Blood flow velocity parameters in the ductus venosus in fetuses with congenital heart defects
Yarygina T.A., Gasanova R.M., Shchegolev A.I., Gus A.I., Shmakov R.G.
Objective: To investigate absolute flow velocity parameters in the ductus venosus of fetuses with congenital heart defects (CHD).
Materials and methods: In this cross-sectional study, we assessed the velocities of a-waves, S-waves, D-waves, and time-averaged maximum velocity (TAMX) in the ductus venosus of a total cohort of 171 fetuses with CHD, including 47 fetuses with right heart defects (subgroup 1) and 124 fetuses with other forms of defects (subgroup 2). A comparative analysis of the obtained indicators was conducted across three gestational intervals: 18–21 weeks (x1), 22–29 weeks (x2), and 30–40 weeks (x3) of pregnancy.
Results: The a-wave velocity below the 5th percentile was observed in 18.1%, 21.3%, and 16.9% of cases in the overall cohort and in subgroups 1 and 2, respectively. In the overall group and subgroup 2, there was a significant increase in the a-wave, S-wave, D-wave, and TAMX velocities in each subsequent gestational interval (p<0.001), consistent with the findings in the healthy population. In subgroup 1, there were no significant changes in a-wave velocity between gestational intervals (p>0.05), whereas increases in S-wave, D-wave, and TAMX values were recorded only between the first and third gestational intervals (x1–x3) (p=0.001). No differences were observed between the gestational intervals x1–x2 and x2–x3 (p>0.05). Comparative analysis of a-wave, S-wave, and D-wave velocities and TAMC did not reveal statistically significant differences between the study subgroups (p>0.05).
Conclusion: A decrease in the velocity of oxygenated blood flow to the fetal heart during the atrial contraction phase (a-wave) was observed in 16–21% of fetuses with cardiac pathology, indicating an increased risk of hypoxic complications. The lack of a physiological increase in flow velocities in the ductus venosus among fetuses with right heart defects underscores the need for additional antenatal monitoring in these patients.
Authors' contributions: Yarygina T.A., Shmakov R.G. – conception and design of the study; Yarygina T.A., Gasanova R.M. – drafting of the manuscript; Yarygina T.A., Shchegolev A.I. – data collection and analysis; Gasanova R.M., Shchegolev A.I., Gus A.I., Shmakov R.G. – editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: The study was conducted within the framework of the applied scientific topic 123020300017-1.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the A.N. Bakulev NMRC of Cardiovascular Surgery, Ministry of Health of Russia.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Yarygina T.A., Gasanova R.M., Shchegolev A.I., Gus A.I., Shmakov R.G.
Blood flow velocity parameters in the ductus venosus in fetuses with congenital heart defects.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (9): 98-108 (in Russian)
https://dx.doi.org/10.18565/aig.2025.214
Keywords
The development of multidisciplinary medical care for children with congenital anomalies of the cardiovascular system in the Russian Federation has led to an annual increase in the number of surgeries performed on newborns with complex congenital heart defects (CHD), while simultaneously reducing the incidence of perioperative complications and adverse outcomes [1, 2].
The primary focus and most important tool for reducing infant mortality in this group of patients is the earliest and most accurate prenatal diagnosis of defects, followed by monitoring the functional state of the fetus and stratifying the risk of perinatal hypoxic complications, the development of heart failure, and a personalized choice of the optimal time and method of delivery [3–5].
Studies conducted both domestically and internationally have shown a significant increase in the risk of placental dysfunction and premature birth among pregnant women diagnosed with fetal cardiac pathology compared to population data and control groups. Furthermore, there is a linear positive correlation between prematurity, asphyxia, and low birth weight and the likelihood of unfavorable outcomes for the child [6–11]. However, in our country, clinical recommendations for the dynamic monitoring of fetuses with various hemodynamic types of CHD have yet to be developed, similar to those already implemented in global practice, where assessing blood flow in the ductus venosus is an integral part of functional prenatal echocardiography [12, 13]. Analyzing the blood flow velocity curve in the ductus venosus allows us to summarize the data on the hemodynamic state of the right heart. The values of the waves in the Doppler spectrum reflect rapid changes in the pressure gradient between the umbilical vein and right atrium during ventricular systole and diastole, as well as atrial systole, throughout the cardiac cycle [14, 15]. It has been established that the S-wave and v-wave occur during ventricular systole, while the D-wave and a-wave appear during ventricular diastole (Figure) [16]. The S-wave reflects an increase in flow through the ductus venosus due to a drop in pressure in the right atrium during the lowering of the atrioventricular (AV) valve rings and ventricular contraction. In late ventricular systole, as the AV valve rings rise, the pressure in the atria increases, leading to a decrease in inflow through the venous duct, corresponding to the v-wave on the blood flow velocity curve. In early ventricular diastole, the AV valves open, the pressure in the atria drops, and the flow velocity in the ductus venous increases, forming the D-wave. In late ventricular diastole, atrial contraction raises the pressure in the atria, reduces inflow through the venous duct, and corresponds to the a-wave on the blood flow velocity curve. The qualitative assessment of the a-wave, particularly regarding the presence of direct (positive), zero, or reverse (negative/reverse) flow during atrial contraction, is most often analyzed in routine obstetric ultrasound examinations.
