Early fetal growth restriction: a new approach to guide the choice of management strategy
Background: Fetal growth restriction (FGR) affects about 10 to 15% of all pregnancies. FGR is a potential cause of preterm birth, preeclampsia (PE), and stillbirth. FGR is associated with a high risk of adverse outcomes both in the neonatal period and long term.Timokhina E.V., Strizhakov A.N., Zafiridi N.V., Fedyunina I.A., Aslanov A.G.
Aim: To identify the threshold of angiogenic markers (sFlt-1, PlGF, and their ratios) to predict fetal deterioration during early FGR.
Materials and methods: This was a prospective study of 80 pregnant women. The study group included 45 patients with early FGR. FGR was diagnosed by a decrease in the estimated fetal weight (EFW) below the 10th percentile and impaired umbilical artery (UA) blood flow. Subgroup IA consisted of 24/45 (53.33%) pregnant women with a stable fetal condition according to Doppler and CTG monitoring, who progressed to full-term delivery. Subgroup IB included 21/45 (46.67%) patients with a progressive fetal deterioration, including an increase in PI up to the absence of end-diastolic blood flow in the UA, a decrease in cerebro-placental ratio and a reduction in PI in MCA, no increase in fetometric ultrasound parameters, and questionable or pathological type of CTG.
Results: In subgroup IA, sFlt-1and PlGF concentrations and sFlt-1/PlGF ratio were 54305 pg/ml, 82.67 pg/ml, and 852.44, respectively. In subgroup IB, sFlt-1and PlGF concentrations and sFlt-1/PlGF ratio were 105001 pg/ml, 34.89 pg/ml, and 2888.92, respectively. Concentrations of sFlt-1, PlGF, and sFlt-1/PlGF in pregnant women with a healthy pregnancy were 11860 pg/ml, 705 pg/ml, and 19.1, respectively. The optimal cut-off values of PlGF and sFlt-1/PlGF were 52.7 pg/ml. and 1118.12.
Conclusion: The study findings showed that patients in the two subgroups of early FGR had statistically significant different levels of angiogenic markers. PlGF level ≥ 52.7 pg/ml and/or sFlt-1/lGF ratio ≥ 1118.12 were predictive for a high risk of fetal deterioration and adverse perinatal outcomes. Our study showed the validity of using the studied angiogenic markers in pregnant women with early FGR. The findings support a recommendation to use these markers in clinical practice.
Keywords
Fetal growth restriction (FGR) remains one of the most significant challenges in obstetrics and perinatology, being one of the leading causes of perinatal morbidity and mortality, as well as long-term sequelae [1–4]. FGR affects 10 to 15% of all pregnancies and is a potential cause of premature birth, preeclampsia (PE), and stillbirth. FGR is characterized by a high risk of adverse outcomes both in the neonatal period and in the distant future. The most significant early consequence is high perinatal mortality in term and preterm infants.
Low-birth-weight infants are at high risk of perinatal morbidity. Early complications include peripartum hypoxia and asphyxia, thymus atrophy, and long-term immunological insufficiency due to hypogammaglobulinemia, a tendency to hypothermia, hyperbilirubinemia, high risk of hypoglycemia, necrotizing enterocolitis, retinopathy of prematurity, and coagulopathy [4, 5]. FGR is characterized by an increased risk of developing respiratory distress syndrome and bronchopulmonary dysplasia, as well as the frequent need for mechanical ventilation [6]. Cerebral palsy, childhood disability, impaired growth, and delayed neurological and intellectual development are significant long-term problems in children who are small by gestational age.
It has long been recognized that many complications associated with FGR lead to lifelong effects. They not only increase the risk of an adverse perinatal outcome but also have serious long-term consequences. It has been shown that the risk of premature death, cerebrovascular events, severe cardiovascular diseases, and metabolic disorders is manifold increased throughout adulthood [7, 8].
To date, neither domestic nor international guidelines suggest a single view of the FGR classification. Generally, two types of FGR are defined: severe early-onset and moderate late-onset. The cutoff point for these forms has been arbitrarily established at 32 weeks [2].
