Changes in placental weight-to-birth weight ratio in cases of fetal congenital heart disease
Yarygina T.A., Gasanova R.M., Dziuba G.S., Makhukova N.A., Barinova I.V., Shchegolev A.I., Gus A.I., Shmakov R.G.
Objective: Analysis of placental weight-to-birth weight ratio in healthy full-term newborns and in babies born with congenital heart disease.
Materials and methods: A comparative analysis of newborns’ weight, placental weight and placental weight-to-birth weight ratio was conducted between the main group, which comprised 100 observations with prenatally diagnosed fetal CHD and the control group, which included 100 healthy newborns.
Results: In the studied groups, there were no statistically significant differences in birth weight: 3412 (409) g (the 65th percentile) weighed newborns with CHD, and 3397 (429) g (the 64th percentile) weighed healthy newborns (p=0.8). Placental weight was significantly lower in the group with CHD, compared with the control group – 460 (98) g (the 42th percentile) versus 513 (104) g (the 57th percentile) (p=0.003). Placental weight-to-birth weight ratio was 0.14 (the 25th percentile) versus 0.15 (the 50th percentile) (p<0.001), as well as there was lower frequency of observations with placental weight-to-birth weight ratio less than the 10th percentile – 31% and 9% (p<0.001), respectively.
Conclusion: The observations showed that there was a disproportionate reduction in placental weight in relation to the newborn’s weight in the group with fetal CHD compared with the control group of healthy children, that may be a pathogenetic basis for a high incidence of post-hypoxic complications in this cohort of patients.
Authors' contributions: Yarygina T.A., Shmakov R.G. – the concept and design of the study; Yarygina T.A., Gasanova R.M., Dziuba G.S., Makhukova N.A. – text writing; Yarygina T.A., Dziuba G.S., Makhukova N.A., Barinova I.V. – material collection and processing; Gasanova R.M., Shchegolev A.I., Gus A.I., Shmakov R.G. – text editing.
Conflicts of interest: The authors confirm that they have no conflicts of interest to declare.
Funding: The study was conducted within the framework of applied scientific research topic No. 123020300017-1.
Ethical Approval: The study was approved by the local Ethics Committee of A.N. Bakulev National Medical Research Center for Cardiovascular Surgery, Ministry of Health of Russia.
Patient Consent for Publication: The patients have signed informed consent for participation in the study and publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Yarygina T.A., Gasanova R.M., Dziuba G.S., Makhukova N.A., Barinova I.V., Shchegolev A.I.,
Gus A.I., Shmakov R.G. Changes in placental weight-to-birth weight ratio in cases of fetal congenital heart disease.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (7): 48-57 (in Russian)
https://dx.doi.org/10.18565/aig.2025.132
Keywords
Preserving and strengthening population health, supporting families with growing number of births and increased life expectancy are national development goals of our country [1]. They determine the necessity for continuous search of ways to reduce perinatal and infant morbidity and mortality as the basis of modern medicine. In this global context, it is necessary to bear in mind that according to the World Health Organization estimates, every year due to developmental defects about 240 000 newborns and 170 000 children between the ages of 1 month and 5 years die in the world [2]. In our country, the incidence rate of congenital anomalies reaches 3.6% among live births, that amounts over 45 thousand children in absolute numbers [3].
From 2010 to 2022, infant mortality rates due to congenital anomalies (developmental defects), deformations and chromosomal abnormalities reduced by almost 2 times – from 18.2 to 9.4 per 10,000 live births in the Russian Federation [4, 5].
At the same time, until the present time cardiovascular anomalies significantly negatively contribute to global health indicators, and despite the fact that the vast majority of fatal outcomes occur in low-income countries [2]. In the countries with advanced healthcare, the survival rate in children with critical congenital heart defects did not reach yet 90% [6]. Taking into account high-level development of cardiac surgery in the Russian Federation, and a wide range of nosological forms of congenital heart defects (CHDs), which can be successfully treated in babies after birth [7], a significant number of patients take a decision to continue pregnancy with the diagnosed cardiac anomalies in fetuses. In our country about 3, 000 babies are born every year, requiring surgical interventions in the first year of life; 1,600 – 1,700 newborns undergo cardiac surgery in the neonatal period [8].
Among all developmental defects, congenital cardiovascular anomalies are one of the main causes of death in stillborn babies [9]. According to the Federal Service for State Statistics (Rosstat), congenital anomalies of the circulatory system ranked second among all congenital anomalies and the causes of early neonatal death. Congenital heart defects account for more than 80% of congenital anomalies of the circulatory system, and were the cause of early neonatal death in 32.0% of all deaths due to congenital anomalies in general in the Russian Federation [10]. Moreover, during the COVID-19 pandemic (in 2020) compared with the pre-COVID period (2019), the incidence of CHD as a primary cause of death in stillborn babies increased from 13.9% to 16.2% among all deaths from congenital anomalies [11], and on the contrary, as a cause of early neonatal death it decreased from 28.3% to 24.6% among all developmental defects [12].
The preoperative conditions for favorable prognosis for infants with CHD include both prenatal diagnosis of fetal anomaly, routing of pregnant women for delivery, management of early neonatal period according to the type of CHD, which is put into practice in the Russian healthcare [13], and not yet well understood antenatal environment, which is controlled and modulated by the placenta, and plays a crucial role in fetal development and has a long-term postnatal programming impact [14, 15].
There are numerous foreign publications, which are devoted to exploration of morphological damage to the placenta in fetal cardiac anomalies. However, the study conducted by our research team is the only one in Russia that found anomalies in macro- and microstructures and low placental weight in more than 50% of cases [16–19]. It was found that low placental weight was significantly associated with the birth of preterm and/or small for gestational age infants with CHD, who have been proven to have extremely high perioperative risks, and therefore, receive maximum volume of neonatal care [19, 20]. However, despite the fact that the vast majority of newborns with cardiovascular pathology are full-term infants, and their weight is appropriate for the gestational age, the scientific data indicate placentation abnormalities, that increases the risk of developing hypoxic complications and correlates with postnatal manifestations of brain pathology and psychomotor development disorders in this group of patients [21–23].
