Pathogenetic features of the preclinical stage ofgestational complications in women with endometriosis
Tezikov Yu.V., Lipatov I.S., Amosov M.S.
Objective: To identify the pathogenetic features of the preclinical stage of pregnancy complications in women with endometriosis.
Materials and methods: The study cohort comprised 160 pregnant women with endometriosis of various locations who underwent a comprehensive examination of changes during pregnancy. Of the total number of participants, 85 were diagnosed with adenomyosis, while 75 were diagnosed with ovarian endometriosis (OE). The control group consisted of 30 healthy pregnant women. To retrospectively determine the pathogenetic features of specific gestational pathologies, the patients were divided into two groups: Group I, which included 84 pregnant women with fetal growth retardation (FGR), and Group II, which comprised 31 women with preterm birth (PB). The examination was conducted at gestational ages of 11–14, 19–22, and 30–34 weeks and included the determination of CRP, TNFα, IL-4, IL-8, CEC, FN, MAPA, PIGF, PAMG-1, and FAMG in the blood.
Results: Obstetric pathology in pregnant women with endometriosis of various locations occurred in 100% of observations and was most frequently represented by early reproductive losses (15.6%), threatened termination of pregnancy (46.2%), FGR (65.2%), and/or chronic fetal hypoxia (45.9%), PB (25.9%), and preeclampsia (13.3%). Pathological shifts in the studied markers enabled the identification of pathogenetic features of obstetric pathology with a preconceptional onset in patients with endometriosis of various localizations, starting from the first trimester of gestation: "proinflammatory state" and "functional endometrial insufficiency" (FEI). In the second trimester, women with adenomyosis and OE were diagnosed with destabilization of the vascular intima and activation of the platelet link, which was defined as "endothelial-hemostatic dysfunction" (EHD). The preclinical stage of PB in pregnant women with endometriosis is characterized by an increase in the proinflammatory state and FEI, followed by the addition of EHD; FGR was characterized by a moderate increase in EHD along with a decrease in the angiogenesis marker PIGF starting from the first trimester.
Conclusion: The identified patterns within the context of "pregnancy and endometriosis" allow for the formulation of a predictive and preventive strategy, considering the high risk of adverse gestational and perinatal outcomes in the presence of structural and functional damage to the reproductive system associated with endometriosis.
Authors' contributions: Tezikov Yu.V. – conception and design of the study, data interpretation, editing of the manuscript; Lipatov I.S., Amosov M.S. – material collection and processing, review of the relevant literature, drafting of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Samara State Medical University.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Tezikov Yu.V., Lipatov I.S., Amosov M.S. Pathogenetic features of
the preclinical stage ofgestational complications in women with endometriosis.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (10): 101-112 (in Russian)
https://dx.doi.org/10.18565/aig.2024.142
Keywords
According to Rosstat, over the past 15 years, the number of patients with endometriosis has increased by 2.5 times. Currently, there are 2 million women with this condition in Russia, reaching 190 million worldwide [1, 2]. In recent years, the term "endometrioid disease" (ED) has appeared more frequently in the literature. Initially proposed to denote severe forms of endometriosis, the definition of endometriosis has broadened owing to the multifaceted nature of its pathogenetic features, which involve various regulatory systems, including neuro-metabolic-endocrine-immune disorders, and the variability of clinical manifestations. This expanded definition reflects the complexity of the pathology's genesis, which affects internal organs and causes anatomical and functional damage to the reproductive system at all hierarchical levels [3, 4].
As endometriosis progresses, a decrease in oocyte quality is observed, along with changes in the molecular basis for proliferation and secretion of the endometrium, embryogenesis, and implantation of the blastocyst [5]. However, over the past few decades, obstetric services have significantly improved the methods for addressing infertility, including endometriosis-associated infertility. Modern assisted reproductive technologies (ART), effective preventive and therapeutic pharmacological agents, interdisciplinary collaboration among specialists, high-tech differentiated surgical strategies, the active use of computer and telecommunication technologies, and artificial intelligence programs have contributed to an increased likelihood of realizing reproductive potential in patients with endometriosis [6].
With the expansion of infertility treatment options, obstetricians and gynecologists now face a new challenge: an increase in the number of pregnant women with high gestational and perinatal risks, as confirmed by data from the World Endometriosis Society [7, 8]. According to Hamdan et al. (2015), there has been a notable increase in the incidence of spontaneous miscarriage among patients with endometriosis [9].
A 30-year national cohort study in Scotland (2018) showed a 2.2-fold increase in the incidence of placenta previa and associated antenatal and postpartum hemorrhage in pregnant women with endometriosis [10]. Pan M. et al. concluded that the incidence of preeclampsia (PE) in patients with endometriosis is twice as high, with the pathology itself acting as an independent factor contributing to this obstetric complication [11]. The meta-analysis by Bruun M. et al. (2018) indicated an increased likelihood of preterm birth (PB) with an odds ratio (OR) of 1.47 (95% CI 1.28–1.69). Moreover, in pregnant women with adenomyosis, the risk of PB was even higher at OR 3.09 (95% CI 1.88–5.09) than in patients with endometriosis in other locations [12]. Systematic reviews and multicenter cohort studies have demonstrated a high incidence of fetal growth retardation (FGR) in pregnant women with endometriosis of various localizations [13, 14]. Additionally, there are several severe complications during gestation that primarily occur in patients with deep infiltrative forms of endometriosis, including intestinal perforation, spontaneous hemoperitoneum, pseudoaneurysm of the uterine arteries and their branches, infection, rupture of endometrioma, and spontaneous uroperitoneum. These complications, which pose potential dangers to the lives of both the mother and fetus, are rare, as evidenced by the limited number of studies [15, 16]. The priority and dominance of the different mechanisms of endometriosis pathogenesis are the subject of ongoing discussion. The course of endometriosis is influenced by local and systemic inflammation, oxidative stress, increased local estrogen activity, luteal phase insufficiency, immunological and vascular-endothelial changes, and impaired uterine contractility [17, 18]. Owing to this unfavorable preconception background, early pregnancy carries a high risk of complications that can affect reproductive potential and result in deviations in the formation of the maternal-fetal placental system (MFPS), contributing to the development of obstetric pathology [19]. Therefore, the issue of "pregnancy and endometriosis" necessitates an understanding of the natural chain of pathogenetic events underlying adverse gestational outcomes, which will guide the search for effective preventive measures as well as predictive and diagnostic methods for obstetric pathology associated with endometriosis in various locations.
This study aimed to identify the pathogenic features of the preclinical stage of pregnancy-related complications in women with endometriosis.
