showed that median values of umbilical cord were the following: the length was 36 (Q1–Q3: 30–45) cm, the diameter was 1.3 (Q1–Q3: 1.0–1,5) cm, UCI=0.24 coils/cm (Q1–Q3: 0.17–0.35). The frequency of detection of the studied pathological signs both in the general study group, and in each group is represented in Table 1, where statistically significant difference between them is indicated.
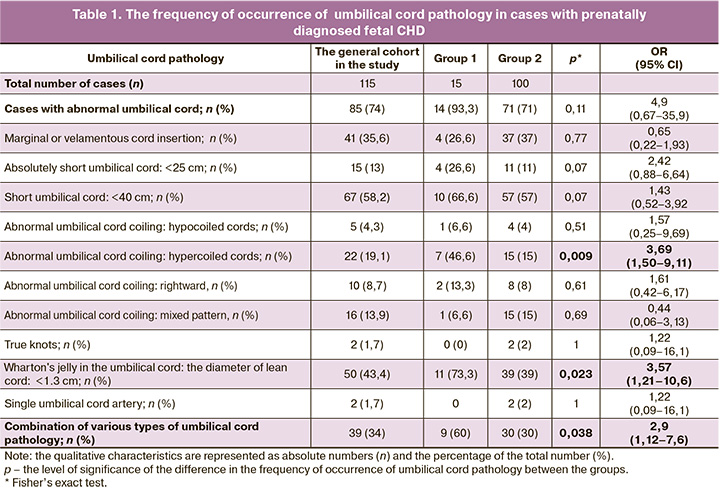
Short umbilical cord was most frequently in cases with fetal CHD – in 67/115 (58.2%) cases and lean cord was in 50/115 (43.4%) cases. Abnormal umbilical cord attachment to the placenta was found in 41/115 (35.6%) observations. Hypo- and hypercoiled cords were in 5/115 (4.3%) and 22/115 (19.1%) cases, respectively. SUA, as well as true knots were found only in 2 out of 115 umbilical cords and reached 1.7%. It is noteworthy that the overall frequency of occurrence of some types of umbilical cord pathology and their combination in one umbilical cord were in 85/115 (74%) and 39/115 (34%) cases, respectively. Long cord was found in none of the cases, that was probably due to measuring the length of the umbilical cord in the Pathology Department, but not immediately after birth, that is, without taking into account the length of the umbilical cord in the newborn baby at birth.
The results of comparative analysis of the frequency of occurrence of umbilical cord pathology (Table 1) showed statistically significant (р<0.05) increase in the number of observations with hypercoiling, reduced diameter and combination of various types of umbilical cord pathology in complicated pregnancies ended with premature and/or small for gestational age infants (group 1) versus group 2, which included full-term infants with birthweight ≥ the 10th percentile. It was determined that odds ratio for preterm and/or small for gestational age babies born with congenital CHD is 4.96 (95% CI 1.56–15.7), 4.3 (95% CI 1.28–14.45) and 3.5 (95% CI 1.14–10.8) in cases of hypercoiling, small diameter of the umbilical cord or multiple pathologies, respectively.
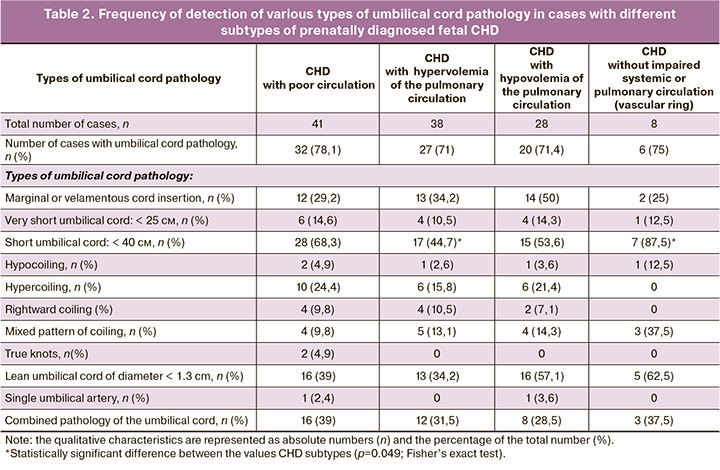
The general cohort was divided into the subtypes of CHD. Subtype 1 included 41/115 (35.7%) cases with poor circulation. Subtype 2 comprised 38/115 (33%) cases with hypervolemia of the pulmonary circulation. Subtype 3 comprised 28/115 (24.3%) cases with hypovolemia of the pulmonary circulation. Subtype 4 was related to 8/115 (7%) cases with vascular ring around the trachea and esophagus without impaired systemic or pulmonary circulation. The results of pairwise test of contingency tables in the groups with various types of umbilical cord pathology using Fisher's exact test are represented in Table 2 and indicate statistically significant differences only in frequency of occurrence of a short umbilical cord between the subtypes 4 and 2 (p=0.049; Fisher’s exact test). The differences between other parameters were not detected.