Since the clinical significance of prenatal assessment and Doppler sonography in predicting placental volume and weight in cases with fetal CHD has not yet been demonstrated [24], postnatal calculation of the placental weight-to-birth weight ratio (or reverse birth weight-to-placental weight ratio) is used for personalized assessment of the risk of neonatal morbidity and mortality [25]. There are published results of analysis of this indicator in cohorts of pregnant women after surgical treatment of aortic aneurysms [26], with carbohydrate metabolism disorders [27], the use of assisted reproductive technologies [28], SARS-CoV-2 infection in pregnancy [29], as well as in fetuses with anterior abdominal wall defects [30]. In addition, the largest number of foreign publications with discordant results are devoted to observations of fetal congenital heart disease [24, 31–37], that emphasizes the need to continue the scientific research in the world and the relevance of this study conducted for the first time in Russia.
The objective of the study was analysis of placental weight-to-birth weight ratio in healthy full-term newborns and in babies born with congenital heart disease.
Мaterials and methods
This multicenter retrospective observational study was carried out at Academician V.I. Krasnopolsky Moscow Regional Research Institute of Obstetrics and Gynecology, Perinatal Cardiological Center of A.N. Bakulev National Medical Research Center for Cardiovascular Surgery, Ministry of Health of Russia and Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia in 2023–2024.
The main studied parameters (endpoints) were the absolute values and birthweight percentiles of newborns, placental weight and placental weight-to birth weight ratio.
The INTERGROWTH-21st growth charts were used for ranging absolute values of newborn’s weight in percentiles [38].
The percentiles of placental weight were determined in accordance with reference intervals obtained from the Russian population [39].
The absolute values of placental weight-to-birth weight ratio were calculated by dividing placental weight (g) by newborn’s birthweight (g). The percentile values of placental weight-to-birth weight ratio were determined by the results obtained in the control group.
The general inclusion criteria in the study were the following: age of a pregnant woman 18–45 years; singleton pregnancy that ended with delivery of a live-born baby with birthweight ≥ the 10-th percentile at 37–40 weeks of gestation; medical records of pathologic findings of the placenta; patients’ consent to participate in the study.
The general non-inclusion criteria were the age of pregnant women below 18 and over 45 years; multiple pregnancy, newborn’s weight at birth <the10th percentile; pregnancy termination or preterm birth (at gestational age of <37 weeks), absence of data on newborn’s weight and the results of pathological examination of the placenta; patient’s refusal to participate in the study.
Inclusion criteria in the main group were newborns with CHD excepting isolated ventricular septal defects.
The main group was divided into 4 subgroups according to hemodynamic abnormalities in newborns with CHD: subgroup 1 – oxygen-depleted blood in the systemic circulation (obstructive lesions of the left heart of the heart); subgroup 2 –
hypervolemia of lesser circulation; subgroup 3 – hypovolemia of lesser circulation (right-sided obstructive heart lesions); subgroup 4 – vascular rings around the trachea and esophagus, without impairments of systemic or pulmonary circulation.
In addition, among these subgroups, the cases with fetal heart defects with the highest risk of placental and neurological complications – hypoplastic left heart syndrome (HLHS) and transposition of the great arteries (TGA) were analyzed separately.
Inclusion criteria in the control group were normal pregnancies. There were no data on the presence of bad habits, aggravated obstetric and gynecological history, chronic or acute diseases in the mother, no pregnancy complications. There were no data on antenatal and postnatal structural pathology and hypoxic complications in the fetus/newborn; and the course of early neonatal period was normal.
The required sample size and the number of observed cases in each group were determined to obtain 90% statistical power (5% α-error (type I error) and 10% β-error (type II error)) [40], taking into account the expected frequency of observations with placental weight-to-birth weight ratio <10th percentile, which was equal to 10% in the control group and 30% in the main group, with the planned ratio of the number of groups 1:1 (control/CHD). The calculated minimum sample size was 164 observations (82 control and 82 with CHD in the fetus).
Given the above, 200 observations were included in the general cohort, of which 100 belonged to the control group and 100 to the main group. The main study group was divided into 4 subgroups according to the hemodynamics in CHD. Subgroup 1 included 37/100 (37%) infants including 7 patients with HLHS. Subgroup 2 included 32/100 (32%) infants including 10 with TGA. Subgroup 3 included 24/100 (24%) infants, and subgroup 4 included 7/100 (7%) infants, respectively.
Statistical analysis
The qualitative characteristics are represented as absolute numbers (n) and the percentage (%) of the total number. The quantitative data are represented as arithmetic mean (М) with standard deviation (SD), and median (Me) with interquartile range (Q1–Q3). The Kholomogorov–Smirnov test was used to compare distributions. Statistical Student’s t-test was used for comparison of differences between two groups. The Mann–Whitey U test was used for non-normal distribution, quantitative variables or unequal variances. Fisher's exact test was used to test the differences in the frequencies of the studied variables between the groups and the subgroups. The odds ratio for births with placental weight-to-birth weight ratio <10th percentile was determined for cases with fetal cardiac anomalies compared with healthy fetuses. The Kruskal–Wallis test (H-test) was used to compare quantitative variables with non-normal distribution between the subgroups with fetal CHD. The significance level was set at 0.05 (5% type 1 error). The null hypothesis (no differences between the groups) was rejected, when the probability (p) did not exceed type I error. MedCalc Statistical Software package, version 16.4.3 (edCalc Software bv, Ostend, Belgium) and GraphPad Prism 8 (GraphPad Software, USA) were used for statistical data processing.