Materials and methods
A total of 160 pregnant women with endometriosis at various locations who underwent comprehensive examination during their pregnancies were divided into two groups. Group I included 85 patients with adenomyosis and Group II included 75 women with ovarian endometriosis (OE). All patients became pregnant spontaneously. Inclusion criteria were confirmed diagnosis of adenomyosis or OE (anamnesis, clinical picture, 2D/3D ultrasound, magnetic resonance imaging, histological confirmation), spontaneous singleton pregnancy, and age < 35 years. The exclusion criteria were lipid metabolism disorders, extragenital pathology (diabetes mellitus types 1 and 2 and other endocrinopathies, autoimmune diseases, cardiovascular diseases, and kidney and liver diseases), genitourinary infections, and pregnancy with the use of ART. The control group comprised 30 healthy pregnant women with uncomplicated pregnancies. The examinations were conducted at gestational ages of 11–14, 19–22, and 30–34 weeks. Serum markers of the pro-inflammatory state, including C-reactive protein (CRP), tumor necrosis factor (TNF)-α, interleukin (IL)-8, and IL-4, were determined. Additionally, the examination included the determination of placental angiogenesis markers (such as placental growth factor, PIGF) as well as serum markers of the vascular-platelet link (such as circulating endothelial cells (CEC), fibronectin (FN), maximum amplitude of platelet aggregation (MAPA), and the functional state of the endometrium (such as placental alpha-microglobulin-1 (PAMG-1) and fertility alpha-2-microglobuliny (FAMG)). Obstetric complication rates were analyzed. The investigations were conducted using Voluson E8 digital system (GE Healthcare, USA), GB 6 fetal monitor (General Meditech, China), Philips Achieva 1.5T MR system (Philips Healthcare, USA), Accent M320 (PZ Cormay S.A., Poland), Torus 1230 (Dixion, Russia), DDS-240 (Deacon, Russia), ChemWell 2910 (Awareness Technology, USA), and Realight 1204 aggregometer (Dixion, Russia). A comprehensive assessment scale [20–22] was used to determine the severity of placental insufficiency (PI). PI diagnosis was confirmed based on a morphological study of placental specimens.
Statistical analysis
Statistical analysis processing was performed using IBM SPSS Statistics version 25 (IBM, USA). The normality of the distribution of continuous variables was tested using the Shapiro–Wilk test. Continuous variables showing a normal distribution were expressed as mean (M) and standard deviation (SD) and presented as M (SD); otherwise, the median (Me) with interquartile range [Q1; Q3] was reported. ANOVA and nonparametric Kruskal–Wallis method were used to compare groups, followed by Mann–Whitney U test with Bonferroni correction (critical value is p<0.05 for two groups, p<0.017 for three groups). The intragroup dynamics of the parameters were assessed using the paired Wilcoxon test. Categorical variables were compared using the Pearson’s χ2 test with Yates correction.
Results
The mean age of patients with adenomyosis and OE was 29.4 (3.3) and 30.6 (3.6) years, respectively (p=0.12); primary infertility was diagnosed in 22.3% (19/85) and 26.6% (20/75) of patients, secondary infertility in 77.7% (66/85) and 73.4% (55/75), χ²=0.20, p=0.65. The duration of infertility in women with adenomyosis was 5.7 (2.1) years, and in OE was 5.2 (2.4) years (p=0.08). The proportion of employed patients with adenomyosis and OE was 90.6% (77/85) and 88% (66/75), respectively, and 9.4% (8/85) and 12% (9/75), χ² = 0.07, p=0.78, respectively. Patients with adenomyosis and OE did not differ in body mass index (BMI) (22.6 (2.5) kg/m2 and 23.4 (2.1) kg/m2, respectively, p=0.14), endometrial thickness before gestation (1.2 (1.5) cm and 1.1 (1.8) cm, respectively, p=0.28). In the control group there were no statistical differences with women with adenomyosis and OE in such parameters of medical and social status as age (28.2 (3.8) years), parity (primiparous – 30% (9/30), multiparous – 70% (21/30)), social status (employed – 86.6% (26/30)), BMI (22.9 (2.6) kg/m2). All women with adenomyosis received hormone therapy with dienogest in a continuous regimen of 2 mg/day for 6–9 months (81.2% (69/85)) as well as the gonadotropin-releasing hormone agonist buserelin for 3–4 months (18.8% (16/85)). Treatment of OE included laparoscopic cystectomy with enucleation of the endometrioma wall, lavage of the cavity, adhesiolysis, and subsequent histological examination of the biomaterial. The mean cyst size was 6.7 (2.5) cm, unilateral location was diagnosed in 70.6% (53/75), and bilateral location was diagnosed in 29.4% (22/75) of patients. According to the OE classification [8], 73.3% (55/75) and 26.7% (20/75) of patients were diagnosed with stage II and III disease, respectively. The fertility index (EFI), taking into account the Least Function Score (LF) and AFS/ASRM classifications, was 7.8 (0.9) points, indicating a 45–60% probability of pregnancy within 1 year and a 60–70% probability within 2 years. For correction of luteal phase insufficiency and preimplantation preparation of the endometrium, all women with adenomyosis (after completion of the main course of hormonal therapy) and OE were prescribed dydrogesterone in a cyclic regimen – from the 16th to the 25th day of the cycle, 10 mg 3 times a day [23, 24]. In the early stages, pregnancy was complicated in groups I and II by moderate hyperemesis gravidarum in 11.8% (10/85) and 10.7% (8/75) (χ²=0.001, p=0.98), non-viable