Discussion
Comprehensive development and improvement of medical care leads to increased survival rates and life expectancy in patients with congenital cardiac pathology [28]. However, even at the stage of prenatal development, the frequency of comorbid pathologies is significantly higher in cases with fetal CHD compared with the general population and can have a negative impact on fetal growth, development and functional state, leading to child’s death or subsequent disability, that necessitates a comprehensive examination of each pregnant woman in detecting cardiovascular anomalies in the fetus [29, 30].
Umbilical cord pathology is one of the factors that increases the likelihood of the birth of premature and small for gestational age infants with congenital heart disease, who are at high risk of pre- and postoperative complications [30]. The importance of thorough examination of the umbilical cord and placenta in cases with fetal malformations has been demonstrated in scientific publications several decades ago. However, our study is the first representation of cases with fetal cardiac pathology in the Russian Federation.
At the antenatal stage, we formed a cohort that included 115 observations of fetal CHD. Then we analyzed and compared the clinical and anamnestic characteristics of mothers, the data on pregnancy outcomes, timing and methods of delivery, weight and newborn’s condition, as well as the results of the umbilical cord examination. The analysis included pathological characteristics of the umbilical cord that according to the results of large-scale foreign meta-analyses, had statistically significant correlation with pregnancy complications worsening prediction of survival and health of newborns, especially with CHD, who will have to undergo complex surgical treatment in the first year or even in the first month of life.
In contrast to available literature data, the frequency of occurrence of umbilical cord abnormalities in the cohort of fetuses with CHD was many times higher than in the population. The data are represented in detail in Table 1. For example, short umbilical cord was detected in 58.2% of cases, and the expected rate was up to 20%; lean umbilical cord was in 43.4% cases, and 6.5% in the population; abnormal cord insertion was in 35.6% of cases with expected 8%; hypocoiling was in 4.3% of cases with expected 2% [31–35].
The frequency of occurrence of hypercoiling, SUA and true knots in the umbilical cord in the cohort of fetuses with CHD conformed to literature data on the general population. Rightward coiling of the umbilical cord was found in less cases than was expected (8.7% versus 15.7%). However, the frequency of occurrence of mixed pattern of cord coiling that increased the likelihood of umbilical cord torsion and blood flow cessation to neighbouring area, was higher [35].
The listed facts confirmed increasing frequency of occurrence of the pathology, that can potentially disrupt blood circulation in the umbilical cord in the Russian cohort of fetuses with CHD in comparison with the population data in foreign literature.
The results of our analysis showed that umbilical cord abnormalities were detected in 74% of observations among the general cohort, that in general conformed to the results reported by Kravchenko E.N. et al. [21]. According to their study, the rate of anomalies was 77.4% among 195 cases of pregnancy termination in the second trimester due to fetal abnormalities incompatible with life. Among them, congenital heart defects were the most common conditions in 66 cases [21]. Similar to the results obtained by us, the study by Kravchenko E.N. et al. [21] reported that abnormal umbilical cord attachment to the placenta and lean umbilical cord were the most common pathologies. However, the detection rate of lean umbilical cord was two times higher in postnatal examination of fetuses with CHD (43.4% versus 20.5%). Also in our study, the incidence rate of hypercoiled (19.1% versus 7.2%) and short umbilical cord (58.2% versus 0%) was higher, that was possibly due to fundamental differences in the gestational age during conduction of the study, since the above anomalies can occur in the second half of pregnancy. On the contrary, it is noteworthy that there were significantly less cases with detected true knots in our study (1.7% versus 14.9%) compared with placental study in the second trimester [21], that can be explained by exclusion of stillbirth and artificial termination of pregnancy from the cohort in our study. It is interesting to note that in severe fetal structural anomalies, significantly higher number of pathologies was detected by researchers in umbilical cords, but not in placentas [21]. Therefore, this fact emphasizes the relevance of our study.