Results
Analysis of clinical and anamnestic data of mothers, the course of gestation and conditions in newborns with CHD were represented in detail in our previously published research papers [19, 41].
According to the results obtained in the control group, the percentiles for the common placental weight-to birth weight ratio in term pregnancies were determined. The data are represented in Table 1.
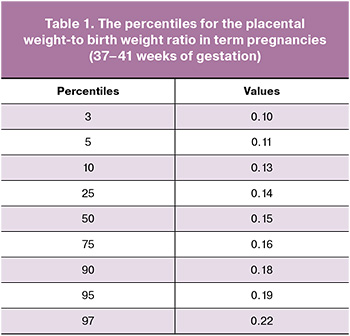
The results of postnatal examination in each group and statistical differences are shown in Table 2.
It was found that there were no statistically significant differences in the mean absolute and percentile values of newborns’ weight: 3412 (409) g (the 65th percentile) in infants with fetal CHD versus 3397 (429) g (the 64th percentile) in healthy infants (р=0.8). While placental weight was significantly lower in the group with diagnosed CHD: 460 (98) g versus 513 (104) g (р=0.003). The median values were equivalent to the 42nd and the 57th percentiles, respectively (р=0.001).
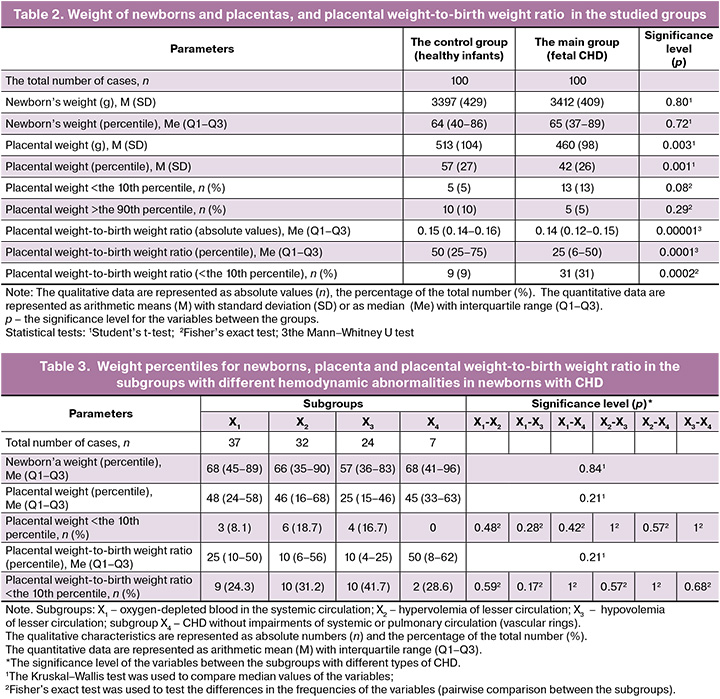
Moreover, there were statistically significant differences between the group with CHD and the control group in medians of the absolute and percentile values of the placental weight-to-birth weight ratio – 0.14 (the 25th percentile) versus 0.15 (the 50th percentile) (р<0.001), as well as in the frequency of cases with placental weight-to-birth weight ratio <the 10th percentile – 31% and 9% (р<0.001), respectively. The odds ratio for placental weight-to-birth weight ratio <the10th percentile in cases with fetal cardiac anomalies compared with healthy fetuses was 4.54 (95% CI: 2.03–10.16) (р<0.001).
There were no significant differences in weight percentiles, placental weight-to-birth weight ratio and the frequency of observations with reduced placental weight-to-birth weight ratio, and placental weight <the 10th percentile between the subgroups with different hemodynamic abnormalities in newborns with CHD.
Analysis showed that the median percentile weight at birth, placental weight and placental weight-to-birth weight ratio were 77 (71–90), 55 (51–60), 25 (25–25) in fetuses with HLHS and 57 (36–84), 32 (9–59), 17 (3–69) in fetuses with TGA, respectively. There were no statistically significant differences between these nosologies of CHD and other observations in the main group (р>0.05 for all indicators).
Discussion
A combination of structural abnormalities of the placenta and fetal cardiovascular system has a negative effect not only in the perinatal period, it also increases the risk of long-term complications, including psychoneurological complications. This is due to placenta-heart-brain onthogenetic relationship [22, 23, 42]. In-depth exploration of placental pathology can be a promising approach for developing prenatal interventions to improve general and neurological outcomes in patients with CHD [22].
It has been convincingly demonstrated that increasing rate of preterm births and intrauterine growth restriction in the presence of fetal CHD [35, 43, 44] is associated with low placental weight and umbilical cord pathology [19, 30, 32, 41]. However, the observational study previously conducted by our group of authors found multiple changes in the placental micro- and macrostructure also in cases with full-term newborns with CHD and normal weight, that was a reason to continue scientific research focused namely on this group of patients.
Calculation of placental weight-to-birth weight ratio is an easy screening method for identifying the patients with antenatal imbalance in the development of fetal and placental tissues, who are at increased risk of postnatal post-hypoxic complications, including neurological complications [45–47].
In our study, the results of analysis of 100 observations with fetal CHD and the group of 100 healthy infants demonstrated that even when the weight for full-term newborns with CHD was appropriate for the gestational age, there was low placental weight (р=0.001) and low placental weight-to-birth weight ratio (р<0.001) <the 10th percentile in each third observation (Table 2) compared with the control group.
In the observational study by Rychik J. et al. [24], reduction in placental weight-to-birth weight ratio <the 10th percentile was found in 77% out of 120 observations of complex fetal CHD.
In our study in Russia, placental weight was <the 10th percentile in 13% of observations, while Nijman M. et al. [22] reported 28% in their prospective analysis of 96 singleton pregnancies with fetal CHD. At the same time, the severity of placental pathology was negatively correlated with cortical gray matter, deep gray matter, brainstem, cerebellum, and total brain volume.