pregnancy in 12.9% (11/85) and 2.7% (2/75) (χ²=4.34, p=0.04), spontaneous miscarriage in 5.9% (5/85) and 9.3% (7/75) (χ²=0.28, p=0.60), threatened miscarriage with retrochorial hematoma in 18.8% (16/85) and 6.7% (5/75) (χ²=4.15, p=0.04), and without hematoma in 32.9% (28/85) and 33.3% (25/75) (χ²=0.01, p=0.91), respectively. Between-group statistical differences were noted in the rates of non-viable pregnancies and threatened miscarriage with retrochorial hematoma, which were more often observed in adenomyosis (p<0.05); however, there were no differences in the rates of early reproductive loss (χ²=0.94, p=0.33) (Table 1). In the later stages, pregnancy was complicated in 100% of cases due to a disorder of the fetoplacental complex (FPC), from placental dysfunction (23% (31/135)) to severe forms of PN (77% (104/135)); characteristic changes were described in previously published studies [20–22]. The combination of PN with other obstetric complications was diagnosed in 38.5% (52/135) of pregnant women, and 55.8% (29/52) and 44.2% (23/52) of patients with adenomyosis and OE, respectively (χ²=0.96, p=0.33). The PB rate was 27.4% (37/135), which was significantly higher than that in the general population. It should be noted that extremely early PB occurred only in pregnant women with adenomyosis (3.0% (2/69)). Early and late PB were diagnosed in the study groups at approximately the same frequency (29.0% (20/69) and 22.7% (15/66) in patients with adenomyosis and OE, respectively (χ²=0.40, p=0.53). It is necessary to consider that PB in endometriosis is both spontaneous and a consequence of other obstetric complications (PE, placental abruption, and premature rupture of membranes (PRM)). PE among patients with EB was diagnosed in 13.3% (18/135) of observations, while among women with adenomyosis – 1.9 times more often than in pregnant women with OE (χ²=1.36, p=0.24). Early manifestation and severe PE are characteristic of pregnant women with adenomyosis. Early moderate PE was diagnosed in 7.2% (5/69) and 3.0% (2/66) of women (χ²=0.51, p=0.47), and severe PE in 8.7% (6/69) and 1.5% (1/66) of patients with adenomyosis and OE, respectively (χ²=2.23, p=0.14). The incidence of fetal pathology associated with PE against the background of adenomyosis and OE was 85.5% (59/69) and 68.2% (45/66), respectively, with a statistically significant predominance in pregnant women with adenomyosis (χ²=4.79, p=0.03). FGR among women with adenomyosis and OE was diagnosed in 72.5% (50/69) and 57.6% (38/66) (χ²=2.67, p=0.10), chronic fetal hypoxia (CFH) in 50.7% (35/69) and 40.9% (27/66) of pregnant women (χ²=0.94, p=0.33), whereas the combination of pathology was observed in 37.7% (26/69) and 30.3% (20/66) of the observations (χ²=0.52, p=0.47). Clinical analysis of FN-associated fetal pathology in pregnant women with adenomyosis and OE demonstrated a higher frequency of FGR and/or CFH among pregnant women suffering from adenomyosis. It is worth noting that early and severe FGR were diagnosed 1.8 and 2.3 times more often among women with adenomyosis than with OE (χ²=1.97, p=0.16 and χ²=4.72, p=0.03). Placenta previa and placental abruption were 1.9 and 2.9 times more often among patients with internal endometriosis; however, no statistically significant differences were observed. Pregnancy complicated by PRM was diagnosed 1.4 times more often in women with adenomyosis than in those with OE (χ²=0.38, p=0.54) (Table 1).
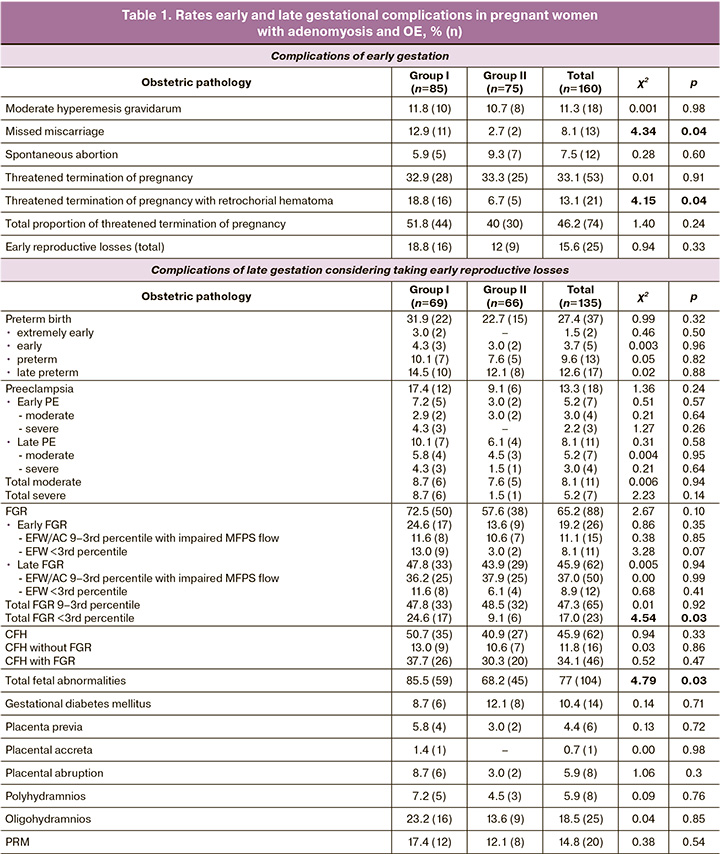
Cesarean section in pregnant women with endometriosis was performed in 62.9% (85/135) of women, and more frequently in patients with adenomyosis, in 71% (49/69), and with OE in 54.4% (36/66) (χ²=3.25, p=0.07).
A clinical assessment of the newborns confirmed the results of the antenatal diagnosis with regard to the state of FPC. A total of 50.4% (68/135) of the children were born with asphyxia, including 11.1% (15/135) of the newborns with moderate asphyxia. The incidence of asphyxia was 0.7% (9/135) and 4.4% (6/135) in the adenomyosis and OE groups, respectively (χ²=0.21; p=0.65). Analysis of the anthropometric parameters of the full-term neonates revealed significant differences compared to the control group in both the adenomyosis and OE groups, with birth weights of 3480 (184), 2628 (285), and 2752 (267) g, respectively (p<0.001). Quetelet's index I was 66.7 (2.8), 54.2 (3.3), and 55.6 (3.1), respectively (p<0.001). At the same time, intergroup comparison between adenomyosis and OE did not show statistically significant differences. Clinical analysis suggests that pregnancy in women with ED) is often complicated by gestational and perinatal complications (PB, 27.4%; FGR, 65.2%; CFH, 45.9%; and PE, 13.3%). Furthermore, early manifestations and severe forms of obstetric pathology are more common in adenomyosis patients. This, along with the specific challenges of the first half of pregnancy, such as a 4.7-fold increase in the occurrence of missed miscarriages and a 2.8-fold increase in retrochorial hematoma formation, indicates a more pronounced disruption of the functional state of the MFPS, which arises from the structural disorganization of the reproductive system in patients with adenomyosis.
To explain the high frequency of severe obstetric pathology and identify the pathogenetic features of the preclinical stage of pregnancy complications in women with endometriosis at various locations, an analysis of laboratory testing results during pregnancy was conducted (Table 2).
