Study of inflammatory and angiogenic biomarker levels in various biological fluids in women with extragenital endometriosis
Kudryavtseva E.V., Mangileva Ya.A., Polushina L.G., Maksimova A.Yu., Kopenkin M.A., Zornikov D.L., Kovalev V.V.
Objective: To analyze the content of biomarkers of inflammation and angiogenesis in oral and peritoneal fluids in women with a common form of extragenital endometriosis including ovarian lesions.
Materials and methods: This was a comparative observational study. Group 1 (n=88) consisted of women with confirmed extragenital endometriosis including endometrioid ovarian cysts; group 2 (n=30) consisted of healthy women without signs of endometriosis. The samples of oral fluid (OF) were collected from all participants of the study, and samples of peritoneal fluid (PF) were collected from women of group 1. The levels of vascular endothelial growth factor (VEGF), tumor necrosis factor alpha (TNF-α), and interleukins (IL), namely IL-1β, IL-6, IL-8, and IL-10 were studied in biological fluids.
Results: The level of VEGF in OF was significantly higher in the patients of group 1, namely 906.4 (281.9–178) pg/ml compared to the patients of group 2, 241.9 (105.225–324.475) pg/ml, p<0.001. The level of IL-8 was also significantly higher in group 1 compared to group 2, 100.85 (23.333–208.7) pg/ml versus 11.34 (6.549–21.205) pg/ml, respectively, p<0.001. The levels of TNF-α, IL-6, and IL-10 were higher in group 2. The analysis of the correlation between the levels of markers in OF and PF showed no correlation for IL-8, IL-10 and TNF-α and the presence of a very weak, nonlinear relationship for IL-1β, IL-6 and VEGF.
Conclusion: The data obtained suggest the independence of inflammatory and angiogenic biomarker profiles in different biological compartments. The results highlight the potential for future research into OF as a potential bio substrate that could indirectly reflect the characteristics of the systemic inflammatory response in patients with extragenital endometriosis.
Authors’ contributions: Kudryavtseva E.V., Kovalev V.V. – developing the concept and design of the study; Mangileva Ya.A., Polushina L.G., Kopenkin M.A., Maksimova A.Yu. – collecting and processing the material; Kudryavtseva E.V., Zornikov D.L. – statistical processing of the data; Kudryavtseva E.V., Mangileva Ya.A. – writing the text; Zornikov D.L., Kovalev V.V. – editing the article.
Conflicts of interest: The authors declare no possible conflicts of interest.
Funding: The study was conducted without sponsorship.
Ethical Approval: The study was approved by the Ethical Review Board of the Ural State Medical University, Ministry of Health of Russia (Protocol No.11 dated 24/12/2021).
Patient Consent for Publication: The patients signed informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Kudryavtseva E.V., Mangileva Ya.A., Polushina L.G., Maksimova A.Yu., Kopenkin M.A.,
Zornikov D.L., Kovalev V.V. Study of inflammatory and angiogenic biomarker levels in various
biological fluids in women with extragenital endometriosis.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (7): 112-120 (in Russian)
https://dx.doi.org/10.18565/aig.2025.116
Keywords
Endometriosis is a common disease among reproductive-aged women characterized by the presence of endometrial tissue outside the uterus, which can lead to painful menstruation, infertility, and other reproductive problems [1, 2]. In recent years, the number of women suffering from endometriosis has steadily increased, which has led to an urgent need to find and develop more effective diagnostic methods. These methods should meet several requirements, including being low-cost and non-invasive [3–5].
Recent studies have shown that endometriosis is associated with changes in the levels of various biomarkers, including cytokines, angiogenesis factors, for example, vascular endothelial growth factor (VEGF), and other molecules that are thought to play a key role in the pathogenesis of the disease [6, 7]. In particular, such molecules as VEGF, tumor necrosis factor alpha (TNF-α), and interleukins (IL) can influence the processes of inflammation, angiogenesis, and tissue damage. These processes may have clinical consequences for women with endometriosis [8, 9].
A number of scientific papers are devoted to the study of the dynamic characteristics of cytokine levels and other biological markers in oral fluid (OF) and peritoneal fluid (PF) [10–12].