The retrospective study using the largest sample size was conducted in the Kingdom of Denmark. The study included the cohort of 924,422 live births at 22–45 weeks of singleton pregnancies from 1997 to 2011. Among them, 7,569 infants had congenital heart defects. Low placental weight and low birth weight was found in these infants compared with the rest of the study population [35].
Z-score for placental weight, which was considered as an indirect indicator of placental function, was associated with both z-score for fetal head circumference, considered by the authors as an indirect indicator of prenatal brain growth, and z-score for newborn’s weight in all subtypes of CHD. In the study by Matthiesen N.B. et al. [35], the mean z-score for placental weight-to-birth weight ratio in CHD was higher compared with the general population, primarily due to baby’s low birth weight.
Low birthweight infants were excluded from our analysis. This led to discordance of the results obtained by us with the results of previously discussed studies [22, 24, 35] and the case-control study by Albalawi A. et al. (2017) [32]. The study included postnatal group of 200 infants, who underwent surgery for CHD within 6 months of life from 2000 to 2013, and the control group of 200 pregnant women with corresponding gestational age проведенной. Their study showed that the risk of fetal growth restriction increased threefold in CHD, and found no differences between placental weight-to-birth weight ratio between the groups [32].
Takemoto R. et al. (2018) [34] conducted the study at the neonatal intensive care unit from 2010 to 2016. It included 733 babies born at 34–41 weeks of gestation. Of them 109 newborns had congenital anomalies, including 37 cases of cardiac anomalies. The authors found no association between placental weight-to-birth weight ratio and CHD.
Miremberg H. et al. (2019) [33] conducted comparative analysis of medical records after termination of pregnancies for medical indications. Their analysis included 32 observations of CHD and 32 cases of fetal central nervous system anomalies. They found that the incidence rate of vascular malperfusion in the placenta in cardiac anomalies was significantly higher – 40.6% versus 12.5% (p=0.02).
A limited number of studies analyzed the differences in the discussed indicators between the nosological types of CHD and represented controversial results. Courtney J. et al. (2020) [35] analyzed the groups of full-term and not small for gestational age infants: 8 with TGA, 16 with HLHS and 18 healthy infants in the control group. The ratio between newborn’s birth weight and placental weight significantly differed in 3 groups (ANOVA, p=0.004). However, post hoc testing showed a significant increase only in the group with TGA compared with the control group due to reduced placental weight.
Jones H.N. et al. (2015) [37] analyzed 16 cases with HLHS and 18 patients in the control group. They found that there was a reduction in the mean weight of newborns, placental weight and percentiles in CHD compared with the control group. The percentage of infants with CHD was 31%. However, there were no differences in placental weight-to-birth weight ratio in these cases compared with normal infant’s weight [37].
The largest analysis of placentation with different nosological forms of CHD was conducted by Desmond A. et al. (2023) [16] from 2011–2021. It included 139 observations, where septal defects were in 27 patients, right heart lesions were in 13, left‐sided heart lesions were in 38, conotruncal and other anomalies were in 41 and 20 patients, respectively.
In comparison with published reference data, the following parameters were <the 10th percentile without significant differences between the types of CHD: birth weight in 27% of newborns, placental weight in more than half of observations, placental weight-to-birth weight ratio in 80% of the cohort [16].
The controversial and incomparable results of the above studies are due to significant discrepancies in study design, inclusion criteria in the study groups and cohort size, indicating the need for large-scale multicenter analyses using a unified methodology [48].
The results represented by our group of authors showed that there were no statistically significant differences in the studied parameters between the subgroups with different hemodynamic types of CHD. Separate analysis of patients with HLHS and TGA, who according to published data are at high risk of cerebral and placental abnormalities, also found no differences compared with other nosological forms of CHD. Appropriate for gestational age birth weight and reduced placental weight-to-birth weight ratio with subsequently increasing risk of general and neurological morbidity can be considered as an analogue of late-onset fetal growth restriction, both from clinical and pathogenetic perspective [16]. In such cases, placental function is decompensated at later stage, nonobligatory influencing fetometric parameters. However, chronic hypoxia affects fetal tissues performing aerobic respiration, primarily the brain, heart, liver, renal cortex, small intestine and retina. This fact indicates the expediency of classifying pregnancies in all women with prenatally diagnosed fetal congenital heart defects as high-risk placenta-related complications [16].
The strengths of this study conducted for the first time in Russia are the following: the sample size of 100 observations in each group, that provided statistical power of 90%; two-year interval, that made it possible unifying diagnosis identification, standardization of the applied methods and using the same class of equipment. In addition, significant positive aspects of the study are the use of Russian nomograms of placental mass, the creation of the first Russian nomograms of the generalized placental weight-to-birth weight ratio for full-term pregnancies based on the data of the control group.
Weaknesses of the study include relatively small sample size, the lack of correlation analysis between placental weight and placental weight-to-birth weight ratio with the results of perinatal Doppler sonography, neuroimaging and cerebral biometry, that will be the aim of subsequent research.
Conclusion
Placental abnormalities in anatomy and function have an important delayed negative impact on the quality and future life expectancy of patients with congenital heart disease. In Russia, our first comparative analysis of placental weight and placental weight-to-birth weight ratio in full-term infants with appropriate for gestational age birth weight found significant decreases in these indicators in infants with congenital heart disease compared with the control group of healthy infants.
Future studies including detailed collection of perinatal risk factors, placental function and histology, genetic and epigenetic approaches, and neuroimaging metrics will be of key importance for understanding the mechanisms of organ and system impairment in fetuses with congenital heart defects. Innovative mechanistic studies and machine learning approaches to elucidate the relationship between the placenta, heart, and brain can lead to future maternal-fetal interventions that will improve survival and neurodevelopmental outcomes in patients with CHD.