Management of FGR concerning the optimal delivery timing requires a careful clinical balance between the risk of antepartum stillbirth due to delaying the delivery and iatrogenic prematurity potentially causing significant morbidity or neonatal death by early intervention. The search for markers that reflect the state of the fetus and could aid clinical decision-making is of utmost importance. In our study, we address this problem.
In recent years, there has been a wealth of evidence that fetal growth restriction is caused by an imbalance of angiogenic and anti-angiogenic factors, characterized by an increase in anti-angiogenic factors such as soluble fms-like tyrosine kinase 1 (sFlt-1, also referred to as sVEGFR-1), and a decrease in pro-angiogenic factors, such as placental growth factor (PlGF) [9], which are directly involved in FGR pathogenesis.
Currently, diagnostic criteria are based on ultrasound, Doppler, and CTG monitoring.
But this strategy ignores the underlying pathological process in the placenta, which leads to an inadequate supply of oxygen and nutrients to the growing fetus.
Consequently, these diagnostic limitations can lead to the recognition of FGR at an advanced stage, hindering its early diagnosis, optimal treatment and potentially leading to severe complications for the fetus.
This study aimed to identify the threshold of angiogenic markers (sFlt-1, PlGF, and their ratios) to predict fetal deterioration during early FGR. The established thresholds will help guide the choice of ultrasound and Doppler imaging, CTG monitoring, and determine the optimal delivery timing.
Materials and methods
This was a prospective study of 80 pregnant women managed at the Perinatal Center of the S.S. Yudin City Clinical Hospital. All patients signed informed consent to participate in the study and for providing blood samples.
The study was reviewed and approved by the Research Ethics Committee of the I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University) on 23.01.2019, an extract from the minutes No. 01-19.
The study group included 45 patients with early FGR. The inclusion criteria were age >18 years, a singleton FGR-complicated pregnancy at gestational age 20–36 weeks, and 6 days. FGR was diagnosed by a decrease in the estimated fetal weight (EFW) below the 10th percentile and impaired UA blood flow (increased PI in the UA).
The control group consisted of 35 patients aged >18 years with a healthy singleton pregnancy at gestational age 20–36 weeks and 6 days.
Criteria for excluding patients from the study were age < 18, multiple gestation pregnancy, smoking, biochemical and ultrasound markers of chromosomal abnormalities in the 1st and 2nd trimesters of pregnancy, preeclampsia (criteria of the clinical guidelines of the Ministry of Health of the Russian Federation “Hypertensive complications during pregnancy, childbirth and the postpartum period. Preeclampsia. Eclampsia” dated June 07, 2016 N15-4/10/2-3483), and refusal to further participate in the study.
Venous blood samples were collected from the study subjects to determine the serum concentration of angiogenic factors – sFlt-1, PlGF, and the sFlt-1/PlGF ratio. Additional blood samples were drawn from the antecubital vein during the standard blood sampling procedure. Blood samples were placed in sterile tubes at 4°C and centrifuged for 10 min at 3000 rpm. After that, serum samples were stored at -40°C until analysis.
The levels of sFlt-1 and PlGF were determined in the laboratory of clinical biochemistry of the N.N. Blokhin RCRC, Ministry of Health of Russia using specific and sensitive enzyme-linked immunosorbent assays Human PlGF and Human VEGF R1/Flt-1 Quantikine ELISA Kit from R&D Systems, USA.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 26.0 software. The normality of the distribution was tested by the Shapiro–Wilk test. Quantitative variables showing normal distribution were expressed as means (M) and standard deviation (SD); otherwise, the median (Me) with interquartile range (Q1; Q3) were reported. Normally distributed continuous variables were compared between two groups with a Student’s and Welch t-test (unequal variance t-test). For non-normally-distributed parameters, the Mann–Whitney U-test was utilized for comparisons. Continuous variables showing normal distribution were compared among three groups with one-way analysis of variance by using the Game's–Howell test (with unequal variances) for post-hoc comparisons. Kruskal–Wallis test was used to compare quantitative parameters between three or more groups with non-normal distribution using the Dunn test for post-hoc comparisons. Categorical variables were presented as counts and percentages and compared with the Pearson χ2 or Fisher’s exact test. Differences were considered statistically significant at p<0.05. ROC curves were constructed to evaluate the diagnostic performance of quantitative variables in predicting a specific outcome. An optimal cut-off value of the quantitative variables was determined, making it possible to classify patients according to the risk of the outcome, which has the best combination of sensitivity and specificity. The quality of the predictive model was assessed based on the area under the ROC curve with a standard error and 95% confidence interval (CI) and the level of statistical significance. 95% confidence intervals were computed using the adjusted Wald method.