A limited study of placentas in fetuses with developmental abnormalities after delivery at 35 weeks of pregnancy was conducted by Amaeva Z.Yu. et al. [22]. The main group included 37 cases without indication of abnormalities, and the control group comprised 15 placentas. In addition, anthropometric characteristics and newborns’ condition was not analyzed in the groups. The major finding in this study was detection of abnormal umbilical cord attachment to placenta in 30.4% of cases, that correlates with the data obtained in our study – 35.6% of cases [22].
A number of foreign publications also reported association between umbilical cord pathology and fetal CHD. So, Miyoshi T. et al. [36] have conducted a retrospective study that included 154 fetuses with CHD and 948 fetuses without CHD. Their study found that the frequency of abnormal attachment and SUA was significantly higher in cases with CHD, especially with cyanotic defects versus other congenital heart defects. Albalawi A. et al. (2017) [37] have conducted case-control study, including 200 cases with CHD (conotruncal defects, right heart and left heart lesions) and 200 healthy infants. The study focused on abnormal attachment of the umbilical cord, that was significantly more often detected in any of the subtypes of congenital heart disease compared with the control group. Though, according to the results of our study, detection of abnormal umbilical cord attachment increased the probability of fetal congenital heart disease at least by 2 times, and this fact prompted the authors to recommend prenatal echocardiography in such cases [37].
Montaña-Jimenez L.P. et al. [38] have conducted postnatal study in the cohort comprising infants with CHD and found that more than 65% of umbilical cords had pathological changes, among which hypercoiled cord (27.9%) and abnormal cord insertion (16.4%) were most often. The represented data also do not contradict the results obtained by us [38].
The fundamental novelty of our study is identification and analysis of the characteristics of abnormalities in a single umbilical cord, which were found in 34% of cases in the general cohort. At the same time, simultaneously 5 pathological changes were found in 1 case. Also, it was extremely important to identify the subgroup of babies with CHD born preterm and small for the gestational age, that enabled to assess the relationship between the newborn’s and the umbilical cord conditions. It is noteworthy to mention significantly high percentage of babies with low birth weight among premature infants (26.6%), the cases with low Apgar scores, as well as operative delivery in this subgroup in the study, that was associated with high frequency of hypercoiling, reduction in umbilical cord diameter, and the presence of multiple abnormalities in one umbilical cord. It was demonstrated that in the presence of the above pathologies, the risk of birth of preterm and small for gestational age infants with CHD increases by 3–5 times, that indicates the need to expand the protocol for ultrasound evaluation of the umbilical cord in continued pregnancy with diagnosed cardiac pathology in the fetus.
Based on modern advances in prenatal imaging techniques for detection of anatomical anomalies or fetal growth during screening tests, international medical associations recommend to conduct extended prenatal examinations: echocardiography and neurosonography [39] aimed at improving the accuracy of prenatal diagnostics and, accordingly, the quality of medical care for pregnant women. Given the literature data on the significant impact of structural abnormalities of the umbilical cord on high risk of growth restriction, asphyxia and death of the fetus with cardiac pathology [40], as well as the results of analysis of of the incidence rate of umbilical cord pathology in infants with CHD, the group of authors of our study considers it justified to develop the extended protocol for ultrasound examination of the placenta and umbilical cord for the patients of this category. In this respect, future research directions may include prenatal study of the characteristics discussed in this publication, identification of correlation between the obtained results and the results of pathomorphological examination of the placenta and assessment of the condition of newborns with cardiac pathology. Since for the time being no reliable differences in the frequency of umbilical cord pathology have been identified between the subtypes of cardiac pathology, each clinical case of fetal CHD must refer to high-risk group of perinatal hypoxia associated with impaired umbilical arteries structure and function.