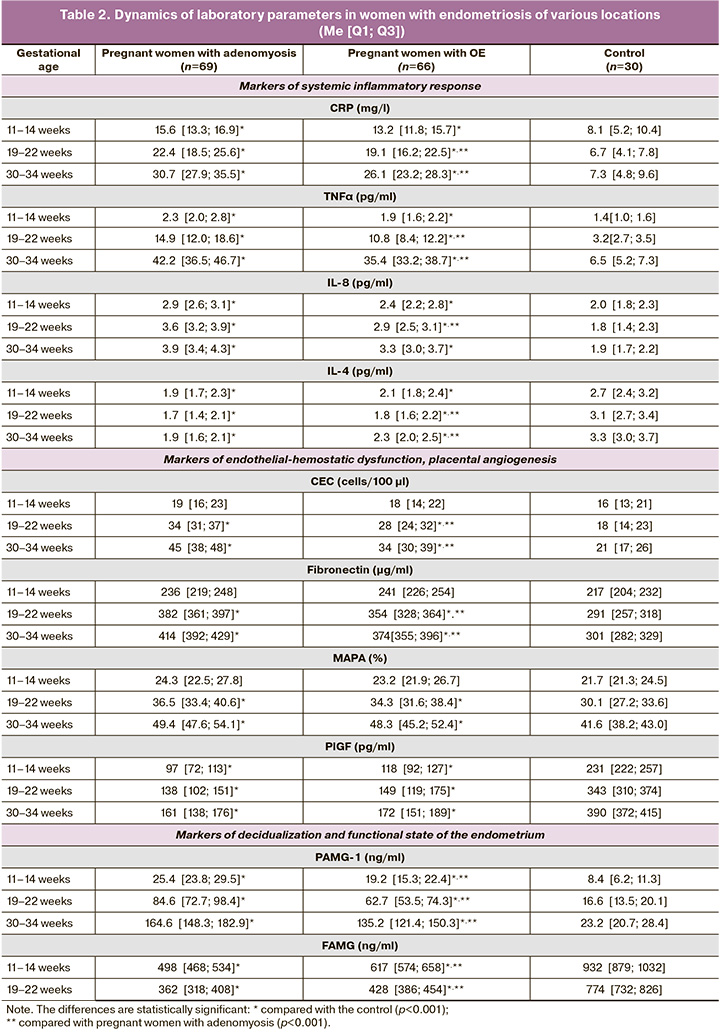
Some sensitive markers of inflammation or cellular alterations that activate the immune system and control cell differentiation, proliferation, and apoptosis include CRP and the cytokine TNFα. From the first trimester of pregnancy, women with endometriosis exhibit elevated levels of these markers, which consistently increase throughout pregnancy. Given the statistical differences in these indicators from the second trimester between patients with adenomyosis and OE, we can infer a more pronounced pro-inflammatory state in pregnant women with adenomyosis (pA-OE (19-22 weeks)<0.001, pA-OE (30-34 weeks)<0.001).
The interest in studying the levels of IL-8, a cytokine with proinflammatory activity, in women with endometriosis at various locations stems from its prognostic value regarding PB, PI, and early reproductive losses, as shown in several studies [25, 26]. Analysis of the dynamics of IL-8 indicated an increase in levels among women with both adenomyosis and OE, and in all three trimesters, the parameter values were significantly higher than those in the control group (p<0.001).
Statistical differences were identified between pregnant women with adenomyosis and OE regarding IL-8 levels in the second (II) and third (III) trimesters (pA-OE (19-22 weeks)< 0.001; pA-OE (30-34 weeks)<0.001). This suggests that cytokine cascade reactions stimulate a systemic inflammatory response in patients with adenomyosis.
Analysis of IL-4 indicated a statistically significant decrease throughout gestation in both pregnant women with adenomyosis and OE compared to the control group (p<0.001). This pattern reflects a disruption in the shift from a Th1 to Th2 immune response in pregnant women with endometriosis. In the control group, proinflammatory cytokine levels are regulated by modulating physiological alterations during trophoblastic invasion and placental proliferation. A sufficient concentration of IL-4 adequately suppresses the immune response of Th1 subpopulation cytokines [27].
In pregnant women with endometriosis at various locations at gestational periods of 19–22 weeks and 30–34 weeks, analyses of CEC and FN revealed a statistically significant prevalence of these markers compared with the control (p<0.001). However, no statistically significant differences were observed during the first trimester of pregnancy. When comparing CEC and FN values among patients with adenomyosis and OE, significant differences were observed in the second and third trimesters (pA-OE (19-22 weeks)<0.001; pA-OE (30-34 weeks)<0.001), indicating destructive changes in the vascular intima in pregnant women with adenomyosis.
Evaluation of platelet functional activity showed no statistical differences in MAPA values between pregnant women with adenomyosis, OE, and the control group during the first trimester. However, in the second and third trimesters, an increase in MAPA values was noted in patients with endometriosis (p<0.001). A moderate increase in MAPA values in the control group from the first to the third trimester suggests physiological hyperaggregation potential.
Angiogenic factors are key markers that reflect the completeness of pro-angiogenesis and vascularization of the placenta, as well as the adaptability of the mother's body and protection of the vascular endothelium. Placental growth factor was the most sensitive parameter. Laboratory results indicated that PlGF levels were significantly lower in the adenomyosis and OE groups than in the control group (p<0.001). Although a less pronounced increase in PlGF concentration was observed in pregnant women with adenomyosis, no statistical differences were observed.
Analysis of parameters reflecting the functional state of the endothelium and components of hemostasis demonstrates direct damage to the vascular intima, an imbalance in the synthesis of regulatory substances by endothelial cells, and a more pronounced hyperaggregation state in pregnant women with endometriosis at various locations. PAMG-1 is an important marker of secretory function of the decidua and regulates the growth and development of the placenta. Its levels increased throughout gestation among pregnant women with both adenomyosis and OE, with statistically significant differences observed by trimester (p<0.001).
High levels of the decidualization modulator PAMG-1 in endometriosis indicate a disruption in the proliferative and metabolic actions of insulin-like growth factors, underscoring the significance of markers in the mechanisms of early placental formation and the functional state of MFPS in the later stages of gestation [28, 29]. The determination of the quantitative values of FAMG is particularly relevant, as its concentration is progesterone-dependent, which is crucial for diagnosing the functional adequacy of the endometrium/decidua. Initially, during the first screening, low levels of FAMG were observed in pregnant women with adenomyosis and OE, with statistical differences compared with the control group (p<0.001). This trend persisted in the second trimester (p<0.001). These findings support the notion of pre-gestational inferiority of the endometrium, manifesting as distorted immunological and metabolic regulation during critical early events, such as decidualization, implantation, trophoblast invasion, and placentation.