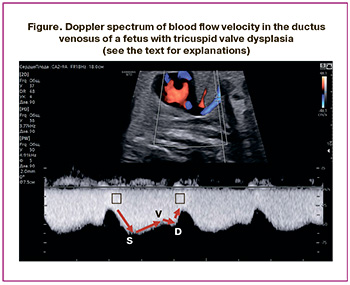
In ultrasound Doppler studies of fetuses without cardiac pathology, the appearance of a negative a-wave in the ductus venosus correlates with the development of acidosis and a high probability of perinatal death, necessitating immediate medical intervention [17–19]. Specialists conducting ultrasound examinations, along with obstetricians and gynecologists managing pregnant women, must consider three potentially coexisting groups of causes for pathological changes in the blood flow velocity curve in the venous duct. The first group includes factors that increase preload, such as organic pathologies causing tricuspid valve insufficiency and an increase in venous return, particularly associated with tumors or vascular malformations of the fetus or placenta, as well as fetal hydrops, regardless of its etiology. The second group involves disruptions in the contractile and/or relaxing functions of the myocardium, while the third group consists of factors that increase afterload, including obstructive lesions of the semilunar valves, coarctation of the aorta, or restriction of the arterial duct, along with increased resistance to blood flow in the umbilical arteries and/or the vascular bed of the placenta [16, 20].
Consequently, the presence of certain pathologies in fetal heart structures can impede the flow of oxygenated blood from the ductus venosus to the right atrium, disrupting its subsequent passage through the oval window into the left atrium, left ventricle, and systemic arterial blood flow. It is believed that a reverse a-wave in the ductus venosus can be observed in right-sided heart lesions, even in the absence of other signs of fetal decompensation, which may affect the effectiveness of heart failure diagnosis [21]. However, in contrast to studies conducted in the 2000s [21, 22], which regarded disturbances in ductus venosus blood flow as manifestations of pathological hemodynamics caused by structural anomalies of the right heart that are unassociated with perinatal death, the results of studies published over the past decade emphasize the importance of assessing blood flow parameters in the ductus venosusin fetuses with CHD within the context of functional prenatal echocardiography for predicting adverse outcomes [12, 14, 23, 24]. In domestic scientific literature, only a few paragraphs in two literature reviews have addressed this topic to date [16, 25], underscoring the need for research aimed at developing personalized protocols for antenatal monitoring of fetuses with cardiac pathology in Russian cohorts. The first stage of studying the hemodynamic features in the ductus venosus of fetuses with CHD involves analyzing absolute flow rates in observations not associated with insufficient fetal growth or disturbances in uteroplacental and fetoplacental blood flow, which defines the purpose of this pilot study.
Materials and methods
We conducted a single-center, cross-sectional study analyzing the results of examinations of pregnant women at the Perinatal Cardiology Center of A.N. Bakulev NMRC of Cardiovascular Surgery in 2024.
The general inclusion criteria were as follows: single-fetus uterine pregnancy at 18–40 weeks of gestation; a live fetus with diagnosed congenital heart disease (CHD), excluding isolated ventricular septal defects; an estimated weight and abdominal circumference ≥10th percentile for gestational age; a pulsatility index in the uterine and umbilical arteries ≤95th percentile for gestational age; documented results of Doppler ultrasound of blood flow in the venous duct; and patient consent to participate in the study [26–29].
The study group was divided into two subgroups. The first subgroup included cases of isolated or combined lesions of the right heart, such as tetralogy of Fallot, tricuspid valve pathology (atresia, stenosis, or dysplasia), pulmonary valve pathology (atresia, stenosis, or valve agenesis), and right ventricular myocardial pathology. The second subgroup included other forms of CHD.
The general exclusion criteria included multiple pregnancies; absence of fetal heart activity at the time of the study; gestational age less than 18 weeks or greater than 40 weeks; estimated fetal weight and/or abdominal circumference below the 10th percentile for gestational age; pulsatility index in the uterine and umbilical arteries above the 95th percentile for gestational age; and formations or vascular malformations of fetal organs, the placenta, and the umbilical cord. Additional exclusion criteria included a lack of recorded results of Doppler ultrasound of blood flow in the ductus venosus and refusal to participate in the study.
All ultrasound examinations were performed using a Voluson E8 Expert (GE Healthcare, Austria) expert-class system with a transabdominal convex multi-frequency (2–8 MHz) transducer by a specialist with >20 years of experience in prenatal diagnostics.