Conclusion
The results of pathological macroscopic examination of placentas after the birth of babies with prenatally diagnosed CHD showed that umbilical cord pathology was detected in 74% of cases. Hypercoiling, small diameter and combination of umbilical cord abnormalities were significantly associated with the birth of premature and small for gestational age infants. The obtained results indicate the need to expand the protocol for ultrasound assessment of the umbilical cord in cases of diagnosed fetal cardiac pathology.
References
- Williford E.M., Yang W., Howley M.M., Ma C., Collins R.T., Weber K.A. et al.; National Birth Defects Prevention Study. Factors associated with infant sex and preterm birth status for selected birth defects from the National Birth Defects Prevention Study, 1997-2011. Birth Defects Res. 2024; 116(1): e2294. https://dx.doi.org/10.1002/bdr2.2294.
- Здравоохранение в России. 2023. Статистический сборник. М.: Росстат; 2023. 181с. [Healthcare in Russia. 2023. Statistical collection. Moscow: Rosstat; 2023. 181p. (in Russian)].
- Щеголев А.И., Туманова У.Н., Шувалова М.П., Фролова О.Г. Сравнительный анализ мертворождаемости в Российской Федерации в 2010 и 2012 г. Российский вестник перинатологии и педиатрии. 2015; 60(3): 58-62. [Shchegolev A.I., Tumanova U.N., Shuvalova M.P., Frolova O.G. Comparative analysis of stillbirth rates in the Russian Federation in 2010 and 2012. Russian Bulletin of Perinatology and Pediatrics. 2015; 60(3): 58-62. (in Russian)].
- Туманова У.Н., Шувалова М.П., Щеголев А.И. Анализ статистических показателей врожденных аномалий как причины ранней неонатальной смерти в Российской Федерации. Российский вестник перинатологии и педиатрии. 2018; 63(6): 60-7. [Tumanova U.N., Shuvalova M.P., Schegolev A.I. Analysis of statistical indicators of congenital anomalies as causes of early neonatal death in the Russian Federation. Russian Bulletin of Perinatology and Pediatrics. 2018; 63(6): 60-7 (in Russian)]. https://dx.doi.org/10.21508/1027-4065-2018-63-5-60-67.
- Щеголев А.И., Туманова У.Н., Чаусов А.А., Шувалова М.П. Сравнительный анализ причин мертворождения в Российской Федерации в 2019 и 2020 годах. Акушерство и гинекология. 2022; 2: 80-90. [Shchegolev A.I., Tumanova U.N., Chausov A.A., Shuvalova M.P. Comparative analysis of stillbirth causes and rates in the Russian Federation in 2019 and 2020. Obstetrics and Gynecology. 2022; (2): 80-90. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.2.80-90.
- Tumanova U.N., Schegolev A.I., Chausov A.A., Shuvalova M.P. Analysis of causes of early neonatal mortality during covid-19 pandemic in 2020 in Russia. Bulletin of RSMU. 2021; 5: 71-7. https://dx.doi.org/10.24075/brsmu.2021.045.
- Ярыгина Т.А., Гасанова Р.М., Леонова Е.И., Марзоева О.В., Сыпченко Е.В., Гус А.И. Особенности допплерографических параметров при оценке церебральной гемодинамики у плодов с врожденными пороками сердца. Детские болезни сердца и сосудов. 2022; 19(2): 117-27. [Yarygina T.A., Gasanova R.M., Leonova E.I., Marzoeva O.V., Sypchenko E.V., Gus A.I. Cerebral hemodynamics in fetuses with congenital heart disease. Children’s Heart and Vascular Diseases. 2022; 19(2): 117-27 (in Russian)]. https://dx.doi.org/10.24022/1810-0686-2022-19-2-117-127.
- Cohen J.A., Rychik J., Savla J.J. The placenta as the window to congenital heart disease. Curr. Opin. Cardiol. 2021; 36(1): 56-60. https://dx.doi.org/10.1097/HCO.0000000000000816.
- Leon R.L., Mir I.N., Herrera C.L., Sharma K., Spong C.Y., Twickler D.M. et al. Neuroplacentology in congenital heart disease: placental connections to neurodevelopmental outcomes. Pediatr. Res. 2022; 91(4): 787-94. https://dx.doi.org/10.1038/s41390-021-01521-7.