Discussion
Normal pregnancy is characterized by a balance between "physiological alteration" factors and "gestational adaptation" mechanisms, which together ensure a harmonious course of pregnancy [30, 31]. In women with endometriosis, this delicate balance is disrupted due to the pathogenic effects of ED on cytotrophoblastic invasion and FPC formation. A periconceptional pro-inflammatory state has been identified in pregnant women with adenomyosis and OE, as evidenced by increased levels of CRP, TNF-α, and IL-8, along with decreased levels of IL-4, reflecting a deficiency in the adaptive mechanisms of gestation. Pathological shifts in these markers were observed as early as the first trimester, with statistically significant differences from the control group throughout the pregnancy. Pathological shifts in inflammation indices, along with changes in markers of decidualization and functional state of the endometrium (PAMG-1, FAMG), were determined from the first trimester of gestation with statistically significant differences compared with the control throughout pregnancy.
Increased oxidative stress and excessive stimulation of CRP synthesis, driven by the increased expression of proinflammatory cytokines, contribute to the modification of molecular signaling pathways that regulate vasculogenesis and angiogenesis. This leads to an imbalance in vascular regulators and an increase in hypercoagulation factors at the implantation site, ultimately resulting in pathological alteration of endothelial cells in the placental vascular network. Laboratory findings corroborate these vascular changes in pregnant women with endometriosis, as evidenced by elevated CEC, FN, and platelet aggregation activity from the second trimester onwards, described as "endothelial-hemostasis dysfunction." Additionally, inhibition of compensatory angiogenesis and vasculogenesis, along with diminished endothelial protective properties in FPC, were noted as critical mechanisms of gestational adaptation. Reduced levels of PlGF were detected in each laboratory assessment of pregnant women with adenomyosis and OE, showing significant differences compared to the controls.
The physiological course of early pregnancy, which determines the subsequent gestational outcomes, is closely linked to successful implantation, which depends on the functional activity of the endometrial glands. This activity is supported by adequate progesterone levels and good sensitivity to gestagens [32]. However, the pathogenesis of ED is influenced by a unique hormone receptor status in affected women, which is characterized by progesterone deficiency and resistance, leading to impaired endometrial receptivity, dysregulation of ovum invasion, and aberrant local immune reactions in patients with adenomyosis and OE. Reduced levels of FAMG and elevated levels of PAMG-1 were documented in pregnant women with adenomyosis and OE, compared to the control group, starting from the first trimester (p<0.001), indicating damage to the endometrial receptor apparatus during the periconceptional period. This set of laboratory findings forms the "pattern of functional endometrial insufficiency."
The reduced effectiveness of gestational adaptation mechanisms shifts the balance toward an already excessive endometriosis-associated alteration of tissues in the embryo (feto)-placental complex.
Stage 2 of the Study
In the second stage of the study, the most frequently observed pregnancy complications in women with endometriosis were analyzed for preclinical laboratory changes in the first and second trimesters. Two comparison groups were established in this study. Group 1 comprised 36 patients with adenomyosis-associated FGR and OE, without any other gestational pathology, who delivered at term. Group 2 consisted of 29 pregnant women with adenomyosis and OE, whose pregnancies ended in spontaneous PB without antenatally diagnosed FGR.
In Group 1, 15 women with early-onset FGR were diagnosed between 28 and 32 weeks of gestation (7 cases with EFW/AC ratio in the 9th to 3rd percentile range with impaired blood flow in the MFPS and 8 cases with EFW less than the 3rd percentile). Additionally, 21 women with late-onset FGR were diagnosed after 32 weeks of gestation (9 cases with an EFW/AC ratio in the 9th to 3rd percentile range and impaired blood flow in MFPS and 12 cases with EFW less than the 3rd percentile).
In Group 2, there was one case of early PB at 31+5 weeks, 12 cases of PB between 32 and 33+6 weeks, and 16 cases of late PB between 34 and 36+3 weeks ( Table 3).
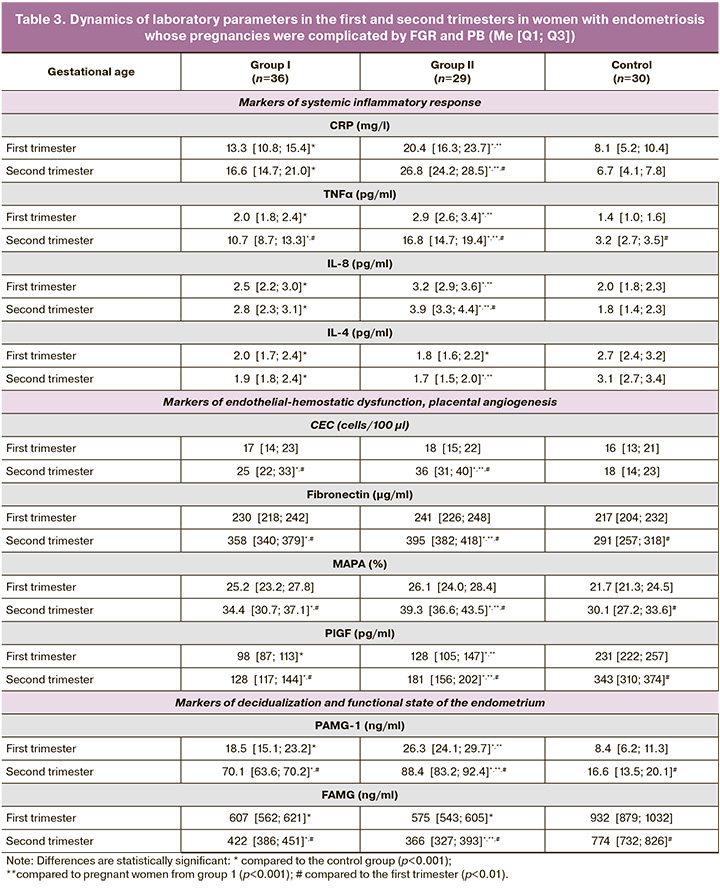
The evaluation of laboratory markers of systemic inflammatory response (CRP, TNFα, IL-8) revealed significant increases in both groups, with statistically significant differences from the control group beginning in the first trimester (p<0.001). A greater increase was observed in women with PB (p<0.001), which was accompanied by a significant decrease in IL-4 levels (p<0.001). Vascular endothelial destabilization markers (CEC and FN), along with hyperaggregation status (MAPA), were elevated between 11–14 weeks and 19–22 weeks of gestation in both groups, with statistically significant differences compared to controls (p<0.001). In the intergroup comparison, the CEC and MAPA values were significantly higher in women with PB during the second trimester (p<0.001). Additionally, PlGF levels were lower in Group 1 than in Group 2 (p<0.001).