The blood flow velocity curve in the ductus venosus was assessed in the isthmus in the mid-sagittal or transverse section of the fetal abdomen, ensuring the absence of respiratory and motor activity. The following protocols were observed: an insonation angle as close to 0 °as possible; a pulse repetition frequency of 50–70 Hz; a horizontal sweep speed of the Doppler spectrum displaying 4–6 complete cardiac cycles; a control volume of the pulse wave Doppler of 2 mm; and measurement of indicators after obtaining at least three consecutive cycles of the same shape.
In automatic mode, the peak velocities (cm/s) of ventricular systole (S wave), ventricular diastole (D wave), atrial systole (a wave), and time-averaged maximum velocity (TAMV) were measured. All indicators <5th percentile or S-, D-wave, and TAMX velocities >95th percentile for the gestational age, based on the reference values of Wu et al. (2025) [14], were considered abnormal.
A comparative analysis of the findings was performed for the entire study group and its subgroups across three gestational intervals: 18–21 weeks (x1), 22–29 weeks (x2), and 30–40 weeks (x3) of pregnancy.
Statistical analysis
Categorical variables are presented as counts (n) and percentages (%). Continuous variables are presented as arithmetic means (M) with standard deviations (SD) and medians (Me) with interquartile ranges (Q1–Q3). The Kolmogorov–Smirnov test was used to assess the distribution type. For distributions other than normal or in the case of unequal variances, the Mann–Whitney U test was employed. Fisher's exact test was used to assess the differences in the frequency of the studied parameters between the subgroups. The Kruskal–Wallis test (H-test) was applied to compare continuous variables with non-normal distributions across the three gestational intervals. The type I error was set at 0.05 for all analyses. The null hypothesis (no differences) was rejected if the probability (p) did not exceed type I error. Statistical analysis was conducted using MedCalc Statistical Software version 16.4.3 (MedCalc Software bv Ostend, Belgium).
Results
Considering the inclusion and exclusion criteria, the final analysis included 171 studies on the blood flow in the venous ducts of fetuses with CHD. Among these, 47 (27.5%) observations were from the first subgroup with damage to the right heart, and 124 (72.5%) observations were from the second subgroup (Table 1).
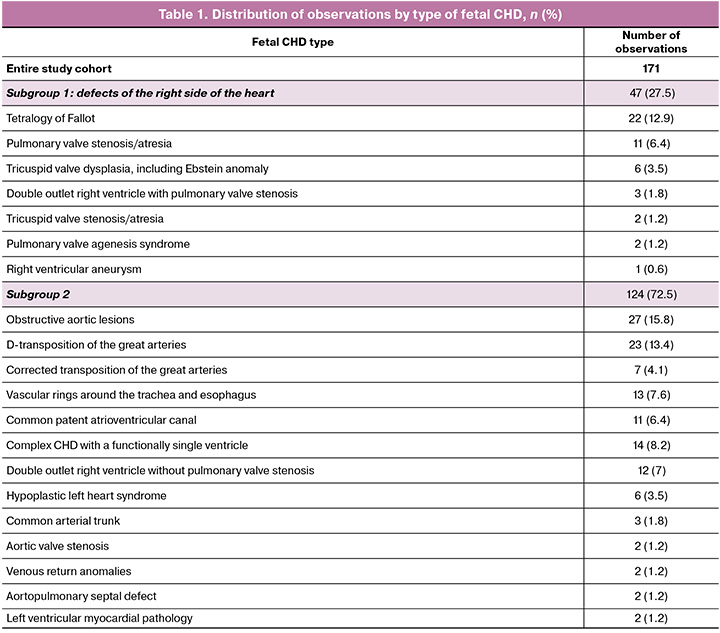
When assessing changes in flow velocities in different phases of the cardiac cycle, it was found that in the entire group of 171 fetuses with CHD and the 2nd subgroup of 124 fetuses without damage to the right sections, an increase in all indicators (S-, D-, a-waves, and TAMX) was noted in each subsequent gestational interval (Table 2) (p<0.001) with statistically significant differences between the periods before and after 30 weeks of pregnancy (x1–x3; x2–x3) (p≤0.010).
At the same time, in the 1st subgroup of 47 fetuses with right heart lesions, no significant changes in the velocity of the a-wave were found between gestational intervals (p>0.05) (Table 2). An increase in the values of the S-and D-wave and TAMX was recorded between the first and third gestational intervals (x1–x3) (p=0.001), while differences between the studied periods of pregnancy (x1–x2 and x2–x3) were not determined (p>0.05) (Table 2).
Comparative analysis of the ductus venosusblood flow rates in different phases of the cardiac cycle and the time-averaged maximum velocity between the 1st and 2nd subgroups of the study, presented in Table 3, revealed the absence of statistically significant differences in all the results obtained (p>0.05).