References
- Указ Президента Российской Федерации от 7 мая 2024 г. № 309 «О национальных целях развития Российской Федерации на период до 2030 года и на перспективу до 2036 года». [Decree of the President of the Russian Federation of 07.05.2024 No. 309 "On the national development goals of the Russian Federation for the period up to 2030 and for the future until 2036" (in Russian)]. https://www.kremlin.ru/acts/bank/50542
- Birth Defect Rates by Country 2025. World population review. Available at: https://worldpopulationreview.com/country-rankings/birth-defect-rates-by-country (дата обращения 02.05.2025).
- Котова Е.Г., Кобякова О.С., Стародубов В.И., Александрова Г.А., Голубев Н.А., Кучерявая Д.А., Огрызко Е.В., Поликарпов А.В., Шелепова Е.А. Основные показатели здоровья матери и ребенка, деятельность службы охраны детства и родовспоможения в Российской Федерации. Статистические материалы. М.: ФГБУ «ЦНИИОИЗ» Минздрава России; 2023. 171 с. [Kotova E.G., Kobyakova O.S., Starodubov V.I., Aleksandrova G.A., Golubev N.A., Kucheryavaya D.A., Ogryzko E.V., Polikarpov A.V., Shelepova E.A. Key indicators of maternal and child health, activities of child protection and maternity services in the Russian Federation. Statistical materials. Moscow: CNIIOIZ Ministry of Health of Russia; 2023. 171 p. (in Russian)].
- Федеральная служба государственной статистики (Росстат). Здравоохранение в России 2023. Статистический сборник. М.: Росстат; 2023. 179 с. [Federal State Statistics Service (Rosstat). Healthcare in Russia 2023. Statistical compilation. Moscow: Rosstat; 2023. 179 p. (in Russian)].
- Щеголев А.И., Павлов К.А., Дубова Е.А., Фролова О.Г. Ранняя неонатальная смертность в Российской Федерации в 2010 г. Архив патологии. 2013; 75(4): 15-9. [Shchegolev A.I., Pavlov K.A., Dubova E.A., Frolova O.G. Early neonatal mortality in the Russian Federation in 2010. Arhiv patologii. 2013; 75(4): 15-9 (in Russian)].
- Cody F., Franklin O., Mc Cay N., Molphy Z., Dicker P., Breathnach F.M. Critical congenital heart disease: contemporary prenatal screening performance and outcomes in a multi-centre perinatology service. BMC Pregnancy Childbirth. 2024; 24(1): 163. https://dx.doi.org/10.1186/s12884-024-06350-0С
- Голухова Е.З. Отчет о научной и лечебной работе Национального медицинского исследовательского центра сердечно-сосудистой хирургии им. А.Н. Бакулева Минздрава России за 2023 год и перспективы развития. Сердечно-сосудистые заболевания. Бюллетень НЦССХ им. А.Н. Бакулева РАМН. 2024; 25 (Спецвыпуск): 5-141. [Golukhova E.Z. Report on the scientific and clinical activity of Bakoulev National Medical Research Center for Cardiovascular Surgery for 2023 and development prospects. The Bulletin of Bakoulev Center. Cardiovascular Diseases. 2024; 25 (Special Issue) (in Russian)]. https://dx.doi.org/10.24022/1810-0694-2024-25S
- ФГБУ «Национальный медицинский исследовательский центр сердечно-сосудистой хирургии им. А.Н. Бакулева» Минздрава России. Методические рекомендации «Резервы для снижения младенческой смертности от врожденных пороков сердца». М.; 2024. 56 с. [A.N. Bakulev National Medical Research Center for Cardiovascular Surgery, Ministry of Hralth of Russia. Methodological guidelines “Reserves for reducing infant mortality from congenital heart defects”. Moscow; 2024. 56 p. (in Russian)].
- Щеголев А.И., Туманова У.Н., Шувалова М.П., Фролова О.Г. Сравнительный анализ мертворождаемости в Российской Федерации в 2010 и 2012 г. Российский вестник перинатологии и педиатрии. 2015; 60(3): 58-62. [Shchegolev A.I., Tumanova U.N., Shuvalova M.P., Frolova O.G. Comparative analysis of stillbirth rates in the Russian Federation in 2010 and 2012. Russian Bulletin of Perinatology and Pediatrics. 2015; 60(3): 58-62 (in Russian)].