- Туманова У.Н., Щеголев А.И. Поражения плаценты в генезе мертворождения (обзор литературы). Международный журнал прикладных и фундаментальных исследований. 2017; 3-1: 77-81. [Tumanova U.N., Shchegolev A.I. Placental lesions as the cause of stillbirth (review). International Journal of Applied and Fundamental Research. 2017; (3-1): 77-81. (in Russian)]. https://dx.doi.org/10.17513/mjpfi.11403.
- Щеголев А.И., Туманова У.Н., Шувалова М.П., Фролова О.Г. Гипоксия как причина мертворождаемости в Российской Федерации. Здоровье, демография, экология финно-угорских народов. 2014; 3: 96-8. [Shchegolev A.I., Tumanova U.N., Shuvalova M.P., Frolova O.G. Hypoxia as a cause of stillbirth in the Russian Federation. Health, Demography, Ecology of Finno-Ugric Peoples. 2014; (3): 96-8. (in Russian)].
- Щеголев А.И., Павлов К.А., Дубова Е.А., Фролова О.Г. Ранняя неонатальная смертность в Российской Федерации в 2010 г. Архив патологии. 2013; 75(4): 15-9. [Shchegolev A.I., Pavlov K.A., Dubova E.A., Frolova O.G. Early neonatal mortality in the Russian Federation in 2010. Arhiv Patologii. 2013; 75(4): 15-9. (in Russian)].
- Щеголев А.И., Туманова У.Н., Чаусов А.А., Шувалова М.П. Мертворождение в Российской Федерации в 2020 году (год пандемии COVID-19). Акушерство и гинекология. 2022; 11: 131-40. [Shchegolev A.I., Tumanova U.N., Chausov A.A., Shuvalova M.P. Stillbirths in the Russian Federation in 2020 (COVID-19 pandemic year). Obstetrics and Gynecology. 2022; (11): 131-40. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.11.131-140.
- Щеголев А.И. Современная морфологическая классификация повреждений плаценты. Акушерство и гинекология. 2016; 4: 16-23. [Shchegolev A.I. Current morphological classification of damages to the placenta. Obstetrics and Gynecology. 2016; (4): 16-23. (in Russian)]. https://dx.doi.org/10.18565/aig.2016.4.16-23.
- Pinar H., Carpenter M. Placenta and umbilical cord abnormalities seen with stillbirth. Сlin. Obstet. Gynecol. 2010; 53: 656-72. https://dx.doi.org/10.1097/GRF.0b013e3181eb68fe.
- Щеголев А.И., Серов В.Н. Клиническая значимость поражений плаценты. Акушерство и гинекология. 2019; 3: 54-62. [Shchegolev A.I., Serov V.N. Clinical significance of placental lesions. Obstetrics and Gynecology. 2019; (3): 54-62. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.3.54-62.
- Туманова У.Н., Ляпин В.М., Козлова А.В., Баев О.Р., Быченко В.Г., Щеголев А.И. Аневризма пуповинной вены: клиническое наблюдение и обзор литературы. Акушерство и гинекология. 2018; 6: 119-25. [Tumanova U.N., Lyapin V.M., Kozlova A.V., Baev O.R., Bychenko V.G., Shchegolev A.I. Umbilical vein aneurysm: a clinical case and a review of literature. Obstetrics and Gynecology. 2018; (6): 119-25. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.6.119-125.
- Ghanchi A., Derridj N., Bonnet D., Bertille N., Salomon L.J., Khoshnood B. Children born with congenital heart defects and growth restriction at birth: a systematic review and meta-analysis. Int. J. Environ. Res. Public Health. 2020; 17(9): 3056. https://dx.doi.org/10.3390/ijerph17093056.
- Katz J.A., Levy P.T., Butler S.C., Sadhwani A., Lakshminrusimha S., Morton S.U. et al. Preterm congenital heart disease and neurodevelopment: the importance of looking beyond the initial hospitalization. J. Perinatol. 2023; 43(7): 958-62. https://dx.doi.org/10.1038/s41372-023-01687-4.