In the entire study group of 171 fetuses with CHD, the S- and D-wave velocities, as well as TAMX, corresponded to the population values in most observations, with the number of observations outside the reference intervals equal to 3 (1.75%), 5 (2.92%), and 6 (3.5%), respectively. These frequencies were significantly lower than those in observations with atrial a-wave velocity <5th percentile, which was recorded in 31/171 (18.1%) fetuses (p<0.0001, Fisher's exact test). The number of such cases did not differ significantly between the subgroups, amounting to 10/47 (21.3%) fetuses with right heart defects (subgroup 1) and 21/124 (16.9%) fetuses in subgroup 2 (p=0.51) (Table 3).

When pairwise comparing the number of observations with a decrease in atrial a-wave velocity <5th percentile (Table 3) in the three studied gestational intervals (18–21 versus 22–29 weeks, 18–21 versus 30–40 weeks, and 22–29 versus 30–40 weeks) using Fisher's exact test, no statistically significant differences were found in the entire group (p=0.79, p=0.46, p=0.81), 1st subgroup (p=1.0, p=1.0, p=0.68) and 2nd subgroup (p=1.0, p=0.36, p=0.56). The number of observations with a-wave velocity decrease <5th percentile compared to the number of observations with S- and D-wave velocity values outside the reference intervals in the entire study group was statistically significantly higher in each of the three studied gestational intervals (Table 4), for TAMX at 18–21 weeks (p=0.002) and 22–29 weeks (p=0.03), respectively. No significant differences were found when a similar analysis was conducted by subgroups (Table 4).

Discussion
At the current level of development in perinatal medicine, echographic monitoring of fetuses with cardiac pathology represents the initial stage of multidisciplinary care for patients with congenital heart defects (CHD). Recent studies indicate that this patient group faces an increased risk of placenta-associated complications and antenatal heart failure compared with the general population. Additionally, hemodynamic changes resulting from the structural pathology of the heart and blood vessels complicate the interpretation of routine Doppler ultrasound methods. This underscores the necessity for scientifically grounded algorithms for the dynamic monitoring of fetuses with CHD, aimed at facilitating early and personalized diagnoses of any disorders. This publication presents the results of the first Russian study analyzing blood flow velocity parameters in the ductus venosus in a cohort of 171 fetuses with CHD, divided into two defect subgroups: those with right heart disease (subgroup 1: 47 fetuses) and those without (subgroup 2: 121 fetuses). This study comprehensively analyzed the absolute values of S-, D-, and a-wave velocities, as well as the time-averaged maximum velocity (TAMX) across different gestational intervals within each subgroup. A comparative analysis highlighted deviations from the population data between subgroups. Observations with insufficient fetal growth, fetoplacental and uteroplacental blood flow disorders, and volumetric malformations of the fetus, placenta, and umbilical cord were excluded to mitigate the impact of confounding factors on hemodynamics in the ductus venosus.
The findings revealed that within the entire cohort and the 2nd subgroup of fetuses without right heart lesions, there was a significant increase in all studied parameters with advancing gestational age, indicating an increase in blood flow velocity in the ductus venosus. This pattern aligns with the results of the largest study published to date, conducted by Wu J. et al. (2025), who observed 8,953 fetuses without identified malformations [14].
Notably, our study found that in both the entire cohort and the 2nd subgroup, velocities increased significantly between gestational intervals of 22–29 weeks and 30–40 weeks. This suggests a substantial influx of oxygenated and nutrient-rich blood during the period of rapid growth of all organs and structures, including the myocardium and brain. Conversely, no statistically significant differences were observed in the first subgroup with right-sided heart defects, in which the atrial a-wave velocity did not vary across the identified gestational intervals. Thus, alterations in intracardiac hemodynamics due to lesions in the right heart chambers hinder the physiological increase in the flow rates in the ductus venosus. This finding reinforces the necessity of categorizing pregnant women with fetal pathologies as being at the highest risk for perinatal hypoxic complications and heart failure, with diverse underlying mechanisms [13, 23, 24, 30]. For instance, Srisupundit K. et al. (2023) [30] noted that significantly enlarged right heart chambers can mechanically compress the left ventricle, limiting its filling. Additionally, when antegrade flow through the right outflow tract and pulmonary trunk is absent, total venous preload is redirected to the left heart, leading to left ventricular dysfunction due to excessive volume load, and ultimately progressing to heart failure [30].
The findings of Wu J. et al. (2025) [14], who also investigated the hemodynamic characteristics of 70 fetuses with right heart disease, are consistent with our study's observation of a significant (21.3%) percentage of cases exhibiting a decrease in a-wave velocity below the 5th percentile of population values in fetuses with similar forms of CHD.