Endometriosis significantly influences the levels of cytokines in the pancreas, which are associated with inflammation and the destruction of tissues affected by the disease. However, detecting these changes directly in the affected area is difficult due to the need for an invasive procedure such as laparoscopy [7, 10, 13]. Examination of PF is likely to provide valuable information about the local inflammatory processes in the abdominal cavity. However, obtaining this information involves an invasive procedure. On the other hand, OF is an alternative biological fluid that can be easily obtained without any invasion [14]. Thus, it can be a potentially useful substrate for studying biomarkers [11, 15]. OF has some advantages over plasma/serum, since the collection of this biomaterial is simple, safe, non-invasive, cost-effective and does not require training of medical personnel [16].
At the same time, it is not clear to what extent changes in biomarkers in OF can reflect the characteristics of the systemic inflammatory processes in extragenital endometriosis, and to what extent they are independent of local changes in PF. The study of the interactions between these components can lead to a better understanding of the underlying mechanisms of the disease.
Simplifying methods for obtaining biological material in order to identify biomarkers in various patient groups at risk can help us better understand the pathophysiology of endometriosis. This information could lead to the development of more accurate and non-invasive diagnostic and prognostic tools, which would significantly improve the patients’ compliance [10, 17].
The aim of the study is to analyze the content of biomarkers of inflammation and angiogenesis in OF and PF in women with a common form of extragenital endometriosis including ovarian lesions.
Materials and methods
This was a comparative observational study which included 118 women. Group 1 consisted of 88 women with confirmed extragenital endometriosis, including endometrioid ovarian cysts; these patients had to undergo surgical treatment (laparoscopy, cystectomy, coagulation of endometriotic lesions). The presence of ovarian endometriosis as well as the presence of heterotopias on the peritoneum was confirmed visually (stage III/IV endometriosis according to R-AFS score), and by histological examination in all women. The comparison group (group 2) included 30 women who did not suffer from infertility and had no signs of extragenital endometriosis during ultrasound assessment.
The study was approved by the Ethical Review Board of the Ural State Medical University, protocol No.11 dated 24/12/2021.
There were the following inclusion criteria in the study: age 18–40 years, consent to participate in scientific research and surgery (for patients of group 1), ovarian endometriosis detected by ultrasound and confirmed by histological examination after laparoscopy (for patients of group 1), the absence of inflammatory diseases of the oral cavity (all study participants were examined by a dentist), the absence of somatic diseases that can significantly affect the level of markers studied, the absence of occupational hazards and habitual intoxications.
The exclusion criteria were inflammatory diseases of the oral cavity, the presence of an autoimmune disease, infectious diseases in the acute stage, oncological diseases, diseases of the liver, kidneys or endocrine system in the sub- or decompensation stage, pregnancy at the time of inclusion in the study, hormone therapy for 6 months before and at the time of inclusion in the study, taking immunosuppressants, smoking, the presence of occupational hazards, refusal to participate in the study.
The samples of OF were collected from all participants of the study, and samples of PF were collected from women of group 1.
OF (saliva) was collected in the morning, on an empty stomach, after preliminary rinsing of the oral cavity with boiled water (OF was collected on the day of surgery in group 1). OF was collected using the passive salivation method in accordance with the recommendations of the World Endometriosis Research Foundation for general biomarker studies [18].
All patients of the studied groups also had a general clinical examination as part of a medical follow-up.
The samples of PF were obtained during surgery after the introduction of a video endoscope and small trocars into the abdominal cavity. After visualization of all organs and confirmation of the presence of endometrioid changes, a surgical aspiration tube was inserted into one of the trocars and a sterile disposable syringe was attached to the end of the tube. PF was aspirated from the pouch of Douglas and other peritoneal pockets. All collected samples were placed in 5 ml Eppendorf tubes for further storage and analysis.
All obtained samples of OF and PF were immediately labeled and placed in a refrigerator for storage at -40° C, no later than 4 hours after collecting. The samples were stored in the biobank of the Ural State Medical University (USMU).
Laboratory studies were performed at the Central Research Laboratory of the USMU. The analysis included the determination of levels of VEGF, TNF-α, IL-1β, IL-6, IL-8, IL-10, which are important markers of inflammation and angiogenesis. All studies were conducted using test systems produced by the Vector-Best Company (Russia). An enzyme immunoassay analyzer Thermo Scientific Multiskan GO (Japan) was used to perform the analysis.