- Ярыгина Т.А., Гасанова Р.М., Марзоева О.В., Сыпченко Е.В., Леонова Е.И., Ляпин В.М., Щеголев А.И., Гус А.И. Анализ патоморфологических особенностей строения плаценты в случаях с пренатально диагностированным врожденным пороком сердца у плода. Акушерство и гинекология. 2024; 6: 75-83. [Yarygina T.A., Gasanova R.M., Marzoeva O.V., Sypchenko E.V., Leonova E.I., Lyapin V.M., Shchegolev A.I., Gus A.I. Analysis of pathomorphological characteristics of the placental structure in cases of prenatally diagnosed fetal congenital heart disease. Obstetrics and Gynecology. 2024; (6): 75-83. (in Russian)]. https://dx.doi.org/10.18565/aig.2024.119.
- Кравченко Е.Н., Коломбет Е.В., Любавина А.Е. Исследование плода и плаценты при врожденных пороках развития, несовместимых с жизнью. Лечащий врач. 2017; (10): 62-5. [Kravchenko E.N., Colombet E.V., Lyubavina A.E. Study of the fetus and placenta in congenital malformations incompatible with life. Lechaschi Vrach. 2017; (10): 62. (in Russian)].
- Амаева З.Ю., Омаров Н., Кантаева Д.К. Особенности последов плодов с врожденными пороками развития при сроке гестации от 35 недель. Уральский медицинский журнал. 2018; 158(3): 76-8. [Amaeva Z.U., Omarov N.S., Kantaeva D.K. Features of afterbirth of fetuses with congenital malformations from 35 weeks of gestation. Ural Medical Journal. 2018; 158(3): 76-8. (in Russian)]. https://dx.doi.org/10.25694/URMJ.2018.03.012.
- Villar J., Cheikh Ismail L., Victora C.G., Ohuma E.O., Bertino E., Altman D.G. et al.; International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH-21st). International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014; 384(9946): 857-68. https://dx.doi.org/10.1016/S0140-6736(14)60932-6.
- Щеголев А.И., Бурдули Г.М., Дубова Е.А., Павлов К.А. Патология пупочного канатика. М., 2011. 72 с. [Shchegolev A.I., Burduli G.M., Dubova E.A., Pavlov K.A. Pathology of the umbilical cord. M.; 2011. 72 p. (in Russian)].
- Радзинский В.Е., ред. Патология пуповины. М.: ГЭОТАР-Медиа; 2011. 196 c. [Radzinsky V.E., ed. Umbilical cord pathology. Moscow: GEOTAR-Media; 2011. 196 p. (in Russian)].
- Afroze K.H., Prabha S.L., Chandrakala V., Deepak M. Sonographic estimation of umbilical cord cross-section area and its reference value in normal pregnancy. J. Clin. Diagn. Res. 2017; 11(8): AC04-AC06. https://dx.doi.org/10.7860/JCDR/2017/30251.10415.
- Щеголев А.И., Туманова У.Н., Ляпин В.М. Извитость пуповины: определение, классификация, клиническое значение. Акушерство и гинекология. 2019; 2: 42-50. [Shchegolev A.I., Tumanova U.N., Lyapin V.M. Umbilical cord coiling: definition, classification, clinical significance. Obstetrics and Gynecology. 2019; (2): 42-50. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.2.42-50.
- Голухова Е.З., Ким А.И., Завалихина Т.В., Нефедова И.Е., Черногривов А.Е., Авакова С.А. Анализ оказания медицинской помощи детям с врожденными пороками сердца в Российской Федерации и предпосылки к созданию регистра в современную эру цифровых медицинских информационных систем. Креативная кардиология. 2023; 17(3): 315-21. [Golukhova E.Z., Kim A.I., Zavalikhina T.V., Nefedova I.E., Chernogrivov A.E., Avakova S.A. Analysis for medical care to children with congenital heart disease in Russian Federation and precondition for Registry in the era of digital medical information systems. Creative Cardiology. 2023; 17(3): 315-21. (in Russian)]. https://dx.doi.org/10.24022/1997-3187-2023-17-3-315-321.