These data suggest an increased risk of adverse perinatal outcomes, as highlighted by foreign studies, which indicate that every fifth observation in this cohort exhibits such risks [23, 24]. For example, Freud L.R. et al. (2015) [24] found that retrograde blood flow in the ductus venosus (indicated by negative a-wave velocity) increased the risk of perinatal death by 4.7 times (2.4–9.1) (p<0.001) in their observations of 243 fetuses with Ebstein's anomaly. In our study, we identified a decrease in the a-wave as the most frequently observed change in flow velocities in the venous duct, occurring in 16.9% of fetuses in the second subgroup without right heart pathologies. Furthermore, a detailed comparative analysis of absolute indicators and the frequency of observations with S-, D-, a-wave, and TAMX velocities in the ductus venosus exceeding the reference intervals did not reveal significant differences between the subgroups. This suggests the potential presence of intracardiac hemodynamic disorders, as an increase in pressure within the right atrium can impede the inflow of highly oxygenated blood from the ductus venosus during atrial contraction, despite no changes in flow velocities during other phases of the cardiac cycle across various forms of CHD. This finding underscores the validity of developing a dynamic monitoring system for fetuses with any form of cardiac pathology. The strengths of this study include the exclusion of potential placental dysfunction influences on the examined parameters, a comprehensive analysis of absolute flow velocities in the ductus venosus across a broad range of CHD nosological forms, and Doppler ultrasonography performed by a single specialist with constant quality control to minimize measurement errors [31].
The limitation of this study is its relatively small sample size. However, this sample size still allowed for the identification of several statistically significant differences in ductus venosus flow velocities among fetuses with heart defects, highlighting the need for continued scientific research. Future stages of the study will aim to evaluate angle-independent indices of the ductus venosus waveform, such as pulsation and peak velocity indices for veins, preload index, and the S/a ratio of peak systolic velocity to peak late diastolic velocity, which are increasingly utilized in routine practice [14]. Additionally, examining the v-wave velocity and related indices that are not automatically determined is of interest, as several publications suggest that these have the strongest correlation with left ventricular cardiac function, including diastolic and global measurements of myocardial performance [32, 33].
All of the above should provide a foundation for achieving patient-specific management of pregnant women with fetal CHD, aiming to maximize the quality of medical care while minimizing the risk of perinatal complications and improving both immediate and long-term outcomes in the child.
Conclusion
We report the first Russian study on blood flow velocity characteristics in the ductus venosus of fetuses with CHD, focusing on observations devoid of combined placental or fetal pathologies that could potentially influence the intracardiac hemodynamics. A decrease in the rate of oxygenated blood flow to the fetal heart during atrial contraction (a-wave) was observed in 16–21% of fetuses with cardiac pathology, indicating an increased risk of hypoxic complications. The absence of a physiological increase in flow rates in the ductus venosus in cases of right heart defects underscores the necessity of developing programs aimed at enhancing antenatal monitoring of the functional state of fetuses within this high-risk group.
References
- Голухова Е.З. Отчет о лечебной и научной работе Национального медицинского исследовательского центра сердечно-сосудистой хирургии им. А.Н. Бакулева Минздрава России за 2024 год. Перспективы дальнейшего развития. Сердечно-сосудистые заболевания. Бюллетень НЦССХ им. А.Н. Бакулева РАМН. 2025; 26 (Cпецвып.): 5-130. [Golukhova E.Z. Report on the clinical and scientific activity of Bakoulev National Medical Research Center for Cardiovascular Surgery for 2024. Development prospects. The Bulletin of Bakoulev Center. Cardiovascular Diseases. 2025; 26(Special Issue): 5-130 (in Russian)]. https://dx.doi.org/10.24022/1810-0694-2025-26S
- ФГБУ «Национальный медицинский исследовательский центр сердечно-сосудистой хирургии им. А.Н. Бакулева» Минздрава России. Методические рекомендации. Резервы для снижения младенческой смертности от врожденных пороков сердца. М.; 2024. 56 с. [A.N. Bakulev National Medical Research Center for Cardiovascular Surgery of the Ministry of Health of Russia. Guidelines. Reserves for reducing infant mortality from congenital heart defects. Moscow; 2024. 56 p. (in Russian)].