Statistical analysis
All statistical calculations were carried out using the R programming language and version 4.4.2. The Shapiro–Wilk test was used to test the hypothesis of the normality distribution. To analyze the quantitative data, the median (Me) and interquartile range (IQR) between the 25th and 75th percentiles were indicated. Absolute and relative frequencies (%) were calculated for categorical variables. The Mann–Whitney U test was used to compare groups based on continuous variables. The chi-square test (c2) was used to compare categorical variables. Spearman’s rank correlation coefficient was used to study the relationship between variables. All the results of the statistical tests were found to be statistically significant at the level of p<0.01.
Results
All the participants in the study were residents of the Sverdlovsk region; all the subjects of the study were Caucasians. The initial characteristics of the patients from the study groups are presented in Table 1.
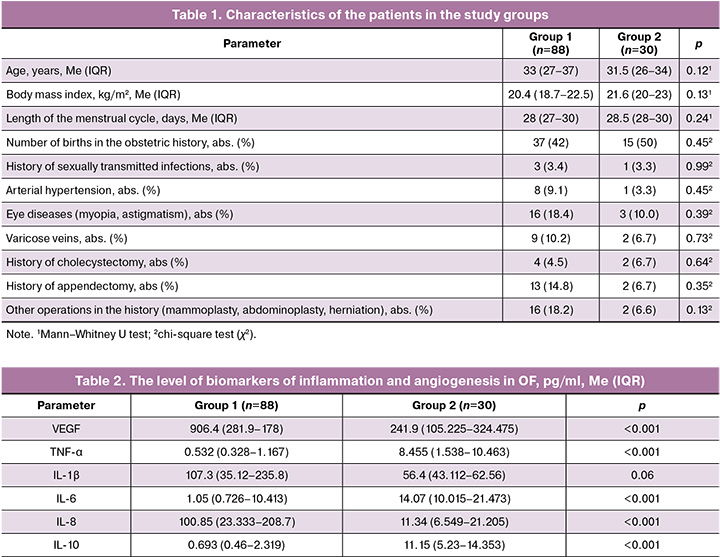
The clinically studied groups were comparable, and there were no statistically significant differences between them.
Initially, we compared the levels of markers in OF. The results are presented in Table 2. Statistically significant differences were found in all parameters except IL-1β.
The differences between the groups according to the studied criteria are clearly presented in the graphs (Fig. 1).

The levels of TNF-α, IL-6 and IL-10 in OF were higher in the group of healthy women (group 2). The indicators of VEGF and IL-8 were significantly higher in patients with extragenital endometriosis (group 1) than in the patients of the comparison group. The level of IL-1β which is one of the most potent inflammatory mediators was also higher in group 1, but the differences did not reach statistical significance (p=0.06).
The level of the markers in PF was determined only in women included in group 1, since conducting invasive procedures only for the purpose of collecting PF for scientific research in healthy women is not ethically justified. The values of the biomarker levels in PF are shown in Table 3.
Then we analyzed the correlation between the levels of biomarkers in OF and PF. The results are shown in Figure 2. A very weak nonlinear relationship was found between the levels of IL-1β and IL-6 in OF and RF (direct dependence) and VEGF (inverse dependence). There was no relationship between TNF-α, IL-8, and IL-10 levels. No statistically significant correlation was found.

Discussion
Currently, more and more researchers agree that endometriosis is not simply the presence of endometrium–like tissue outside the uterus, but a chronic systemic inflammatory disease [1, 19]. Therefore, changes in the level of inflammatory markers in endometriosis can be observed not only near endometriotic lesions, for example, in PF, but also in other biological fluids. It is believed that inflammation associated with immune system dysfunction is one of the main pathogenic mechanisms in the development of diseases accompanied by abnormal cell proliferation and infiltration [19]. This study provided the analysis of the levels of inflammation and angiogenesis biomarkers in OF and PF in women with ovarian endometriosis. It is important to consider the data obtained in relation to the pathogenesis of endometriosis and the function of the biomarkers.
Our results showed that the levels of TNF-α, IL-6 and IL-10 in PF were significantly higher in the group of healthy women (group 2) than ones in the group of patients with endometriosis (group 1). This may be indicative of a disturbed normal balance of inflammatory markers in women with endometriosis. One of the possible causes for the decrease in the levels of these cytokines in saliva of women with extragenital endometriosis is the peculiarities of the immune response due to the chronic inflammation.