- Туманова У.Н., Шувалова М.П., Щеголев А.И. Анализ статистических показателей врожденных аномалий как причины ранней неонатальной смерти в Российской Федерации. Российский вестник перинатологии и педиатрии 2018; 63(6): 60-7. [Tumanova U.N., Shuvalova M.P., Schegolev A.I. Analysis of statistical indicators of congenital anomalies as causes of early neonatal death in the Russian Federation. Russian Bulletin of Perinatology and Pediatrics. 2018; 63(6): 60-7 (in Russian)]. https://dx.doi.org/10.21508/1027-4065-2018-63-5-60-67
- Щеголев А.И., Туманова У.Н., Чаусов А.А., Шувалова М.П. Сравнительный анализ причин мертворождения в Российской Федерации в 2019 и 2020 годах. Акушерство и гинекология. 2022; 2: 80-90. [Shchegolev A.I., Tumanova U.N., Chausov A.A., Shuvalova M.P. Comparative analysis of stillbirth causes and rates in the Russian Federation in 2019 and 2020. Obstetrics and Gynecology. 2022; (2): 80-90 (in Russian)]. https://dx.doi.org/10.18565/aig.2022.2.80-90
- Туманова У.Н., Щеголев А.И., Чаусов А.А., Шувалова М.П. Анализ причин ранней неонатальной смертности в Российской Федерации в 2020 г. (год пандемии COVID-19). Вестник РГМУ. 2021; 5: 71-7. [Tumanova U.N., Schegolev A.I., Chausov A.A., Shuvalova M.P. Analysis of causes of early neonatal mortality during covid-19 pandemic in 2020 in Russia. Bulletin of RSMU. 2021; 5: 71-7. (in Russian)]. https://dx.doi.org/10.24075/brsmu.2021.045
- Голухова Е.З., Ким А.И., Завалихина Т.В., Нефедова И.Е., Черногривов А.Е., Авакова С.А. Анализ оказания медицинской помощи детям с врожденными пороками сердца в Российской Федерации и предпосылки к созданию регистра в современную эру цифровых медицинских информационных систем. Креативная кардиология. 2023; 17(3): 315-21. [Golukhova E.Z., Kim A.I., Zavalikhina T.V., Nefedova I.E., Chernogrivov A.E., Avakova S.A. Analysis for medical care to children with congenital heart disease in Russian Federation and precondition for Registry in the era of digital medical information systems. Creative Cardiology. 2023; 17(3): 315-21 (in Russian)]. https://dx.doi.org/10.24022/1997-3187-2023-17-3- 315-321
- Туманова У.Н., Щеголев А.И. Поражения плаценты в генезе мертворождения (обзор литературы). Международный журнал прикладных и фундаментальных исследований. 2017; 3-1: 77-81. [Tumanova U.N., Shchegolev A.I. Placental lesions as the cause of stillbirth (review). International Journal of Applied and Fundamental Research. 2017; 3-1: 77-81 (in Russian)]. https://dx.doi.org/10.17513/mjpfi.11403
- Щеголев А.И., Серов В.Н. Клиническая значимость поражений плаценты. Акушерство и гинекология. 2019; 3: 54-62. [Shchegolev A.I., Serov V.N. Clinical significance of placental lesions. Obstetrics and Gynecology. 2019; (3): 54-62 (in Russian)]. https://dx.doi.org/10.18565/aig.2019.3.54-62
- Desmond A., Imany-Shakibai H., Wong D., Kwan L., Satou G., Sklansky M., Afshar Y. Prenatal congenital heart disease and placental phenotypes: preserved neonatal weight despite small placentas. JACC Adv. 2023; 2(4): 100383. https://dx.doi.org/10.1016/j.jacadv.2023.100383
- Leon R.L., Sharma K., Mir I.N., Herrera C.L., Brown S.L., Spong C.Y. et al. Placental vascular malperfusion lesions in fetal congenital heart disease. Am. J. Obstet. Gynecol. 2022; 227(4): 620.e1-620.e8. https://dx.doi.org/10.1016/j.ajog.2022.05.038
- Licht D.J., Jacobwitz M., Lynch J.M., Ko T., Boorady T., Devarajan M. et al. Impaired maternal-fetal environment and risk for preoperative focal white matter injury in neonates with complex congenital heart disease. J. Am. Heart. Assoc. 2023; 12(7): e025516. https://dx.doi.org/10.1161/JAHA.122.025516
- Ярыгина Т.А., Гасанова Р.М., Марзоева О.В., Сыпченко Е.В., Леонова Е.И., Ляпин В.М., Щеголев А.И., Гус А.И. Анализ патоморфологических особенностей строения плаценты в случаях с пренатально диагностированным врожденным пороком сердца у плода. Акушерство и гинекология. 2024; 6: 75-83. [Yarygina T.A., Gasanova R.M., Marzoeva O.V., Sypchenko E.V., Leonova E.I., Lyapin V.M., Shchegolev A.I., Gus A.I. Analysis of pathomorphological characteristics of the placental structure in cases of prenatally diagnosed fetal congenital heart disease. Obstetrics and Gynecology. 2024; (6): 75-83 (in Russian)]. https://dx.doi.org/10.18565/aig.2024.119
- Барышникова И.Ю., Гасанова Р.М. Недоношенный ребенок с врожденным пороком сердца: перинатальные факторы риска развития кардиохирургических осложнений. Детские болезни сердца и сосудов. 2023; 4(20): 235-41. [Baryshnikova I.Yu., Gasanova R.M. Premature baby with congenital heart disease: perinatal risk factors for complications after cardiac surgery. Children’s Heart and Vascular Diseases. 2023; 20(4): 235-41 (in Russian)]. https://dx.doi.org/10.24022/1810-0686-2023-20-4-235-241
- Panigrahy A., Votava-Smith J.K., Licht D.J. Need for 'one-stop-shop' heart-brain-placental imaging in fetal congenital heart disease: fetal hemodynamics portend neurodevelopmental outcome. J. Am. Coll. Cardiol. 2024; 83(13): 1240-2. https://dx.doi.org/10.1016/j.jacc.2024.02.022