Results
To identify angiogenic factors predicting the course of early FGR, we analyzed 45 pregnant women in the study group and 35 women in the control group.
The mean age of pregnant women in the study group [30.95 (5.07)] was statistically significantly higher than that in the control group [27.37 (4.9)] (p=0.033). The groups were comparable in somatic, obstetric, and gynecological status, parity, and BMI. No significant differences were found in blood count, biochemical blood tests, and urinalysis.
The gestational age at the time of assessment of angiogenic markers in the study and control groups was 30.5 (28.5; 33.5) and 31.5 (26.5; 34.5) weeks, respectively (p=0.644).
The study group was divided into two subgroups categorized by pregnancy clinical course and outcome.
Subgroup IA consisted of 24/45 (53.33%) pregnant women with a stable fetal condition according to Doppler and CTG monitoring, who progressed to full-term delivery. The gestational age at the time of delivery was 38.2 (0.63) weeks.
Subgroup IB included 21/45 (46.67%) patients with progressive fetal deterioration, including an increase in PI up to the absence of end-diastolic blood flow in the UA, a decrease in cerebro-placental ratio and a reduction in PI in MCA, no increase in fetometric ultrasound parameters, and questionable or pathological type of CTG. These indicators required early delivery for perinatal indications. The gestational age at the time of delivery in this subgroup was 29.11 (4.04) weeks, which is statistically significantly less than in subgroup IA (p<0.001).
The preterm birth rate in subgroup IB was 100%. In subgroup IA and control group, all deliveries were full term.
Caesarean section rate in subgroup IB was statistically significantly higher than in subgroup IA and the control group – 19/21 (90.48%), 5/24 (20.83%) and 4/35 (11.43%), respectively (p<0.001, pIB control <0.001, pIB-IA = 0.022).
All pregnant women of subgroup IB underwent emergency cesarean section compared to subgroup IA and the control group, where all cesarean deliveries were elective.
The mean birthweight of children in subgroup IA, IB, and the control group was 2736.2 (125.5) 946.67 (539.21), and 3475.26 (203.56) g, respectively. The differences were statistically significant (p<0.001, pIA control <0.001, pIB control <0.001, pIB-IA <0.001).
In subgroup IA, IB, and the control group, the first-minute Apgar score was 7 (7; 8), 6 (5; 6), and 8 (8; 8), respectively. The differences were statistically significant (p <0.001, pIA control <0.016, pIB control <0.001, pIB-IA <0.013). In subgroup IA, IB, and the control group, the fifth-minute Apgar score was 8 (8; 9), 7 (6; 7), and 9 (9; 9), respectively. Statistically significant differences were found (p <0.001, pIA control <0.023, pIB control <0.001, pIB-IA <0.006).
In the studied groups, we assessed serum concentrations of angiogenic factors sFlt-1, PlGF, and their ratio (Table).
In subgroup IA (women with a stable fetus) the concentration of sFlt-1 was 54305 pg/ml (12352; 102394) (Fig. 1), PlGF – 82.67 pg/ml (61.03; 101.6) (Fig. 2), the sFlt-1/PlGF ratio is 852.44 (161.6; 1015.2) (Fig. 3).