TNF-α is an important mediator of inflammation that promotes the activation of inflammatory cells and enhances the production of other cytokines. At the same time, TNF-α is involved in a number of processes that have antitumor and cytotoxic effects [6]. A relatively higher TNF-α level in PF in healthy women may reflect a normal immune system response to various stimuli. Its decrease in OF in patients with endometriosis may indicate a weakened systemic anti-inflammatory response or a dysfunction of the whole immune system. Our results are somewhat contradictory to previous scientific studies, which have shown that TNF-α activates a nuclear factor that is the main regulator of the inflammatory response and plays an important role in the development of endometriosis [20]. However, in most studies that indicate the important role of TNF-α in the pathogenesis of endometriosis, its increase was noted in two biological fluids – in PF and in follicular fluid, rather than in OF or in blood serum, as demonstrated in the scientific review by Brulport A. et al. (2024) [17]. The evaluation of this marker, for example, in serum and plasma showed low sensitivity and specificity for endometriosis. In modern scientific literature, there are directly opposite points of view about the level of this indicator. Some studies demonstrated an increase in this marker, while others showed a decrease [17, 21]. However, there have been no studies investigating the association between this marker and endometriosis in OF.
IL-6 plays a key role in inflammation, regulates the activation of T cells and the production of other cytokines. In case of endometriosis, IL-6 increases inflammation in the endometriotic lesions and promotes angiogenesis, as shown in the study by Chen S. et al. (2023), where IL-6 was described as one of the key factors stimulating angiogenesis and degradation of the extracellular matrix [22]. In our study, the level of IL-6 in OF in women with endometriosis was low, namely 1.05 (0.726–10.413) pg/ml compared with 14.07 (10.015–21.473) pg/ml in healthy women. The level of IL-6 in PF of women with ovarian endometriosis was much higher than one in OF, and amounted to 38.225 (16.7525–129.85) pg/ml. It is likely that the local rather than systemic increase in IL-6 plays a more significant role in the pathogenesis of endometriosis. For example, in the study by Kovalak E.E. et al. (2023), IL-6 levels in blood serum were analyzed among other markers. This indicator did not show statistically significant differences when compared to the control group [21]. In addition, a high level of IL-6 in PF may not be an etiopathogenetic factor, but a consequence of the presence of endometriotic lesions.
IL-10 is a cytokine with potent anti–inflammatory properties that help control excessive inflammation. In our study, the level of IL-10 in OF was higher in women from group 2, namely 11.15 (5.23–14.353) pg/ml, compared to 0.693 (0.46–2.319) pg/ml in women in group 1. A relative increase in IL-10 levels in OF in healthy women may indicate the regulation of inflammatory processes and immune balance. In endometriosis, its role is more complicated: IL-10 can suppress excessive inflammatory activity, but it also helps maintain immune tolerance in the endometriotic lesions, which allows endometriotic cells to exist and develop. This is indirectly confirmed by the relatively high level of IL-10 in PF, namely 19.22 (11.08–27.275) pg/ml. A local increase in the level of the multifunctional cytokine IL-10 was demonstrated in the study by Nanda A. et al. (2020). The authors compared the cytokine profile of PF in women with endometriosis and tubal infertility factor and found that IL-10 expression was higher in women with endometriosis [7]. However, in our opinion, this study is not entirely correct, since the control group consisted not of healthy women, but of women with tubal infertility factor, who may have altered levels of various biomarkers of PF and reduced levels of anti-inflammatory cytokines, which include IL-10.
The levels of VEGF and IL-8 in OR in the group of patients with endometriosis were significantly higher, which indirectly confirms their active role in the pathogenesis of the disease.
VEGF is the main factor contributing to neovascularization. In endometriosis, VEGF promotes the development of new vessels in the foci of the disease, which allows endometriosis cells to survive and actively spread [6]. This explains the increased levels of VEGF in OF in women with endometriosis, which supports the hypothesis of its role in angiogenesis in endometriosis. It is known that therapeutic agents leading to a decrease in the systemic level of VEGF demonstrate clinical efficacy in endometriosis [8, 23, 24].
