Analysis of pathomorphological characteristics of the placental structure in cases of prenatally diagnosed fetal congenital heart disease
Yarygina T.A., Gasanova R.M., Marzoeva O.V., Sypchenko E.V, Leonova E.I., Lyapin V.M., Shchegolev A.I., Gus A.I.
Objective: The purpose of the study was to determine placental pathologic changes associated with live-born premature and small-for-gestational-age (SGA) infants in cases of prenatally diagnosed fetal congenital heart disease (CHD).
Materials and methods: A comparative retrospective analysis of the results of pathologic examination of placentas was carried out in the cohort of 115 cases with fetal CHD, that was formed prenatally and divided into groups depending on the gestational age at delivery and birth weight of newborns. Group 1 consisted of 15 cases with premature and/or SGA infants. Group 2 consisted of 100 newborns with birth weight ≥ the 10th percentile.
Results: Examination of placentas in the general cohort showed small for gestational age in 59.1% of cases, abnormal shape in 53%, infarctions in 15.6%, delayed maturation of the vascular tree in 13.3%, meconium staining of the membranes in 8 .7%, massive fibrin deposition in 7.8%, multiple calcifications in 2.6% of cases. There were differences in the placental weight between the groups: the values less that the 3rd percentile and the 10th percentile were in 80% vs 40%, 93.3% vs 54%, in group 1 and group 2, respectively (p<0.01). It was found that the odds ratio for giving birth to premature and/or SGA babies with CHD, when placental weight was less that the 3rd and the 10th percentile, was 6.0 and 11.9, respectively.
Conclusion: According to the results of the pathologic examination of the placenta in cases of congenital heart disease, multiple macro- and microscopic signs of pathological changes were found in the fetus. A significant association between small placental weight and the birth of SGA or premature infant with cardiac pathology indicates the need to expand the scope of prenatal ultrasound examination for searching the markers of placental dysfunction in the diagnosis of fetal cardiac anomalies.
Authors' contributions: Yarygina T.A., Gasanova R.M., Shchegolev A.I., Gus A.I. – the concept and design of the study; Yarygina T.A., Marzoeva O.V., Sypchenko E.V., Leonova E.I., Lyapin V.M. – writing the text of the article; Yarygina T.A., Leonova E.I., Lyapin V.M., Shchegolev A.I. – material collection and processing; Gasanova R.M., Shchegolev A.I., Gus A.I. – editing the text of the article.
Conflicts of interest: The authors confirm that they have no conflicts of interest to declare.
Funding: The study was conducted within the framework of applied scientific research topic No. 123020300017-1.
Ethical Approval: The study was approved by the local Ethics Committee of A.N. Bakulev National Medical Research Center for Cardiovascular Surgery, Ministry of Health of Russia.
Patient Consent for Publication: The patients have signed informed consent for participation in the study and publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Yarygina T.A., Gasanova R.M., Marzoeva O.V., Sypchenko E.V., Leonova E.I., Lyapin V.M., Shchegolev A.I., Gus A.I. Analysis of pathomorphological characteristics of the placental structure
in cases of prenatally diagnosed fetal congenital heart disease.
Akusherstvo i Gynecologia/Obstetrics and Gynecology. 2024; 6: 75-83 (in Russian)
https://dx.doi.org/10.18565/aig.2024.119
Keywords
Placenta is a complex organ that has both a direct influence, which is limited by the length of pregnancy, on antenatal growth and development, and a long-term influence, which is mediated by the mechanisms of fetal programming, on the quality and duration of human life [1–3].
The rapid development of chorionic and embryonic vessels in the first 10 weeks of pregnancy and the subsequent functional relationship between the placenta and fetal heart, the so-called the heart-placental axis, determine a significant frequency of association between cardiac pathology and placental abnormalities [4].
To date, the results of scientific studies have convincingly demonstrated the correlation of low placental weight with prematurity, fetal growth restriction (FGR) and the birth of a small for gestational age (SGA) infant [5, 6]. At the same time, the frequency of cases of FGR and SGA among the fetuses with cardiac pathology significantly exceeds the average statistical indicators [7, 8]. For example, a large-scale analysis that was carried out by the researchers in the Kingdom of Denmark of a cohort of 924,422 live-born infants, included a group of 7,569 infants with heart defects. It was found that they had lower placental weight and birth weight as compared to the rest of population [9].