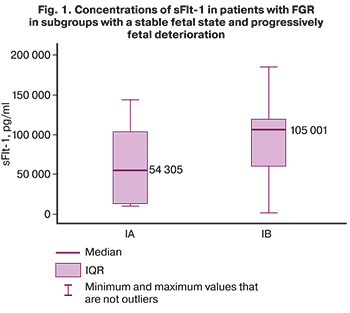
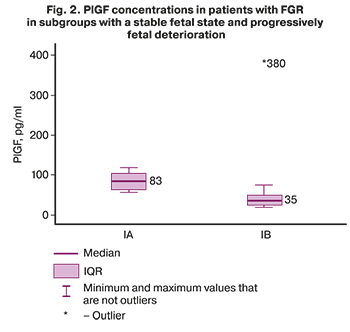
In subgroup IB (patients with a progressive fetal deterioration) the concentration of sFlt-1 was 105001 pg/ml (59766; 118014) (Fig. 1), PlGF – 34.89 pg/ml (24.57; 49) (Fig. 2), the sFlt-1/PlGF ratio is 2888.92 (1615.08; 4273.2) (Fig. 3).
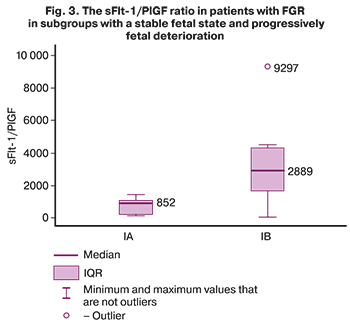
The concentration of sFlt-1 in pregnant women with a healthy pregnancy was 11860 pg/ml (6420; 15720), PlGF was 705 pg/ml (324.6; 951), and sFlt-1/PlGF was 19.1 (7.43; 39, 44).
The difference in sFlt-1 concentrations was statistically insignificant (p=0.18), while differences in the concentration of PlGF and sFlt-1/PlGF were statistically significant (p=0.01 and p=0.004 respectively).
Given that the concentrations of PlGF and sFlt-1/PlGF were statistically significantly different in the subgroups, we performed a ROC analysis to determine the threshold values for predicting FGR decompensation.
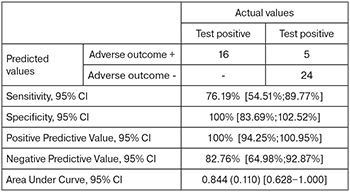
The area under the ROC curve (Fig. 4), corresponding to the relationship between the prognosis of fetal deterioration in FGR and PlGF, was 0.844 (0.110) with 95% CI: 0.628–1.000. The resulting model was statistically significant (p=0.011).
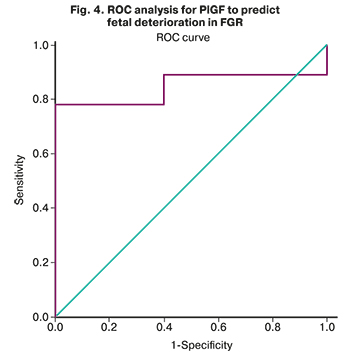
The PlGF cut-off was 52.7 pg/ml. PlGF level less than or equal to this value was predictive of high risk of fetal deterioration. The sensitivity and specificity of the method were 76.19% and 100%, respectively.
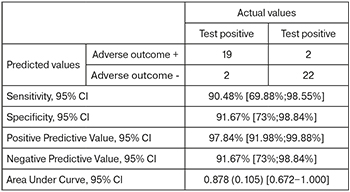
The area under the ROC curve (Fig. 5), corresponding to the relationship between the prognosis of fetal deterioration and sFlt-1/PlGF, was 0.878 (0.105) with 95% CI: 0.672–1.00. The resulting model was statistically significant (p=0.006).
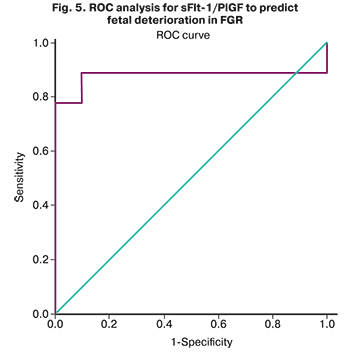
The sFlt-1/PlGF cut-off was 1118.12. Levels of sFlt-1/PlGF equal to or greater than this value were predictive of deterioration of growth-restricted fetuses. The sensitivity and specificity of the method were 90.48 and 91.67%, respectively.