IL-8 is an important chemokine that stimulates neutrophil migration and supports inflammation [22]. IL-8 level in OF in patients of group 1 were significantly higher than one in group 2, namely 100.85 (23.333–208.7) and 11.34 (6.549–21.205) pg/ml, respectively. The level of this indicator was significantly higher in PF in patients of group 1 than one in OF in patients of group 2, namely 37.59 (23.333–174.875) pg/ml. The role of IL-8 in endometriosis, as one of the most significant mediators in this disease, has been highlighted in several scientific studies [22, 25]. The significant contribution of IL-8 to the progression of ectopic endometrium proliferation has been demonstrated, in particular, through the use of long-acting, recirculating antibodies against IL-8 in animal models, which led to a reduction in endometriotic lesions [25]. In addition, some studies have shown that the level of IL-8 in the follicular fluid can be a good indicator of embryo quality in women with endometriosis [26].
Although the level of IL-1β in the group of patients with endometriosis did not reach statistical significance, we observe a tendency to its higher level in group 1 than in group 2, namely 107.3 (35.12–235.8) and 56.4 (43.113–62.56) pg/ml, respectively. IL-1β is a potent inflammatory mediator that stimulates the production of other cytokines, promotes cellular stress and angiogenesis. In case of endometriosis, interleukin-1β can increase the permeability of blood vessels, promote inflammation and angiogenesis in endometriotic lesions.
In our study, we found essential differences in the cytokine profile of OF between women with endometriosis and healthy women. Most of the markers we studied showed highly significant statistical differences. Further study of the level of these markers in PF seems promising from the point of view of developing noninvasive methods for the diagnosis of endometriosis. Currently, international scientific publications describe only one commercially available test for the noninvasive diagnosis of endometriosis based on the use of OF (saliva). This test, called Endotest, involves analyzing the microRNA profile of saliva [11]. However, a number of researchers are skeptical about this test due to a number of related problems, including complex machine learning for using a set with a large number of points, as well as validation of the test in a limited population (it is known that microRNAs differ between different ethnic groups) [27, 28]. The use of cytokine levels in OF for noninvasive diagnosis of endometriosis has several advantages. Firstly, the cost of these tests is significantly lower compared to other methods. Secondly, the results of studies conducted on different patient populations are more consistent and reproducible.
The analysis of the relationship between the levels of markers in OF and PF showed no correlation for IL-8, IL-10 and TNF-α and the presence of a very weak, nonlinear relationship for IL-1β, IL-6 and VEGF (the correlation coefficient for them was 0.316, 0.213 and -0.26, respectively). Even if there is statistical significance, the correlation coefficient value is less than 0.3, which indicates a lack of correlation [29]. It is possible that local changes in the levels of certain cytokines and angiogenesis factors in OF may be a consequence of the inflammation and angiogenesis in the body, including changes in their levels in PF. However, these relationships require further study.
OF can be considered as an additional source of information when taking an integrated approach to studying endometriosis. But in order to assess the diagnostic value of this test, further research is necessary. This research should include a wider range of biomarkers and a more comprehensive analysis, as well as comparisons with clinical manifestations of the disease. We believe that changes in markers of inflammation and angiogenesis in OF in women without oral diseases may reflect some systemic changes in the immune system. These changes may enhance proliferation and angiogenesis, and reduce apoptosis, creating conditions for the life support, growth, and development of ectopic endometrioid tissue. The level of these indicators in PF more reflects the local inflammatory process and may be not only a pathogenetic factor in the development of endometriosis, but also a consequence of its presence. As previously emphasized in the scientific literature, it is essential to distinguish between systemic and local compartments when identifying endometriosis biomarkers [17]. Contradictory scientific research data is to some extent explained by the use of various biological fluids.
The changes in the mediators examined in this research can be seen in a variety of illnesses, not only endometriosis [22]. When developing noninvasive diagnostic methods, it is essential to create combined models. Their sensitivity and specificity are likely to be significantly higher compared to using single indicators alone.
Conclusion
In this study, we assessed the levels of biomarkers for inflammation and angiogenesis in different body fluids in women with a common form of extragenital endometriosis, including ovarian lesions.
Differences in cytokine profiles were found between patients with extragenital endometriosis and healthy women. These differences were particularly evident in terms of the levels of VEGF and IL-8. However, correlation analysis demonstrated the absence or extremely weak relationship between the levels of the same markers in different biological fluids.