- Gimeno L., Brown K., Harron K., Peppa M., Gilbert R., Blackburn R. Trends in survival of children with severe congenital heart defects by gestational age at birth: a population-based study using administrative hospital data for England. Paediatr. Perinat. Epidemiol. 2023; 37(5): 390-400. https://dx.doi.org/10.1111/ppe.12959
- Patel S.R., Michelfelder E. Prenatal diagnosis of congenital heart disease: the crucial role of perinatal and delivery planning. J. Cardiovasc. Dev. Dis. 2024; 11(4): 108. https://dx.doi.org/10.3390/jcdd11040108
- Барышникова И.Ю., Гасанова Р.М. Недоношенный ребенок с врожденным пороком сердца: перинатальные факторы риска развития кардиохирургических осложнений. Детские болезни сердца и сосудов. 2023; 4(20): 235-41. [Baryshnikova I.Yu., Gasanova R.M. Premature baby with congenital heart disease: perinatal risk factors for complications after cardiac surgery. Children’s heart and vascular diseases. 2023; 20(4): 235-41 (in Russian)]. https://dx.doi.org/10.24022/1810-0686-2023-20-4-235-241
- Ярыгина Т.А., Леонова Е.И., Гасанова Р.М., Марзоева О.В., Сыпченко Е.В., Гус А.И. Пренатальное выявление факторов, ассоциированных с нарушением психомоторного развития у детей с врожденными пороками сердца. Детские болезни сердца и сосудов. 2022; 19(4): 285-96. [Yarygina T.A., Leonova E.I., Gasanova R.M., Marzoeva O.V., Sypchenko E.V., Gus A.I. Prenatal identification of factors associated with impaired psychomotor development in children with congenital heart disease. Children’s heart and vascular diseases. 2022; 19(4): 285-96 (in Russian)]. https://dx.doi.org/10.24022/1810-0686-2022-19-3-285-296
- Ярыгина Т.А., Гасанова Р.М., Марзоева О.В., Сыпченко Е.В., Леонова Е.И., Ляпин В.М., Щеголев А.И., Гус А.И. Анализ патоморфологических особенностей строения плаценты в случаях с пренатально диагностированным врожденным пороком сердца у плода. Акушерство и гинекология. 2024; 6: 75-83. [Yarygina T.A., Gasanova R.M., Marzoeva O.V., Sypchenko E.V., Leonova E.I., Lyapin V.M., Shchegolev A.I., Gus A.I. Analysis of pathomorphological characteristics of the placental structure in cases of prenatally diagnosed fetal congenital heart disease. Obstetrics and Gynecology. 2024; (6): 75-83 (in Russian)]. https://dx.doi.org/10.18565/aig.2024.119
- Ярыгина Т.А., Гасанова Р.М., Леонова Е.И., Марзоева О.В., Сыпченко Е.В., Гус А.И. Особенности допплерографических параметров при оценке церебральной гемодинамики у плодов с врожденными пороками сердца. Детские болезни сердца и сосудов. 2022; 19(2): 117-27. [Yarygina T.A., Gasanova R.M., Leonova E.I., Marzoeva O.V., Sypchenko E.V., Gus A.I. Cerebral hemodynamics in fetuses with congenital heart disease. Children’s heart and vascular diseases. 2022; 19(2): 117-27 (in Russian)]. https://dx.doi.org/10.24022/1810-0686-2022-19-2-117-127
- Туманова У.Н., Шувалова М.П., Щеголев А.И. Анализ статистических показателей врожденных аномалий как причины ранней неонатальной смерти в Российской Федерации. Российский вестник перинатологии и педиатрии. 2018; 63(6): 60-7. [Tumanova U.N., Shuvalova M.P., Schegolev A.I. Analysis of statistical indicators of congenital anomalies as causes of early neonatal death in the Russian Federation. Russian Bulletin of Perinatology and Pediatrics. 2018; 63(6): 60-7 (in Russian)]. https://doi.org/10.21508/1027-4065-2018-63-5-60-67
- Щеголев А.И., Туманова У.Н., Фролова О.Г. Региональные особенности мертворождаемости в Российской Федерации. В кн.: Крупнов Н.М., ред. Актуальные вопросы судебно-медицинской экспертизы и экспертной практики в региональных бюро судебно-медицинской экспертизы на современном этапе. Сборник трудов конференции. Рязань: Эмпирикон; 2013: 163-9. [Shchegolev A.I., Tumanova U.N., Frolova O.G. Regional features of stillbirth in the Russian Federation. In: Krupnov N.M., ed. Current issues of forensic medical examination and expert practice in regional bureaus of forensic medical examination at the present stage. Proceedings of the conference. Ryazan: Empiricon; 2013: 163-9 (in Russian)].