The study findings showed that with a stable and progressively deteriorating fetal condition with FGR, there are statistically significant differences in the PlGF concentration and the sFlt-1/PlGF ratio.
Therefore, these markers can be used to predict the state of growth-restricted fetuses.
The result of the ROC analysis showed that the threshold value indicating a high risk of fetal deterioration in FGR and an unfavorable perinatal outcome was 52.7 pg/ml for PlGF and 1118.12 for sFlt-1/PlGF.
Discussion
Even though the etiology of FGR is not fully understood, it is generally accepted that this condition is caused by a defect in trophoblast invasion and impaired remodeling of the spiral arteries in the first half of pregnancy, which leads to insufficient placental perfusion in the second half of pregnancy.
The period of trophoblast invasion is the main factor determining the quality of placental implantation. During this period, the spiral arteries are transformed into low resistance vessels. Incomplete spiral artery remodeling prevents adequate blood flow in the intervillous space since the contractile properties of the arteries are preserved. This leads to hypoperfusion-reperfusion, which, in turn, causes damage to the architecture of the villi and impairs exchange between the mother and the fetus, which ultimately leads to FGR [10].
More than ten years ago, it was shown that placental soluble fms-like tyrosine kinase 1 (sFlt1), an antagonist of VEGF and placental growth factor (PlGF), is a key factor regulating angiogenic homeostasis during pregnancy [11]. It is markedly elevated in FGR-complicated pregnancy, especially in early FGR. sFlt1 is a splice variant of the VEGF receptor Flt1 lacking the transmembrane and cytoplasmic domains but retaining the ligand-binding domain. Flt-1 (or VEGFR-1) is a receptor for the vascular endothelial growth factor family present on vascular endothelial cell membranes, which is also expressed in the placenta, mainly by syncytiotrophoblastomas. Flt-1 and the insert kinase domain receptor (KDR or VEGFR-2) have a high affinity for vascular endothelial growth factor-A (VEGF-A), which is an essential factor involved in placental vascular development, proliferation and endothelial cell survival, vascular permeability, and endothelial cell fenestration.
PlGF is a member of the VEGF family, has pro-angiogenic activity, and is abundantly expressed in the placenta. It binds to Flt-1, but not KDR, and acts to enhance the effect of VEGF-A [12].
In response to hypoxia arising in FGR, alternative splicing of the Flt-1 gene is generated in the form of sFlt-1 mRNA, and sFlt-1 is produced and secreted by the placenta into the maternal bloodstream, causing a decrease in the bioavailability of the pro-angiogenic factors VEGF and PLGF by binding to them, thereby further increasing aggravating the imbalance towards an antiangiogenic state.
Modern literature on perinatology lacks studies investigating the role of angiogenic markers in isolated FGR; most of them are devoted to studying pregnancy complicated by PE.
From 2010 to 2014, a prospective, multicenter, observational PROGNOSIS study including1270 pregnant women was conducted in 14 countries. The study aimed to assess whether a low sFlt-1/PlGF ratio predicts the absence of preeclampsia in the short term. It was found that low sFlt-1/PlGF ratios predict the absence of preeclampsia within one week after the first visit, and high ratios predict the presence of preeclampsia within 4 weeks.
The researchers showed that sFlt-1/PlGF ratio of 38 or lower could be used to predict the short-term absence of preeclampsia in women in whom the syndrome is suspected clinically. sFlt-1/PlGF ratio above 38 was predictive for adverse fetal outcomes (perinatal mortality, delivery at term less than 34 weeks, FGR, placental abruption, respiratory distress syndrome, necrotizing enterocolitis, and intraventricular hemorrhage) and a diagnosis of preeclampsia within four weeks [13].
Since FGR also has a placentogenic nature and is a placenta-associated complication, we conducted a study to investigate these markers in early FGR. It should be noted that all studies on FGR are associated with PE, and we have undertaken an assessment of pregnancy complications without obvious manifestations of PE.