The data obtained suggest the independence of local and systemic processes of inflammation and angiogenesis in extragenital endometriosis. The use of OF as a bio substrate for assessing immune changes needs further research as part of a comprehensive study that includes simultaneous assessment of clinical symptoms and systemic biomarkers.
References
- Horne A.W., Missmer S.A. Pathophysiology, diagnosis, and management of endometriosis. BMJ. 2022: e070750. https://dx.doi.org/10.1136/bmj-2022-070750
- Адамян Л.В., Андреева Е.Н. Эндометриоз и его глобальное влияние на организм женщины. Проблемы репродукции. 2022; 28: 54-64. [Adamyan L.V., Andreeva E.N. Endometriosis and its global impact on a woman’s body. Russian Journal of Human Reproduction. 2022; 28(1): 54 64 (in Russian)]. https://dx.doi.org/10.17116/repro20222801154
- Shimura K., Tarumi Y., Fujii M., Ogawa K., Maeda E., Tanaka Y. et al. Low-nutrient environment-induced changes in inflammation, cell proliferation, and PGC-1α expression in stromal cells with ovarian endometriosis. Reprod. Sci. 2022; 30: 1094-102. https://dx.doi.org/10.1007/s43032-022-01089-5
- Didziokaitė G., Biliute G., Gudaite J., Kvedarienė V. Oxidative stress as a potential underlying cause of minimal and mild endometriosis-related infertility. Int. J. Mol. Sci. 2023; 24(4): 3809. https://dx.doi.org/10.3390/ijms24043809
- Дубровина С.О., Берлим Ю.Д., Александрина А.Д., Вовкочина М.А., Богунова Д.Ю., Гимбут В.С., Божинская Д.М. Современные представления о диагностике и лечении эндометриоза. Акушерство и гинекология. 2023: 2: 146-53. [Dubrovina S.O., Berlim Yu.D., Aleksandrina A.D., Vovkochina M.A., Bogunova D.Yu., Gimbut V.S., Bozhinskaya D.M. Modern ideas about the diagnosis and treatment of endometriosis. Obstetrics and Gynecology. 2023; (2): 146-53 (in Russian)]. https://dx.doi.org/10.18565/aig.2023.43
- Li W., LibinWeng. Examination on risk factors of infertility caused by EMT and their correlation with VEGF, TNF-α, IL-6, IL-10, and IL-17. Contrast Media Mol. Imaging. 2022; 2022: 4421418. https://dx.doi.org/10.1155/2022/4421418
- Nanda A., Thangapandi K., Banerjee P., Dutta M., Wangdi T., Sharma P. et al. Cytokines, angiogenesis, and extracellular matrix degradation are augmented by oxidative stress in endometriosis. Ann. Lab. Med. 2020; 40(5): 390-7. https://dx.doi.org/10.3343/alm.2020.40.5.390
- Liu Y., Fu H., Zuo L. Anti-inflammatory activities of a new VEGF blocker, conbercept. Immunopharmacol. Immunotoxicol. 2021; 43(5): 594-8. https://dx.doi.org/10.1080/08923973.2021.1959608
- Akhmedova S.R., Omarov N.S.M. Determination of cytokine status indicators and angiogenesis factors in patients with external genital endometriosis and vitamin D deficiency. Periodico Tche Quimica. 2020; 17(34): 251-9. https://dx.doi.org/10.52571/PTQ.v17.n34.2020.268_P34_pgs_251_259.pdf
- Кудрявцева Е.В., Геец А.В., Мангилева Я.А., Чижова А.В., Пацюк О.В. Современные неинвазивные методы диагностики эндометриоза. Уральский медицинский журнал. 2023; 22(4): 140-7. [Kudryavtseva E.V., Geets A.V., Mangileva Y.A., Chizhova A.V., Patsyuk O.V. Modern non-invasive diagnosis of endometriosis. Ural Medical Journal. 2023; 22(4): 140-7 (in Russian)]. https://dx.doi.org/10.52420/2071-5943-2023-22-4-140-147
- Ferrier C., Bendifallah S., Suisse S., Dabi Y., Touboul C., Puchar A. et al. Saliva microRNA signature to diagnose endometriosis: a cost‐effectiveness evaluation of the Endotest®. BJOG 2022; 130(4): 396-406. https://dx.doi.org/10.1111/1471-0528.17348