- Best K.E., Tennant P.W.G., Rankin J. Survival, by birth weight and gestational age, in individuals with congenital heart disease: a population-based study. J. Am. Heart. Assoc. 2017; 6(7): e005213. https://dx.doi.org/10.1161/JAHA.116.005213
- Moon-Grady A.J., Donofrio M.T., Gelehrter S., Hornberger L., Kreeger J., Lee W. et al. Guidelines and recommendations for performance of the fetal echocardiogram: an update from the American society of echocardiography. J. Am. Soc. Echocardiogr. 2023; 36(7): 679-723. https://dx.doi.org/10.1016/j.echo.2023.04.014
- Haxel C.S., Johnson J.N., Hintz S., Renno M.S., Ruano R., Zyblewski S.C. et al. Care of the fetus with congenital cardiovascular disease: from diagnosis to delivery. Pediatrics. 2022; 150(Suppl 2): e2022056415C. https://dx.doi.org/10.1542/peds.2022-056415C
- Wu J., Ruan Y., Gao X., Wang H., Guan Y., Hao X. et al. The reference ranges for fetal ductus venosus flow velocities and calculated waveform indices and their predictive values for right heart diseases. J. Perinat. Med. 2025; 53(4): 491-502. https://dx.doi.org/10.1515/jpm-2024-0577
- Chemla D., Berthelot E., Assayag P., Attal P., Hervé P. [Pathophysiology of right ventricular hemodynamics]. Rev. Mal. Respir. 2018; 35(10): 1050-62. [Article in French]. https://dx.doi.org/10.1016/j.rmr.2017.10.667
- Ярыгина Т.А., Гасанова Р.М., Марзоева О.В., Сыпченко Е.В., Гус А.И. Все о венозном протоке – в помощь практикующим специалистам. Акушерство и гинекология. 2023; 9: 22-32. [Yarygina T.A., Gasanova R.M., Marzoeva O.V., Sypchenko E.V., Gus A.I. All those practitioners should know about ductus venosus. Obstetrics and Gynecology. 2023; (9): 22-32 (in Russian)]. https://dx.doi.org/10.18565/aig.2023.127
- Ярыгина Т.А., Гус А.И. Задержка (замедление) роста плода: все, что необходимо знать практикующему врачу. Акушерство и гинекология. 2020; 12: 14-24. [Yarygina T.A., Gus A.I. Fetal growth restriction (retardation): everything the practitioner should know. Obstetrics and Gynecology. 2020; (12): 14-24 (in Russian)]. https://dx.doi.org/10.18565/aig.2020.12.14-24
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Недостаточный рост плода, требующий предоставления медицинской помощи матери (задержка роста плода). М.; 2022. 73 с. [Ministry of Health of the Russian Federation. Clinical guidelines. Insufficient fetal growth requiring the provision of medical care to the mother (fetal growth retardation). Moscow; 2022. 73 p. (in Russian)].
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Многоплодная беременность. М.; 2021. 58 с. [Ministry of Health of the Russian Federation. Clinical guidelines. Multiple pregnancy. Moscow; 2021. 58 p. (in Russian)].
- Seravalli V., Miller J.L., Block-Abraham D., Baschat A.A. Ductus venosus Doppler in the assessment of fetal cardiovascular health: an updated practical approach. Acta Obstet. Gynecol. Scand. 2016; 95(6): 635-44. https://dx.doi.org/10.1111/aogs.12893
- Berg C., Kremer C., Geipel A., Kohl T., Germer U., Gembruch U. Ductus venosus blood flow alterations in fetuses with obstructive lesions of the right heart. Ultrasound Obstet. Gynecol. 2006; 28(2): 137-42. https://dx.doi.org/10.1002/uog.2810
- Gembruch U., Meise C., Germer U., Berg C., Geipel A. Venous Doppler ultrasound in 146 fetuses with congenital heart disease. Ultrasound Obstet. Gynecol. 2003; 22(4): 345-50. https://dx.doi.org/10.1002/uog.242
- Kahramanoglu O., Eyisoy O.G., Demirci O. Prenatal predictors and early postnatal outcomes in fetuses diagnosed with tricuspid atresia. Diagnostics (Basel). 2024; 14(24): 2855. https://dx.doi.org/10.3390/diagnostics14242855
- Freud L.R., Escobar-Diaz M.C., Kalish B.T., Komarlu R., Puchalski M.D., Jaeggi E.T. et al. Outcomes and predictors of perinatal mortality in fetuses with ebstein anomaly or tricuspid valve dysplasia in the current era: a multicenter study. Circulation. 2015; 132(6): 481-9. https://dx.doi.org/10.1161/CIRCULATIONAHA.115.015839
- Цибизова В.И., Аверкин И.И., Бицадзе В.О., Козленок А.В., Грехов Е.В., Первунина Т.М., Петров К.В., Сапрыкина Д.О., Блинов Д.В. Новая эра в оценке функционального состояния сердца плода. Акушерство, гинекология и репродукция. 2021; 15(2): 208-17. [Tsibizova V.I., Averkin I.I., Bitsadze V.O., Kozlenok A.V., Grekhov E.V., Pervunina T.M., Petrov K.V., Saprykina D.O., Blinov D.V. A new epoch in assessing fetal heart condition. Obstetrics, Gynecology and Reproduction. 2021; 15(2): 208-17 (in Russian)]. https://dx.doi.org/10.17749/2313-7347/ob.gyn.rep.2021.225