The above is of particular importance for clinical practice in management of the cases of fetal congenital heart disease (CHD), since, even taking into account steady development of cardiac surgery, gestational age at the time of birth, body weight and newborn’s condition are the predictors of survival and subsequent physical and neuropsychological development of the infant [10, 11]. This fact was clearly demonstrated in population study by Gimeno L. et al. [12], which was devoted to the survival trend among infants in England from 2004 to 2016. The cohort of 5 953 598 live-born infants included 21291 cases of CHD. The study found an increase in five-year survival in all groups of children without heart defects, as well as in the group of children with congenital heart disease, who were born at ≥ 39 weeks’ gestation. For infants with CHD born at 24–31 and 37–38 weeks’ gestation, there was a weak evidence of improvement in survival. In the group of infants born at 32–36 weeks’ gestation, there was no increase in the survival during the studied period [12].
Since understanding the morphofunctional basis of placental dysfunction is a foundation for the development prediction and prevention methods for perinatal hypoxic complications, in the last decade the number of mainly foreign publications that are devoted to the analysis of the changes in the placenta in association with fetal cardiac anomalies, has been steadily increasing. In cases of fetal CHD, significant differences in the macro- and microscopic structure of the placenta have been demonstrated [8, 13, 14].
It should be added that in the Russian Federation congenital anomalies rank second among the causes of fetal demise and stillbirth, and reached 4.7% in 2010, 7.2% in 2012 among all stillborn babies. The percentage of congenital heart defects was 16.7% of the total number of congenital anomalies, which were the cause of stillbirth in 2012 [15]. On the other hand, it is important to note that in 2020 (the year of the COVID-19 pandemic), stillbirth rate increased due to placental pathology by 5.6% compared to 2019 [16].
O'Hare C.B. et al. (2023) reported increased frequency of immature villi, which occurred three times more in full-term pregnancy and the diagnosis of fetal CHD, compared to healthy population, and was associated with a three-fold increased risk of respiratory support in neonates with CHD [14].
According to the literature data, impaired implantation, placental lesions, and umbilical cord abnormalities can lead to neurological disorders and brain damage in newborns [17].
According to Nijman M. et al., the severity of placental pathology negatively correlates with the volumes of cortical gray matter, cerebellum and total brain volume in full-term newborns with CHD [18]. However, the final data explaining the relationship between placental anomalies and postpartum outcomes in cases of fetal cardiac pathology are still not obtained. Due to this, the research is actively going on [13].
At the same time, in the Russian Federation, only two literature reviews were devoted to the issue under discussion – one by Tsibizova V.I. et al. (2022) [19], and another by our group of authors [20]. The only research by Kravchenko E.N. et al. (2017) [21] was devoted to the study of 150 placentas and umbilical cords after termination of pregnancy in the second trimester due to fetal malformations of various organs and systems. Among them, there were 66 cases of CHD, which were not grouped separately.
Also, according to the Federal Service for State Statistics (Rosstat), congenital heart defects were registered as the initial cause of early neonatal death in 35.9% of all developmental anomalies and in 6.5% of the total structure of the causes of death in 2010 in the Russian Federation [22]. At the same time, in 17.2% of cases of early neonatal death, placental abnormality was the cause of death. In the subsequent years (2012–2016), the rate of CHD as the cause of early neonatal death among all congenital anomalies reached 32.0% [23].
However, at the time of writing this article, we found no any source analyzing the relationship between fetal growth in fetuses with CHD and placental characteristics.
The above determines the relevance of our study aimed at determination of placental pathologic changes associated with small for gestational age (SGA) or preterm birth in cases of prenatally diagnosed fetal congenital heart disease.
Material and methods
A multicenter retrospective study was carried out at the Perinatal Cardiology Center of A.N. Bakulev National Medical Research Center for Cardiovascular Surgery, Ministry of Health of Russia and Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia in 2023.