- Dabi Y., Suisse S., Puchar A., Delbos L., Poilblanc M., Descamps P. et al. Endometriosis-associated infertility diagnosis based on saliva microRNA signatures. Reprod. Biomed. Online. 2023; 46(1): 138-49. https://dx.doi.org/10.1016/j.rbmo.2022.09.019
- Борисова А.В., Чаговец В.В., Козаченко А.В., Стародубцева Н.Л., Кононихин А.С., Салимова Д.Ф., Коган Е.А., Адамян Л.В., Франкевич В.Е., Сухих Г.Т. Сравнительный анализ липидного состава перитонеальной жидкости и плазмы крови у пациенток с наружным генитальным эндометриозом и миомой матки. Акушерство и гинекология. 2017; 6: 74-82. [Borisova A.V., Chagovets V.V., Kozachenko A.V., Starodubtseva N.L., Kononikhin A.S., Salimova D.F., Kogan E.A., Adamyan L.V., Frankevich V.E., Sukhikh G.T. Comparative analysis of the lipid composition of peritoneal fluid and blood plasma in patients with external genital endometriosis and uterine myoma. Obstetrics and Gynecology. 2017; (6): 74-82 (in Russian)]. https://dx.doi.org/10.18565/aig.2017.6.74-82
- Копенкин М.А., Басова Е.А., Полушина Л.Г., Базарный В.В. Стабильность аналитов ротовой жидкости при разных условиях центрифугирования. Вестник УГМУ. 2024; 2: 7-18. [Kopenkin M.A., Basova E.A., Polushina L.G., Bazarny V.V. Stability of oral fluid analytes under different centrifugation conditions. USMU Medical Bulletin. 2024; 2: 7-18 (in Russian)].
- Bakun O. A modern noninvasive methods of diagnosis genital endometriosis associated with infertility. Clinical Anatomy and Operative Surgery. 2024; 23(1): 153-9. https://dx.doi.org/10.24061/1727-0847.23.1.2024.21
- Perricos A., Proestling K., Husslein H., Kuessel L., Hudson Q.J., Wenzl R. et al. Hsa-mir-135a shows potential as a putative diagnostic biomarker in saliva and plasma for endometriosis. Biomolecules. 2022; 12(8): 1144. https://dx.doi.org/10.3390/biom12081144
- Brulport A., Bourdon M., Vaiman D., Drouet C., Pocate-Cheriet K., Bouzid K. et al. An integrated multi-tissue approach for endometriosis candidate biomarkers: a systematic review. Reprod. Biol. Endocrinol. 2024; 22(1): 21. https://dx.doi.org/10.1186/s12958-023-01181-8
- Rahmioglu N., Fassbender A., Vitonis A.F., Tworoger S.S., Hummelshoj L., D’Hooghe T.M. et al. World endometriosis research foundation endometriosis phenome and Biobanking harmonization project: III. Fluid biospecimen collection, processing, and storage in endometriosis research. Fertil. Steril. 2014; 102(5): 1233-43. https://dx.doi.org/10.1016/j.fertnstert.2014.07.1208
- Lamceva J., Uljanovs R., Strumfa I. The main theories on the pathogenesis of endometriosis. Int. J. Mol. Sci. 2023; 24(5): 4254. https://dx.doi.org/10.3390/ijms24054254
- Zhang P., Wang G. Progesterone resistance in endometriosis: current evidence and putative mechanisms. Int. J. Mol. Sci. 2023; 24(8): 6992. https://dx.doi.org/10.3390/ijms24086992
- Kovalak E.E., Karacan T., Zengi O., Akgül Ö.K., Özyürek Ş.E., Güraslan H. Evaluation of new biomarkers in stage III and IV endometriosis. Gynecol. Endocrinol. 2023; 39(1): 2217290. https://dx.doi.org/10.1080/09513590.2023.2217290
- Chen S., Liu Y., Zhong Z., Wei C., Liu Y., Zhu X. Peritoneal immune microenvironment of endometriosis: role and therapeutic perspectives. Front. Immunol. 2023; 14: 1134663. https://dx.doi.org/10.3389/fimmu.2023.1134663