Our study showed that angiogenic markers have statistically significant differences in the two FGR subgroups, depending on the state of the fetus. PlGF levels ≥ 52.7 pg/ml and sFlt-1/PlGF levels ≥ 1118.12 were predictive of a high risk of deterioration of growth-restricted fetuses. Thus, these markers can act as a laboratory predictor of early FGR.
When analyzing the literature on this topic, there were no analogs to our study.
O.V. Makarov et al. [14] conducted a study to investigate the basics of placental angiogenesis, the causes of placental insufficiency, and fetal development. The study included patients with placental insufficiency and FGR and patients with a healthy pregnancy. The authors determined their angiogenic factors and found statistically significant differences in serum concentrations in patients with placental insufficiency and FGR compared with healthy pregnant women. The authors concluded that placental insufficiency is characterized by an imbalance of angiogenic factors, an increase in the level of antiangiogenic factors, and a decrease in the concentrations of a pro-angiogenic factor.
Conclusion
Our study showed the validity of using the studied angiogenic markers in pregnant women with an early FGR. The results obtained are of practical importance and support a recommendation to use these markers in clinical practice. The established thresholds values help guide the choice of the type and frequency of diagnostic studies (ultrasound, Doppler, CTG monitoring) and determine the optimal delivery time to improve perinatal outcomes.
Of the limitations of our work, it should be noted that the sample is still insufficient. But our pilot study aimed to confirm the hypothesis about the possible clinical use of angiogenic markers and their ratio for the management of pregnant women with early FGR. Further, sufficiently powered studies are required to update clinical guidelines for an unresolved problem such as FGR.
A multivariate analysis of clinical data, FGR risk factors, ultrasound and Doppler data, and angiogenic markers lends support for further research.
References
- Friedman A.M., Cleary K.L. Prediction and prevention of ischemic placental disease. Semin. Perinatol. 2014; 38(3): 177-82. https://dx.doi.org/10.1053/j.semperi.2014.03.002.
- ACOG Practice bulletin no. 134: fetal growth restriction. Obstet. Gynecol. 2013; 121(5): 1122-33. https://dx.doi.org/10.1097/01.AOG.0000429658. 85846.f9.
- Hunt K., Kennedy S.H., Vatish M. Definitions and reporting of placental insufficiency in biomedical journals: a review of the literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016; 205: 146-9. https://dx.doi.org/10.1016/j.ejogrb.2016.08.029.
- Тимохина Е.В., Стрижаков А.Н., Зафириди Н.В., Губанова Е.С. Инновационный подход к прогнозированию и терапии преэклампсии – мировой опыт. Акушерство и гинекология. 2019; 5: 5-10. [Timokhina E.V., Strizhakov A.N., Zafiridi N.V., Gubanova E.S. Innovative approach to prediction and therapy of preeclampsia: global experience. Odstetrics and Gynecology. 2019; 5: 5-10. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.5.5-10.
- Halliday H.L. Neonatal management and long-term sequelae. Best Pract. Res. Clin. Obstet. Gynaecol. 2009; 23(6): 871-80. https://dx.doi.org/10.1016/j.bpobgyn.2009.06.005.
- Mendez-Figueroa H., Truong V.T., Pedroza C., Chauhan S.P. Morbidity and mortality in small-for-gestational-age infants: A Secondary Analysis of Nine MFMU Network Studies. Am. J. Perinatol. 2017; 34(4): 323-32. https://dx.doi.org/10.1055/s-0036-1586502.
- Bakalis S., Peeva G., Gonzalez R., Poon L.C., Nicolaides K.H. Prediction of small-for-gestational-age neonates: screening by biophysical and biochemical markers at 30-34 weeks. Ultrasound Obstet. Gynecol. 2015; 46(4): 446-51. https://dx.doi.org/10.1002/uog.14863.
- Birdir C., Fryze J., Frölich S., Schmidt M., Köninger A., Kimmig R. et al. Impact of maternal serum levels of Visfatin, AFP, PAPP-A, sFlt-1 and PlGF at 11-13 weeks gestation on small for gestational age births. J. Matern. Fetal Neonatal Med. 2017; 30(6): 629-34. https://dx.doi.org/10.1080/14767058.2016.1182483.