Markers of endometrial functional insufficiency showed higher PAMG-1 levels in women with PB in both the 11–14 week and 19–22-week assessments, while lower FAMG levels were observed in this group at 19–22 weeks compared to those in Group 1 (p<0.001) and the control group (p<0.001).
These results suggest that women in both groups exhibited increased markers of systemic inflammatory response from the end of the first trimester, more pronounced in those with PB, which aligns with the known role of systemic inflammation in ED. Markers of endothelial hemostasis dysfunction were diagnostically relevant from the second trimester, with greater marker levels in women with PB. A significant decrease in PlGF levels was observed in women with FGR. Impaired endometrial receptivity was evident at 11-14 weeks in both groups, with more severe damage mechanisms developing during the second trimester in women with PB.
Conclusion
Structural and functional changes in the reproductive system caused by endometriosis significantly worsen childbearing prognosis. This is associated with an early onset of obstetric complications, characterized by a "proinflammatory state," "functional endometrial insufficiency," and "endothelial-hemostasis dysfunction." Women with endometriosis are prone to early reproductive loss, threatened preterm labor, PE, PB, and FGR. These complications are related to early pathological changes in the embryo (fetus)-placental system, exacerbated by dysregulatory disorders in the MFPS during later stages of pregnancy.
Among the most common gestational and perinatal complications in women with endometriosis, FGR (65.2%) and PB (27.4%) were the most common. Understanding these patterns provides a foundation for developing preventive strategies, including the creation of mathematical models to predict complications such as FGR, PB, and PE, enabling timely preventive measures such as progesterone therapy and low-dose acetylsalicylic acid administration.
References
- Улумбекова Г.Э., Худова И.Ю. Оценка демографического, социального и экономического эффекта применения гормональной терапии при эндометриозе и аномальных маточных кровотечениях. ОРГЗДРАВ: новости, мнения, обучение. Вестник ВШОУЗ. 2022; 8(1): 82-113. [Ulumbekova G.E., Khudova I.Yu. Demographic, social and economic effects of hormonal therapy in endometriosis and abnormal uterine bleeding. HEALTHCARE MANAGEMENT: News, Views, Education. Bulletin of VSHOUZ. 2022; 8(1): 82-113. (in Russian)]. https://dx.doi.org/10.33029/2411-8621-2022-8-1-82-113.
- Becker C.M., Bokor A., Heikinheimo O., Horne A., Jansen F., Kiesel L. et. al. ESHRE guideline: endometriosis. Hum. Reprod. 2022; 2022(2): 1-26. https://dx.doi.org/10.1093/hropen/hoac009.
- Тезиков Ю.В., Стрижаков А.Н., Липатов И.С., Калинкина О.Б., Аравина О.Р., Амосов М.С. Оптимизация тактики ведения пациенток с эндометриозом яичников и бесплодием. Акушерство и гинекология. 2021; 7: 122-32. [Tezikov Yu.V., Strizhakov A.N., Lipatov I.S., Kalinkina O.B., Aravina O.R., Amosov M.S. Optimizing management strategy in infertile patients with ovarian endometriosis. Obstetrics and Gynecology. 2021; (7): 122-32. (in Russian)]. https://dx.doi.org/10.18565/aig.2021.7.122-132.
- Ярмолинская М.И., Шалина М.А., Нагорнева С.В. Аденомиоз: современные подходы к классификации. Журнал акушерства и женских болезней. 2023;72(1): 97-108. [Yarmolinskaya M.I., Shalina M.A., Nagorneva S.V. Modern approaches to classification of adenomyosis. Journal of Obstetrics and Women's Diseases. 2023; 72(1): 97-108. (in Russian)]. https://dx.doi.org/10.17816/JOWD121307.
- Somigliana E., Garcia-Velasco J.A. Treatment of infertility associated with deep endometriosis: definition of therapeutic balances. Fertil. Steril. 2015; 104(4): 764-70. https://dx.doi.org/10.1016/j.fertnstert.2015.08.
- Соломатина А.А., Садовникова Е.А., Тюменцева М.Ю., Аргун М.З., Чабиева Л.Б., Штыров С.В., Братчикова О.В. Эндометриоз яичников малой величины. Состояние овариального резерва до и после органосохраняющих операций. Вопросы гинекологии, акушерства и перинатологии. 2019; 18(1): 20-7. [Solomatina A.A., Sadovnikova E.A., Tyumenceva M.Yu., Argun M.Z., Chabieva L.B., Shtyrov S.V., Bratchikova O.V. Small-size ovarian endometriosis. The state of ovarian reserve before and after organ-sparing surgery. Gynecology, Obstetrics and Perinatology. 2019; 18(1): 20-7. (in Russian)]. https://dx.doi.org/10.20953/1726-1678-2019-1-20-27.
- Давыдов А.И., Ищенко А.И., Унанян А.Л., Пивазян Л.Г., Азаренков Г.В., Закарян А.А., Денисова А.С., Таирова М.Б. Склеротерапия этанолом и лапароскопическая цистэктомия при эндометриоидных кистах яичников. Сравнительный анализ эффективности и репродуктивных исходов. Вопросы гинекологии, акушерства и перинатологии. 2021; 20(6): 110-9. [Davydov A.I., Ishchenko A.I., Unanyan A.L., Pivazyan L.G., Azarenkov G.V., Zakaryan A.A., Denisova A.S., Tairova M.B. Ethanol sclerotherapy and laparoscopic cystectomy for ovarian endometriomas. Comparative analysis of efficacy and reproductive outcomes. Gynecology, Obstetrics and Perinatology. 2021; 20(6): 110-9. (in Russian)]. https://dx.doi.org/10.20953/1726-1678-2021-6-110-119.
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Эндометриоз. 2020. [Ministry of Health of the Russian Federation. Clinical guidelines. Endometriosis. 2020. (in Russian)].
- Hamdan M., Omar S.Z., Dunselman G., Cheong Y. Influence of endometriosis on assisted reproductive technology outcomes. A systematic review and meta-analysis. Obstet Gynecol. 2015; 125(1): 79-88. https://dx.doi.org/10.1097/AOG.0000000000000592.
- Saraswat L., Ayansina D.T., Cooper K.G., Bhattacharya S., Miligkos D., Horne A.W. et. al. Pregnancy outcomes in women with endometriosis: a national record linkage study. BJOG. 2017; 124(3): 444-452. https://dx.doi.org/10.1111/1471-0528.13920.
- Pan M.L., Chen L.R., Tsao H.M., Chen K.H. Risk of gestational hypertension-preeclampsia in women with preceding endometriosis: A nationwide population-based study. PLoS One. 2017; 12(7): 1-13. https://dx.doi.org/10.1371/journal.pone.0181261.