- Sun S., Zhang H., Zhong P., Xu Z. The effect of letrozole combined with dydrogesterone for endometriosis in China: a meta-analysis. Biomed. Res. Int. 2021; 9946060. https://dx.doi.org/10.1155/2021/9946060
- Aliabad R.A., Hassanpour K., Norooznezhad A.H. Cannabidiol as a possible treatment for endometriosis through suppression of inflammation and angiogenesis. Immun. Inflamm. Dis. 2024; 12(8): e1370. https://dx.doi.org/10.1002/iid3.1370
- Nishimoto-Kakiuchi A., Sato I., Nakano K., Ohmori H., Kayukawa Y., Tanimura H. et al. A long-acting anti-IL-8 antibody improves inflammation and fibrosis in endometriosis. Sci. Transl. Med. 2023; 15(684): eabq5858. https://dx.doi.org/10.1126/scitranslmed.abq5858
- Singh A.K., Dutta M., Chattopadhyay R., Chakravarty B., Chaudhury K. Intrafollicular interleukin-8, interleukin-12, and adrenomedullin are the promising prognostic markers of oocyte and embryo quality in women with endometriosis. J. Assist. Reprod. Genet. 2016; 33: 1363-72. https://doi.org/10.1007/s10815-016-0782-5
- Scheck S.M., Henry C., Bedford N., Abbott J., Wynn‐Williams M., Yazdani A. et al. Non‐invasive tests for endometriosis are here; how reliable are they, and what should we do with the results? Austr. N. Z. J. Obstet. Gynaecol. 2024; 64(2):168-70. https://dx.doi.org/10.1111/ajo.13765
- Chou L.C., Fu C.Y. An empirical analysis of land property lawsuits and rainfalls. Springerplus. 2016; 5: 1. https://dx.doi.org/10.1186/s40064-015-1659-2
- Akoglu H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018; 18(3): 91-3. https://dx.doi.org/10.1016/j.tjem.2018.08.001
Received 30.04.2025
Accepted 18.06.2025
About the Authors
Elena V. Kudryavtseva, Dr. Med. Sci., Associate Professor, Professor at the Department of Obstetrics and Gynecology, A.B. Blokhin Ural Institute of Healthcare Management, 620075, Russia, Yekaterinburg, K. Liebknecht str., 8-B, elenavladpopova@yandex.ru, https://orcid.org/0000-0003-2797-1926Yana A. Mangileva, Obstetrician-Gynecologist, UGMK-Health LLC, 620144, Russia, Yekaterinburg, Sheinkman str., 113, bld. 2, yanaamangileva@mail.ru,
https://orcid.org/0000-0001-5693-0264
Larisa G. Polushina, PhD, Senior Researcher at the Central Research Laboratory, Ural State Medical University, Ministry of Health of Russia, 620024, Russia, Yekaterinburg, Repin str., 3, polushina-larisa@bk.ru, https://orcid.org/0000-0002-4921-7222
Arina Yu. Maksimova, PhD, Researcher at the Central Research Laboratory, Ural State Medical University, Ministry of Health of Russia, 620024, Russia, Yekaterinburg,
Repin str., 3, oreshek92@list.ru, https://orcid.org/0000-0001-8412-4315
Maxim A. Kopenkin, Junior Researcher at the Central Research Laboratory, Ural State Medical University, Ministry of Health of Russia, 620024, Russia, Yekaterinburg,
Repin str., 3, maximkopenkin@yandex.ru, https://orcid.org/0000-0002-6092-3734
Danila L. Zornikov, PhD, Associate Professor, Department of Medical Microbiology and Clinical Laboratory Diagnostics, Ural State Medical University, Ministry of Health
of Russia, 620024, Russia, Yekaterinburg, Repin str., 3, zornikovdl@yandex.ru, https://orcid.org/0000-0001-9132-215X
Vladislav V. Kovalev, Dr. Med. Sci., Professor, Head of the Department of Obstetrics and Gynecology, A.B. Blokhin Ural Institute of Healthcare Management,
620075, Russia, Yekaterinburg, K. Liebknecht str., 8-B, vvkovalev55@gmail.com, https://orcid.org/0000-0001-8640-8418
Corresponding author: Elena V. Kudryavtseva, elenavladpopova@yandex.ru