- Ярыгина Т.А., Леонова Е.И., Гасанова Р.М., Марзоева О.В., Сыпченко Е.В., Гус А.И. Пренатальное выявление факторов, ассоциированных с нарушением психомоторного развития у детей с врожденными пороками сердца. Детские болезни сердца и сосудов. 2022; 4(19): 285-96. [Yarygina T.A., Leonova E.I., Gasanova R.M., Marzoeva O.V., Sypchenko E.V., Gus A.I. Prenatal identification of factors associated with impaired psychomotor development in children with congenital heart disease. Children’s Heart and Vascular Diseases. 2022; 19(4): 285-96. (in Russian)]. https://dx.doi.org/10.24022/1810-0686-2022-19-4-285-296.
- Туманян М.Р., Свободов А.А., Левченко Е.Г., Крылова А.С. Маловесные дети с врожденными пороками сердца: опыт лечения Центра им. А.Н. Бакулева и анализ мировой литературы. Бюллетень НЦССХ им. А.Н. Бакулева РАМН. 2021; 2(22): 221-30. [Tumanyan M.R., Svobodov A.A., Levchenko E.G., Krylova A.S. Low-weight children with congenital heart defects: the treatment experience in Bakoulev Center and analysis of international literature. Bulletin of the Bakoulev National Medical Research Center for Cardiovascular Surgery. 2021; 2(22): 221-30. (in Russian)]. https://dx.doi.org/10.24022/1810-0694-2021-22-2-221-230.
- Siargkas A., Tsakiridis I., Pachi C., Mamopoulos A., Athanasiadis A., Dagklis T. Impact of velamentous cord insertion on perinatal outcomes: a systematic review and meta-analysis. Am. J. Obstet. Gynecol MFM. 2023; 5(2): 100812. https://dx.doi.org/10.1016/j.ajogmf.2022.100812.
- Siargkas A., Tsakiridis I., Pachi C., Mamopoulos A., Athanasiadis A., Dagklis T. Impact of marginal cord insertion on perinatal outcomes: a systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM. 2023; 5(4): 100876. https://dx.doi.org/10.1016/j.ajogmf.2023.100876.
- Olaya-C M., Vargas W., Martinez R.A., Peñaloza I.F., Sanchez M., Madariaga I. et al. Impact of umbilical cord length on fetal circulatory system by Doppler assessment. J. Ultrasound. 2020; 23(4): 585-92. https://dx.doi.org/10.1007/s40477-020-00495-2.
- Гагаев Ч.Г., Ермакова О.А., Орлова Ю.В., Пронина Е.С., Тежаева М.Б. Тощая пуповина: клиническое значение, возможности диагностики. Сибирский журнал клинической и экспериментальной медицины. 2010; 25(4-2): 133-4. [Gagaev Ch.G., Ermakova O.A., Orlova Yu.V., Pronina E.S., Tezhaeva M.B. Lean umbilical cord: clinical significance, diagnostic possibilities. Siberian Journal of Clinical and Experimental Medicine. 2010; 25(4-2): 133-4. (in Russian)].
- Olaya-C M., Gil F., Salcedo J.D., Salazar A.J., Silva J.L., Bernal J.E. Anatomical pathology of the umbilical cord and its maternal and fetal clinical associations in 434 newborns. Pediatr. Dev. Pathol. 2018; 21(5): 467-74. https://dx.doi.org/10.1177/1093526618758204.
- Miyoshi T., Shiraishi I., Katsuragi S., Tanaka H., Kamiya C., Iwanaga N. et al. Fetal congenital heart defects and abnormality of placenta and umbilical cord. Placenta. 2013; 34(10): A3. https://dx.doi.org/10.1016/j.placenta.2013.07.014.
- Albalawi A., Brancusi F., Askin F., Ehsanipoor R., Wang J., Burd I. et al. placental characteristics of fetuses with congenital heart disease. J. Ultrasound Med. 2017; 36(5): 965-72. https://dx.doi.org/10.7863/ultra.16.04023.
- Montaña-Jimenez L.P., Lasalvia P., Diaz Puentes M., Olaya-C M. Congenital heart defects and umbilical cord abnormalities, an unknown association? J. Neonatal Perinatal Med. 2022; 15(1): 81-8. https://dx.doi.org/10.3233/NPM-210799.