- Papageorghiou A.T., Kennedy S.H., Salomon L.J., Ohuma E.O., Cheikh Ismail L., Barros F.C. et al. International standards for early fetal size and pregnancy dating based on ultrasound measurement of crown–rump length in the first trimester of pregnancy. Ultrasound Obstet. Gynecol. 2014: 44(6): 641-8. https://dx.doi.org/10.1002/uog.13448
- Papageorghiou A.T., Ohuma E.O., Altman D.G., Todros T., Cheikh Ismail L., Lambert A. et al. International standards for fetal growth based on serial ultrasound measurements: the fetal growth longitudinal study of the INTERGROWTH–21st project. Lancet. 2014; 384(9946): 869-79. https://dx.doi.org/10.1016/S0140-6736(14)61490-2
- Gómez O., Figueras F., Fernández S., Bennasar M., Martínez J.M., Puerto B. et al. Reference ranges for uterine artery mean pulsatility index at 11-41 weeks of gestation. Ultrasound Obstet. Gynecol. 2008; 32(2): 128-32. https://dx.doi.org/10.1002/uog.5315
- Ciobanu A., Wright A., Syngelaki A., Wright D., Akolekar R., Nicolaides K.H. Fetal Medicine Foundation reference ranges for umbilical artery and middle cerebral artery pulsatility index and cerebroplacental ratio. Ultrasound Obstet. Gynecol. 2019; 53(4): 465-72. https://dx.doi.org/10.1002/uog.20157
- Srisupundit K., Luewan S., Tongsong T. Prenatal diagnosis of fetal heart failure. Diagnostics (Basel). 2023; 13(4): 779. https://dx.doi.org/10.3390/diagnostics13040779
- Seravalli V., Masini G., Ponziani I., Di Tommaso M., Pasquini L. Ductus venosus Doppler assessment: do the results differ between the sagittal and the transverse approach? J. Matern. Fetal. Neonatal. Med. 2022; 35(25): 9661-6. https://dx.doi.org/10.1080/14767058.2022.2050364
- Sanapo L., Turan O.M., Turan S., Ton J., Atlas M., Baschat A.A. Correlation analysis of ductus venosus velocity indices and fetal cardiac function. Ultrasound Obstet. Gynecol. 2014; 43(5): 515-9. https://dx.doi.org/10.1002/uog.13242
- Fratelli N., Amighetti S., Bhide A., Fichera A., Khalil A., Papageorghiou A.T. et al. Ductus venosus Doppler waveform pattern in fetuses with early growth restriction. Acta Obstet. Gynecol. Scand. 2020; 99(5): 608-14. https://dx.doi.org/10.1111/aogs.13782
Received 12.08.2025
Accepted 26.08.2025
About the Authors
Tamara A. Yarygina, PhD, Head of the Ultrasound Diagnostics Department, Academician V.I. Krasnopolsky Moscow Regional Research Institute of Obstetrics and Gynecology, 101000, Russia, Moscow, Pokrovka str., 22a; Researcher at the Perinatal Cardiology Center, A.N. Bakulev National Medical Research Center of Cardiovascular Surgery, Ministry of Health of Russia, 121552, Russia, Moscow, Roublyevskoe Shosse, 135; Associated Professor at the Department of Ultrasound Diagnostics of the Faculty of Continuing Medical Education of the Medical Institute, Patrice Lumumba Peoples’ Friendship University of Russia, 127015, Russia, Moscow, Pistsovaya str., 10,+7(495)414-78-75, tamarayarygina@gmail.com, https://orcid.org/0000-0001-6140-1930
Rena M. Gasanova, Dr. Med. Sci., Head of the Perinatal Cardiology Center, A.N. Bakulev National Medical Research Center for Cardiovascular Surgery, Ministry of Health of Russia, 121552, Russia, Moscow, Roublyevskoe Shosse, 135; Physician of Ultrasound Diagnostics, Department of Ultrasound and Functional Diagnostics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, rmgasanova@bakulev.ru, https://orcid.org/0000-0003-3318-1074
Alexander I. Shchegolev, Dr. Med. Sci., Professor, Head of the 2nd Pathoanatomical Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, +7(495)531-44-44, ashegolev@oparina4.ru,
https://orcid.org/0000-0002-2111-1530
Alexander I. Gus, Dr. Med. Sci., Professor, Chief Researcher at the Department of Ultrasound and Functional Diagnostics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4; Head of the Department of Ultrasound Diagnostics of the Faculty of Continuing Medical Education of the Medical Institute, Patrice Lumumba Peoples’ Friendship University of Russia, 127015, Russia, Moscow, Pistsovaya str., 10, a_gus@oparina4.ru, https://orcid.org//0000-0003-1377-3128
Roman G. Shmakov, Dr. Med. Sci., Professor of the Russian Academy of Sciences, Director, Academician V.I. Krasnopolsky Moscow Regional Research Institute of Obstetrics and Gynecology, 101000, Russia, Moscow, Pokrovka str., 22a; Head of the Department of Obstetrics and Gynecology, Faculty of Advanced Medical Studies, M.F. Vladimirsky Moscow Regional Research Clinical Institute, mdshmakov@mail.ru https://orcid.org/0000-0002-2206-1002
Corresponding author: Tamara A. Yarygina, tamarayarygina@gmail.com