- Nijman M., van der Meeren L.E., Nikkels P.G.J., Stegeman R., Breur J.M.P.J., Jansen N.J.G. et al.; CHD LifeSpan Study Group. Placental pathology contributes to impaired volumetric brain development in neonates with congenital heart disease. J. Am. Heart Assoc. 2024; 13(5): e033189. https://dx.doi.org/10.1161/JAHA.123.033189
- Ortinau C.M., Newburger J.W. Placenta-heart-brain connection in congenital heart disease. J. Am. Heart. Assoc. 2024; 13(5): e033875. https://dx.doi.org/10.1161/JAHA.124.033875
- Rychik J., Goff D., McKay E., Mott A., Tian Z., Licht D.J. et al. Characterization of the placenta in the newborn with congenital heart disease: distinctions based on type of cardiac malformation. Pediatr. Cardiol. 2018; 39(6): 1165-71. https://dx.doi.org/10.1007/s00246-018-1876-x
- Itoh T., Matsuda Y., Itoh H., Ogawa M., Sasaki K., Kanayama N. Intrauterine fetal and neonatal death between small for date and non-small for date in small for gestational age infants. Int. J. Med. Sci. 2019; 16(4): 501-6. https://dx.doi.org/10.7150/ijms.31153
- Zheng M.H., Chen D., Shang J.F., Liu R., Zhou H.T. [Clinical characteristics and placental pathology analysis of 14 cases of pregnancy with aortic dissection/ aortic aneurysm]. [Article in Chinese]. Zhonghua Bing Li Xue Za Zhi. 2023; 52(5): 480-5. https://dx.doi.org/10.3760/cma.j.cn112151-20230129-00078
- Choo S., Vrijer B. de, Regnault T.R.H., Brown H.K., Stitt L., Richardson B.S. The impact of maternal diabetes on birth to placental weight ratio and umbilical cord oxygen values with implications for fetal-placental development. Placenta. 2023; 136: 18-24. https://dx.doi.org/10.1016/j.placenta.2023.02.008
- Jia Q., Guo X., Cao Q., Di M., Yao F., Lei H. et al. Assisted reproductive technology causes reduced expression of amino acid transporters in human full-term placentas. Pathol. Res. Pract. 2022; 239: 154169. https://dx.doi.org/10.1016/j.prp.2022.154169
- Radan A.P., Baud D., Favre G., Papadia A., Surbek D., Baumann M. et al. Low placental weight and altered metabolic scaling after severe acute respiratory syndrome coronavirus type 2 infection during pregnancy: a prospective multicentric study. Clin. Microbiol. Infect. 2022; 28(5): 718-22. https://dx.doi.org/10.1016/j.cmi.2022.02.003
- De Paepe M.E., Mao Q., Chu S., Zhang Y., Luks F.I. Clinicoplacental correlates of amniocyte vacuolization in association with gastroschisis. Placenta. 2017; 57: 87-93. https://dx.doi.org/10.1016/j.placenta.2017.06.002
- Snoep M.C., Aliasi M., Meeren L.E. van der, Jongbloed M.R.M., DeRuiter M.C., Haak M.C. Placenta morphology and biomarkers in pregnancies with congenital heart disease – a systematic review. Placenta. 2021; 112: 189-96. https://dx.doi.org/10.1016/j.placenta.2021.07.297
- Albalawi A., Brancusi F., Askin F., Ehsanipoor R., Wang J., Burd I. et al. Placental characteristics of fetuses with congenital heart disease. J. Ultrasound Med. 2017; 36(5): 965-72. https://dx.doi.org/10.7863/ultra.16.04023
- Miremberg H., Gindes L., Schreiber L., Sternfeld A.R., Bar J., Kovo M. The association between severe fetal congenital heart defects and placental vascular malperfusion lesions. Prenat. Diagn. 2019; 39(11): 962-7. https://dx.doi.org/10.1002/pd.5515
- Takemoto R., Anami A., Koga H. Relationship between birth weight to placental weight ratio and major congenital anomalies in Japan. PLOS One. 2018; 13(10): e0206002. https://dx.doi.org/10.1371/journal.pone.0206002
- Matthiesen N.B., Henriksen T.B., Agergaard P., Gaynor J.W., Bach C.C., Hjortdal V.E., Østergaard J.R. Congenital heart defects and indices of placental and fetal growth in a nationwide study of 924 422 liveborn infants. Circulation. 2016; 134(20): 1546-56. https://dx.doi.org/10.1161/CIRCULATIONAHA.115.019089
- Courtney J., Troja W., Owens K.J., Brockway H.M., Hinton A.C., Hinton R.B. et al. Abnormalities of placental development and function are associated with the different fetal growth patterns of hypoplastic left heart syndrome and transposition of the great arteries. Placenta. 2020; 101: 57-65. https://dx.doi.org/10.1016/j.placenta.2020.09.007
- Jones H.N., Olbrych S.K., Smith K.L., Cnota J.F., Habli M., Ramos-Gonzales O. et al. Hypoplastic left heart syndrome is associated with structural and vascular placental abnormalities and leptin dysregulation. Placenta. 2015; 36(10): 1078-86. https://dx.doi.org/10.1016/j.placenta.2015.08.003
- Villar J., Ismail L.C., Victora C.G., Ohuma E.O., Bertino E., Altman D.G. et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the newborn cross-sectional study of the INTERGROWTH-21st project. Lancet. 2014; 384(9946): 857-68. https://dx.doi.org/10.1016/S0140-6736(14)60932-6
- Баринова И.В., Котов Ю.Б., Скляренко Г.А., Гурьева В.М., Бурумкулова Ф.Ф., Мельников А.П., Шидловская Н.В. Диагностическая ценность массы плаценты как критерия функционального состояния фетоплацентарного комплекса. Российский вестник акушера-гинеколога. 2010; 10(5): 3-6. [Barinova I.V., Kotov Iu.B., Skliarenko G.A., Gur’eva V.M., Burumkulova F.F., Mel’nikov A.P., Shidlovskaya N.V. Diagnostic value of placental mass as a criterion of the functional state of the fetoplacental complex. Russian Bulletin of Obstetrician-Gynecologist. 2010; 10(5): 3-6 (in Russian)].
- Rosner B. Fundamentals of biostatistics. 7th ed. Boston, MA: Brooks/Cole; 2011. 859 p.