- Hagmann H., Thadhani R., Benzing T., Karumanchi S.A., Stepan H. The promise of angiogenic markers for the early diagnosis and prediction of preeclampsia. Clin. Chem. 2012; 58(5): 837-45. https://dx.doi.org/10.1373/clinchem.2011.169094.
- Burton G.J., Woods A.W., Jauniaux E., Kingdom J.C. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta. 2009; 30(6): 473-82. https://dx.doi.org/10.1016/j.placenta.2009.02.009.
- Maynard S.E., Min J.Y., Merchan J., Lim K.H., Li J., Mondal S. et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Invest. 2003; 111(5): 649-58. https://dx.doi.org/10.1172/JCI17189.
- Shibuya M. Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. J. Biochem. 2013; 153(1): 13-9. https://dx.doi.org/10.1093/jb/mvs136.
- Zeisler H., Llurba E., Chantraine F., Vatish M., Staff A.C., Sennström M. et al. Predictive value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. N. Engl. J. Med. 2016; 374(1): 13-22. https://dx.doi.org/10.1056/NEJMoa1414838.
- Макаров О.В.,Волкова Е.В., Лысюк Е.Ю., Копылова Ю.В. Фетоплацентарный ангиогенез у беременных с плацентарной недостаточностью. Акушерство, гинекология и репродукция. 2013; 7(3): 13-9. [Makarov O.V., Volkova E.V., Lysyuk E.Yu., Kopylova Yu.V. Fetoplacental angiogenesis in pregnant women with placental insufficiency. Obstetrics, Gynecology and Reproduction. 2013; 7(3):13-9. (in Russian)].
Received 24.02.2021
Accepted 18.06.2021
About the Authors
Elena V. Timokhina, Dr. Med. Sci., Professor at the Department of Obstetrics, Gynecology and Perinatology, I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University), +7(499)782-30-45, elena.timokhina@mail.ru, 119991, Russia, Moscow, B. Pirogovskaya str., 2-4.Alexander N. Strizhakov, Academician of the RAS, Professor, Head of the Department of Obstetrics, Gynecology and Perinatology, I.M. Sechenov First MSMU,
Ministry of Health of Russia (Sechenov University), +7(499)782-30-45, kafedra-agp@mail.ru, 119991, Russia, Moscow, B. Pirogovskaya str., 2-4.
Nicoleta V. Zafiridi, Ph.D. Student at the Department of Obstetrics, Gynecology and Perinatology, I.M. Sechenov First MSMU, Ministry of Health of Russia
(Sechenov University), +7(499)782-30-45, zafiridisniki@mail.ru, 119991, Россия, Москва, ул. Б. Пироговская, д. 2, стр. 4.
Irina A. Fedyunina, Ph.D., Teaching Assistant at the Department of Obstetrics, Gynecology and Perinatology, I.M. Sechenov First MSMU, Ministry of Health of Russia
(Sechenov University), +7(499)782-30-45, irina.fedjunina@mail.ru, 119991, Russia, Moscow, B. Pirogovskaya str., 2-4.
Alexander G. Aslanov, Ph.D., Associate Professor at the Department of Obstetrics, Gynecology and Perinatology, I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University), +7(499)782-30-45, aslanov7@bk.ru, 119991, Russia, Moscow, B. Pirogovskaya str., 2-4.
Corresponding author: Elena V. Timokhina, elena.timokhina@mail.ru
Authors' contributions: Timokhina E.V., Strizhakov A.N. – conception of the study; Zafiridi N.V. – data collection, manuscript drafting; Timokhina E.V., Aslanov A.G. – manuscript editing; Zafiridi N.V., Fedyunina I.A. – statistical analysis and visualization.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Timokhina E.V., Strizhakov A.N., Zafiridi N.V., Fedyunina I.A., Aslanov A.G. Early fetal growth restriction: a new approach to guide the choice of management strategy.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 9: 42-49 (in Russian)
https://dx.doi.org/10.18565/aig.2021.9.42-49