- Bruun M.R., Arendt L.H., Forman A., Ramlau-Hansen C.H. Endometriosis and adenomyosis are associated with increased risk of preterm delivery and a small-for-gestational-age child: a systematic review and meta-analysis. Acta Obstet. Gynecol. Scand. 2018; 97(9): 1073-90. https://dx.doi.org/10.1111/aogs.13364.
- Qin J., Liu X., Sheng X., Wang H., Gao S. Assisted reproductive technology and the risk of pregnancy-related complications and adverse pregnancy outcomes in singleton pregnancies: A meta-analysis of cohort studies. Fertil. Steril. 2016; 105: 73-85. https:// dx.doi.org/10.1016/j.fertnstert.2015.09.007.
- Чантиева Т.М., Попов А.А., Овсянникова М.Р. Акушерские, неонатальные осложнения и исход родов в зависимости от фенотипа эндометриоза и объема хирургического лечения. Российский вестник акушера-гинеколога. 2023; 23(3): 29-40. [Chantieva T.M., Popov A.A., Ovsyannikova M.R. Obstetric, neonatal complications and birth outcome depending on the phenotype of endometriosis and the extent of surgical treatment. Russian Bulletin of Obstetrician-Gynecologist. 2023; 23(3): 29-40. (in Russian)]. https://dx.doi.org/10.17116/rosakush20232303129.
- Tsunemitsu A., Tsutsumi T., Ikura Y. Deciduosis of the appendix during pregnancy. Int. Med. 2021; 60(10): 1641-4. https://dx.doi.org/10.2169/internalmedicine.5960-20.
- Zilberman A., Eisenberg V., Yoeli R., Soriano D., Syvan E., Golan G. et. al. Uterine artery pseudoaneurysm in a pregnant patient with retrocervical endometriosis. J. Minim. Invasive Gynecol. 2020; 27(5): 1209-13. https://dx.doi.org/10.1016/j.jmig.2020.03.010.
- Липатов И.С., Тезиков Ю.В., Протасов А.Д., Мартынова Н.В., Калинкина О.Б., Приходько А.В., Зубковская Е.В., Жернакова Е.В. Пути оптимизации лечения осложненной эктопии шейки матки у нерожавших женщин. Лечение и профилактика. 2017; 2(22): 14-21. [Lipatov I.S., Tezikov Yu.V., Protasov A.D., Martynova N.V., Kalinkina O.B., Prihod'ko A.V., Zubkovskaya E.V., Zhernakova E.V. Ways to optimize treatment of complicated cervical ectropion in nulliparous women. Treatment and Prevention. 2017; 2(22): 14-21. (in Russian)].
- Vercellini P., Vigano P., Bandini V., Buggio L., Berlanda N., Somigliana E. Association of endometriosis and adenomyosis with pregnancy and infertility. Fertil. Steril. 2023; 119(5): 727-40. https://dx.doi.org/10.1016/j.fertnstert.2023.03.018.
- Овсянникова М.Р., Попов А.А., Чантиева Т.М. Фатальные осложнения в период беременности и родов у женщин с глубоким инфильтративным эндометриозом: что нового в общеклинической практике. Российский вестник акушера-гинеколога. 2023; 23(4): 9-19. [Ovsyannikova M.R., Popov A.A., Chantieva T.M. Fatal complications during pregnancy and childbirth in women with deep infiltrative endometriosis: what’s new in general clinical practice. Russian Bulletin of Obstetrician-Gynecologist. 2023; 23(4): 9-19. (in Russian)]. https://dx.doi.org/10.17116/rosakush2023230419.
- Стрижаков А.Н., Липатов И.С., Тезиков Ю.В. Плацентарная недостаточность: Патогенез, прогнозирование, диагностика, профилактика, акушерская тактика. Самара: ООО «Офорт»; 2014. 239 с. [Strizhakov A.N., Lipatov I.S., Tezikov Yu.V. Placental insufficiency: pathogenesis, prognosis, diagnosis, prevention, obstetric tactics. Samara: Ofort LLC; 2014. 239p. (in Russian)].
- Стрижаков А.Н., Тезиков Ю.В., Липатов И.С., Шарыпова М.А., Анпилогова И.В., Азизов К.У. и др. Стандартизация диагностики и клиническая классификация хронической плацентарной недостаточности. Вопросы гинекологии, акушерства и перинатологии. 2014; 13(3): 5-12. [Strizhakov A.N., Tezikov Yu.V., Lipatov I.S., Sharypova M.A., Anpilogova I.V., Azizov K.U. et al. Standardization of diagnosis and clinical classification of chronic placental insufficiency. Gynecology, Obstetrics and Perinatology. 2014; 13(3): 5-12. (in Russian)].
- Тезиков Ю.В., Липатов И.С., Рябова С.А., Тезикова Т.А., Ефимова Л.В., Ракитина В.Н. Перинатальная хрономедицина: биоритмостаз плода при неосложненной беременности и плацентарной недостаточности. Известия Самарского научного центра Российской академии наук. 2014; 16(5-4): 1467-70. [Tezikov Yu.V., Lipatov I.S., Ryabova S.A., Tezikova T.A., Efimova L.V., Rakitina V.N. Perinatal сhronomediсine: fetus biorhythmostasis at uncomplicated pregnancy and placental insufficiency. News of the Samara Scientific Center of the Russian Academy of Sciences. 2014; 16(5-4): 1467-70. (in Russian)].
- Сухих Г.Т., Серов В.Н., Адамян Л.В., Баранов И.И., Беженарь В.Ф., Габидуллина Р.И., Дубровина С.О., Козаченко А.В., Подзолкова Н.М., Сметник А.А., Тапильская Н.И., Уварова Е.В., Ших Е.В., Ярмолинская М.И. Алгоритмы ведения пациенток с эндометриозом: согласованная позиция экспертов Российского общества акушеров-гинекологов. Акушерство и гинекология. 2023; 5: 159-76. [Sukhikh G.T., Serov V.N., Adamyan L.V., Baranov I.I., Bezhenar V.F., Gabidullina R.I., Dubrovina S.O., Kozachenko A.V., Podzolkova N.M., Smetnik A.A., Tapilskaya N.I., Uvarova E.V., Shikh E.V., Yarmolinskaya M.I. Algorithms for the management of patients with endometriosis: an agreed position of experts from the Russian Society of Obstetricians and Gynecologists. Obstetrics and Gynecology. 2023; (5): 159-76. (in Russian)]. https://dx.doi.org/10.18565/aig.2023.132.