- Ярыгина Т.А., Гасанова Р.М., Марзоева О.В., Сыпченко Е.В., Леонова Е.И., Ляпин В.М., Щеголев А.И., Гус А.И. Патологическое строение пуповины при врожденном пороке сердца у плода. Акушерство и гинекология. 2024; 8: 48-57. [Yarygina T.A., Gasanova R.M., Marzoeva O.V., Sypchenko E.V., Leonova E.I., Lyapin V.M., Shchegolev A.I., Gus A.I. Anatomical pathology of the umbical cord in cases of fetal congenital heart disease. Obstetrics and Gynecology. 2024; (8): 48-57 (in Russian)]. https://dx.doi.org/10.18565/aig.2024.133
- Leon R.L., Mir I.N., Herrera C.L., Sharma K., Spong C.Y., Twickler D.M. et al. Neuroplacentology in congenital heart disease: placental connections to neurodevelopmental outcomes. Pediatr. Res. 2022; 91(4): 787-94. https://dx.doi.org/10.1038/s41390-021-01521-7
- Gimeno L., Brown K., Harron K., Peppa M., Gilbert R., Blackburn R. Trends in survival of children with severe congenital heart defects by gestational age at birth: a population-based study using administrative hospital data for England. Paediatr. Perinat Epidemiol. 2023; 37(5): 390-400. https://dx.doi.org/10.1111/ppe.12959
- Aliasi M., Snoep M.C., van Geloven N., Haak M.C. Birthweight and isolated congenital heart defects – a systematic review and meta-analysis. BJOG. 2022; 129(11): 1805-16. https://dx.doi.org/10.1111/1471-0528.17164
- Иванова Л.А., Титкова Е.В. Особенности строения плацентарного комплекса и основные причины перинатальных потерь. Педиатр. 2018; 9(1): 5-10. [Ivanova L.A., Titkova E.V. Peculiarities of the construction of the placentary complex and the main causes of perinatal losses. Pediatrician (St. Petersburg). 2018; 9(1): 5-10 (in Russian)]. https://dx.doi.org/10.17816/PED915-10
- Lichtwald A., Ittermann T., Friedrich N., Lange A.E., Winter T., Kolbe C. et al. Impact of maternal pre-pregnancy underweight on cord blood metabolome: an analysis of the population-based survey of neonates in Pomerania (SNiP). Int. J. Mol. Sci. 2024; 25(14): 7552. https://dx.doi.org/10.3390/ijms25147552
- Asaka M.K., Nishimura T., Kuwabara H., Itoh H., Takahashi N., Tsuchiya K.J. Interleukin-23 levels in umbilical cord blood are associated with neurodevelopmental trajectories in infancy. PLOS One. 2024; 19(4): e0301982. https://dx.doi.org/10.1371/journal.pone.0301982
- Moon-Grady A.J. Why would a cardiologist be interested in the placenta? JACC Adv. 2023; 2(4): 100403. https://dx.doi.org/10.1016/j.jacadv.2023.100403
Received 16.05.2025
Accepted 04.06.2025
About the Authors
Tamara A. Yarygina, PhD, Head of the Ultrasound Diagnostics Department, Academician V.I. Krasnopolsky Moscow Regional Research Institute of Obstetrics and Gynecology, 101000, Russia, Moscow, Pokrovka str., 22a; Researcher at the Perinatal Cardiology Center, A.N. Bakulev National Medical Research Center of Cardiovascular Surgery, Ministry of Health of Russia, 121552, Russia, Moscow, Roublyevskoe Shosse, 135; Associated Professor at the Department of Ultrasound Diagnostics of the Faculty of Continuing Medical Education of the Medical Institute, Patrice Lumumba Peoples’ Friendship University of Russia, 127015, Russia, Moscow, Pistsovaya str., 10,+7(495)414-78-75, tamarayarygina@gmail.com, https://orcid.org/0000-0001-6140-1930
Rena M. Gasanova, Dr. Med. Sci., Head of the Perinatal Cardiology Center, A.N. Bakulev National Medical Research Center for Cardiovascular Surgery, Ministry of Health of Russia, 121552, Russia, Moscow, Roublyevskoe Shosse, 135; Physician of Ultrasound Diagnostics, Department of Ultrasound and Functional Diagnostics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, rmgasanova@bakulev.ru, https://orcid.org/0000-0003-3318-1074
Galina S. Dziuba, Researcher at the Pathology Department, Academician V.I. Krasnopolsky Moscow Regional Research Institute of Obstetrics and Gynecology, 101000, Russia, Moscow, Pokrovka str., 22a, Gala22_88@mail.ru, https://orcid.org/0009-0002-7459-4369
Natalya A. Makhukova, Junior Researcher at the Pathological Anatomy Department, Academician V.I. Krasnopolsky Moscow Regional Research Institute of Obstetrics and Gynecology, 101000, Russia, Moscow, Pokrovka St., 22a, n.a.makhukova@mail.ru, https://orcid.org/0009-0007-9824-1804
Irina V. Barinova, Dr. Med. Sci., Head of the Pathoanatomical Department, Academician V.I. Krasnopolsky Moscow Regional Research Institute of Obstetrics and Gynecology, 101000, Russia, Moscow, Pokrovka str., 22a, barinova.irina.vladimirovna@gmail.com, https://orcid.org/0000-0003-0447-1734
Alexander I. Shchegolev, Dr. Med. Sci., Professor, Head of the 2nd Pathoanatomical Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, +7(495)531-44-44, ashegolev@oparina4.ru,
https://orcid.org/0000-0002-2111-1530
Alexander I. Gus, Dr. Med. Sci., Professor, Chief Researcher at the Department of Ultrasound and Functional Diagnostics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4; Head of the Department of Ultrasound Diagnostics of the Faculty of Continuing Medical Education of the Medical Institute, Patrice Lumumba Peoples’ Friendship University of Russia, 127015, Russia, Moscow, Pistsovaya str., 10, a_gus@oparina4.ru, https://orcid.org//0000-0003-1377-3128
Roman G. Shmakov, Dr. Med. Sci., Professor of the Russian Academy of Sciences, Director, Academician V.I. Krasnopolsky Moscow Regional Research Institute of Obstetrics and Gynecology, 101000, Russia, Moscow, Pokrovka str., 22a; Head of the Department of Obstetrics and Gynecology, Faculty of Advanced Medical Studies, M.F. Vladimirsky Moscow Regional Research Clinical Institute, mdshmakov@mail.ru https://orcid.org/0000-0002-2206-1002
Corresponding author: Tamara A. Yarygina, tamarayarygina@gmail.com