- Tantbirojn P., Saleemuddin A., Sirois K., Crum C.P., Boyd T.K., Tworoger S. et al. Gross abnormalities of the umbilical cord: related placental histology and clinical significance. Placenta. 2009; 30(12): 1083-8. https://dx.doi.org/10.1016/j.placenta.2009.09.005.
- Snoep M.C., Bet B.B., Zwanenburg F., Knobbe I., Linskens I.H., Pajkrt E. et al. Factors related to fetal demise in cases with congenital heart defects. Am. J. Obstet. Gynecol. MFM. 2023; 5(8): 101023. https://dx.doi.org/10.1016/j.ajogmf.2023.101023.
Received 30.05.2024
Accepted 17.06.2024
About the Authors
Tamara A. Yarygina, PhD, Head of the Ultrasound Diagnostics Department, Academician V.I. Krasnopolsky Moscow Regional Research Institute of Obstetrics and Gynecology, 101000, Russia, Moscow, Pokrovka str., 22a; Researcher at the Perinatal Cardiology Center, A.N. Bakulev National Medical Research Center of Cardiovascular Surgery, Ministry of Health of Russia, 121552, Russia, Moscow, Roublyevskoe Shosse, 135; Associated Professor at the Department of Ultrasound Diagnostics of the Faculty of Continuing Medical Education of the Medical Institute, Patrice Lumumba Peoples’ Friendship University of Russia, 127015, Russia, Moscow, Pistsovaya str., 10,+7(495)414-78-75, tamarayarygina@gmail.com, https://orcid.org/0000-0001-6140-1930
Rena M. Gasanova, Dr. Med. Sci., Head of the Perinatal Cardiology Center, A.N. Bakulev National Medical Research Center for Cardiovascular Surgery, Ministry of Health
of Russia, 121552, Russia, Moscow, Roublyevskoe Shosse, 135; Physician of Ultrasound Diagnostics, Department of Ultrasound and Functional Diagnostics,
Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow,
Academician Oparin str., 4, rmgasanova@bakulev.ru, https://orcid.org/0000-0003-3318-1074
Olga V. Marzoeva, PhD, Doctor of Ultrasound Diagnostics, Researcher at the Perinatal Cardiology Center, A.N. Bakulev National Medical Research Center for Cardiovascular Surgery, Ministry of Health of Russia, 121552, Russia, Moscow, Roublyevskoe Shosse, 135, +7(495)414-78-75, ovmarzoeva@bakulev.ru, https://orcid.org/0000-0003-4475-0105
Elena V. Sypchenko, PhD, Doctor of Ultrasound Diagnostics at Perinatal Cardiology Center, A.N. Bakulev National Medical Research Center for Cardiovascular Surgery, Ministry of Health of Russia, 121552, Russia, Moscow, Roublyevskoe Shosse, 135, +7(495)414-78-75, evsypchenko@bakulev.ru, https://orcid.org/0000-0002-8809-7913
Elena I. Leonova, Doctor of Ultrasound Diagnostics at Perinatal Cardiology Center, A.N. Bakulev National Medical Research Center for Cardiovascular Surgery,
Ministry of Health of Russia, 121552, Russia, Moscow, Roublyevskoe Shosse, 135, +7(495)414-78-75, eileonova@bakulev.ru https://orcid.org/0000-0002-6140-7950
Vyacheslav M. Lyapin, Pathologist of the Pathology Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Academician Oparin str., 4, +7(495)531-44-44, v_lyapin@oparina4.ru
Alexander I. Shchegolev, Dr. Med. Sci., Professor, Head of the 2nd Pathoanatomical Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Academician Oparin str., 4, +7(495)531-44-44, ashegolev@oparina4.ru,
https://orcid.org/0000-0002-2111-1530
Alexander I. Gus, Dr. Med. Sci., Professor, Chief Researcher at the Department of Ultrasound and Functional Diagnostics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Academician Oparin str., 4; Head of the Department
of Ultrasound Diagnostics of the Faculty of Continuing Medical Education of the Medical Institute, Patrice Lumumba Peoples’ Friendship University of Russia, 127015, Russia, Moscow, Pistsovaya str., 10, a_gus@oparina4.ru, https://orcid.org//0000-0003-1377-3128
Corresponding author: Tamara A. Yarygina, tamarayarygina@gmail.com