- Тезиков Ю.В., Липатов И.С., Зуморина Э.М., Азаматов А.Р., Тютюнник В.Л., Кан Н.Е., Чекаловец А.Л., Борисова А.И., Голоднова А.М. Клинико-патогенетическое обоснование двухэтапной профилактики преэклампсии у женщин высокого риска с применением инсулиносенситайзера для преконцепционной подготовки. Акушерство и гинекология. 2023; 9: 60-71. [Tezikov Yu.V., Lipatov I.S., Zumorina E.M., Azamatov A.R., Tyutyunnik V.L., Kan N.E., Chekalovets A.L., Borisova A.I., Golodnova A.M. Clinical and pathogenetic rationale for two-stage prevention of preeclampsia in high-risk women using an insulin sensitizer for preconception preparation. Obstetrics and Gynecology. 2023; (9): 60-71. (in Russian)]. https://dx.doi.org/10.18565/aig.2023.139.
- Щербаков В.И., Поздняков И.М., Ширинская А.В., Волков М.В. Роль провоспалительных цитокинов в патогенезе преждевременных родов и преэклампсии. Российский вестник акушера-гинеколога. 2020; 20(2): 15-21. [Shcherbakov V.I., Pozdnyakov I.M., Shirinskaya A.V., Volkov M.V. Role of pro-inflammatory cytokines in the pathogenesis of preterm birth and preeclampsia. Russian Bulletin of Obstetrician-Gynecologist. 2020; 20(2): 15-21. (in Russian)]. https://dx.doi.org/10.17116/rosakush20202002115.
- Vilotic A., Nacka-Aleksic M., Pirkovic A., Bojic-Trbojevic Z., Dekanski D., Krivokuca M.J. IL-6 and IL-8: An overview of their roles in healthy and pathological pregnancies. Int. J. Mol. Sci. 2022; 23(23): 1-32. https://dx.doi.org/10.3390/ijms232314574.
- Piccinni M., Raghupathy R., Saito S., Szekeres-Bartho J. Cytokines, hormones and cellular regulatory mechanisms favoring successful reproduction. Front. Immunol. 2021; 28(12): 717808. https://dx.doi.org/10.3389/fimmu.2021.717808.
- Посисеева Л.В., Панова О.В. Роль плацентарных белков у беременных при подготовке к родам. Российский вестник акушера-гинеколога. 2022; 22(1): 5-10. [Posiseeva L.V., Panova O.V. The role of placental proteins in pregnant women during preparation for childbirth. Russian Bulletin of Obstetrician-Gynecologist. 2022; 22(1): 5-10. (in Russian)]. https://dx.doi.org/10.17116/rosakush2022220115.
- Липатов И.С., Тезиков Ю.В., Шмаков Р.Г., Азаматов А.Р., Мартынова Н.В. «Беременность – естественная модель метаболического синдрома»: результаты динамического исследования физиологической гестации. Акушерство и гинекология. 2020; 9: 88-96. [Lipatov I.S., Tezikov Yu.V., Shmakov R.G., Azamatov A.R., Martynova N.V. Pregnancy is a natural model of metabolic syndrome: results of a dynamic study of physiological gestation Obstetrics and Gynecology. 2020; (9): 88-96. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.9.88-96.
- Тезиков Ю.В., Липатов И.С., Калинкина О.Б., Гогель Л.Ю., Белоконева Т.С., Мартынова Н.В., Жернакова Е.В., Юсупова Р.Р., Мингалиева Л.К. Стратификация беременных на ранних сроках гестации путем объективизации факторов «физиологической альтерации», механизмов гестационной адаптации и эмбриоплацентарной дисфункции. Наука и инновации в медицине. 2016; 1(4): 6-13. [Tezikov Yu.V., Lipatov I.S., Kalinkina O.B., Gogel' L.Yu.,
- Belokoneva T.S., Martynova N.V., Zhernakova E.V., Yusupova R.R., Mingalieva L.K. Stratification of pregnant women at early gestational ages by means of objectivation of «physiological alteration» factors, mechanisms of gestational adaptation and fetoplacental dysfunction. Science and Innovations in Medicine 2016; 1(4): 6-13. (in Russian)].
- Липатов И.С., Тезиков Ю.В., Азаматов А.Р., Шмаков Р.Г. Общность клинических проявлений преэклампсии и метаболического синдрома: поиск обоснования. Акушерство и гинекология. 2021; 3: 81-9. [Lipatov I.S., Tezikov Yu.V., Azamatov A.R., Shmakov R.G. Identity of preeclampsia and metabolic syndrome clinical manifestations: searching for substantiation. Obstetrics and Gynecology. 2021; (3): 81-9. (in Russian)]. https://dx.doi.org/10.18565/aig.2021.3.81-89.
- Günther V., Allahqoli L., Deenadayal-Mettler A., Maass N., Mettler L., Gitas G. et al. Molecular determinants of uterine receptivity: comparison of successful implantation, recurrent miscarriage, and recurrent implantation failure. Int. J. Mol. Sci. 2023; 24(24): 17616. https://dx.doi.org/10.3390/ijms242417616.
Received 14.06.2024
Accepted 30.09.2024
About the Authors
Yurii V. Tezikov, Professor, Dr. Med. Sci., Head of the Department of Obstetrics and Gynecology of the Institute of Clinical Medicine, Samara State Medical University,Ministry of Health of Russia, 443099, Russia, Samara, Chapaevskaya str., 89, +7(846)958-24-18, yra.75@inbox.ru, Researcher ID: С-6187-2018, SPIN-code: 2896-6986,
Author ID: 161372, Scopus Author ID: 6603787595, https://orcid.org/0000-0002-8946-501X
Igor S. Lipatov, Professor, Dr. Med. Sci., Professor at the Department of Obstetrics and Gynecology of the Institute of Clinical Medicine, Samara State Medical University, Ministry of Health of Russia, 443099, Russia, Samara, Chapaevskaya str., 89, +7(846)958-24-18, i.lipatoff2012@yandex.ru, Researcher ID: С-5060-2018, SPIN-code: 9625-2947, Author ID: 161371, Scopus Author ID: 6603787595, https://orcid.org/0000-0001-7277-7431
Mikhail S. Amosov, Teaching Assistant at the Department of Obstetrics and Gynecology of the Institute of Clinical Medicine, Samara State Medical University, Ministry of Health of Russia, 443099, Russia, Samara, Chapaevskaya str., 89, +7(846)958-24-18, jyckee@mail.ru, Researcher ID: KQU-5863-2024, SPIN-code: 5800-6716,
Author ID: 1095765, Scopus Author ID: 57223148034, https://orcid.org/0000-0002-7487-3280