In the first step of the study, observational analysis of the results of postnatal examination of placentas in the cohort of cases with prenatally diagnosed fetal CHD was done. The second step was comparative «case-control» assessment of the frequency of placental pathologic changes between the groups of the general cohort, which were grouped taking into account the birth weight and gestational age at the time of delivery.
The general inclusion criteria in the study were the cases of live birth and prenatally diagnosed CHD excluding isolated ventricular septal defects (VSDs), reported results of pathologic examination of placentas, patient’s informed consent.
The general non-inclusion criteria were prenatally non-diagnosed CHD, multiple pregnancy, stillbirth, absence of the results of placental examination, patient’s disagreement to participate in the study. The cases of isolated VSDs were excluded from the study, since the defects, such as physiological intracardiac shunts (fetal communications), do not change intrauterine hemodynamics, do not lead to volume overload, do not increase cardiac preload and afterload, and moreover, do not change systolic and diastolic function of the heart, which provides blood circulation in the functional mother-placenta-fetus system.
Group 1 included birth cases of babies born with CHDs, who had increased risk of perinatal, post-operative and delayed complications. Inclusion criteria in group 1 were birth cases of SGA (birth weight < the 10th percentile [24]), term or preterm babies (gestational age <37 weeks) regardless of the body weight.
Inclusion criteria in group 2 were birth cases of term babies with prenatally diagnosed CHD and birth weight ≥ the 10th percentile for gestational age.
The study and description of the morphological features of placental damage were carried out in accordance with the Consensus Statement of the Amsterdam Placental Workshop Group (APWGCS) [25].
The percentiles of placental weight were determined in accordance with the data in the study by Flatley C. et al. (2022) [26].
Statistical analysis
Statistical data processing was performed using standard data processing libraries and statistical models Numpy, Pandas, Matplotlib and Seaborn with Python programming language, V.3.11. The qualitative characteristics are presented as absolute numbers (n), the share of the total number (%). The quantitative data are represented as arithmetic mean (M) with standard deviation (SD), median (Me) with interquartile range (Q1–Q3). The Shapiro–Wilk test was used for testing normality of distribution. Student’s t-test was used for statistical processing of the results. The Mann–Whitney U test was used in case of non-normal distribution of the quantitative features or inequalities for the dispersion. Fisher's exact test was used to assess the differences in the frequency of the studied parameters between the groups. The odds ratio (OR) with 95% confidence interval (CI) was calculated to estimate the probability of birth of premature and/or small for gestational age babies with CHD in pathological changes in the placenta, and to determine the presence and strength of the relationship between these facts. For statistical criteria, type I error was set at 0.05. The null hypothesis (no differences) was rejected, if probability (p) did not exceed the type I error.
Results
The general cohort of the study included 115 birth cases of infants with prenatally diagnosed CHD, which were divided by the hemodynamic subtypes as follows: 41/115 (35.7%) cases of low systemic blood flow; 38/115 (33%) cases of hypervolemia in pulmonary circulation; 28/115 (24,3%) cases of hypovolemia in pulmonary circulation and 8/115 (7%) cases of vascular rings around the trachea and the esophagus, without impairments of systemic or pulmonary circulation.
The median birth weight of newborns in the general cohort was 3240 g, that was equivalent to the 52th percentile with interquartile range 2848–3606 g: 27–86 percentiles) [24]. Group 1 included 15 observations (13% of the group of the study): 9 premature babies born at 33+6–36+6 weeks’ gestation, of them, 4 babies were small for gestational age, and 6 term babies, who were small for gestational age. Group 2 included the remaining 100 observations (87% of the group of the study).
The analysis of clinical and anamnestic maternal characteristics is represented in detail in Table 1. It showed no statistically significant differences between the groups. In the general cohort, the frequency of external genital pathology in pregnant women and the percentage of premature and operative delivery (Table 1) did not exceed the values in the general population in the Russian Federation [27].
With this pathology, the percentage of operative delivery in group 1 was significantly higher compared to group 2 (60% versus 28%, р=0.0019), despite the fact that initially there was an equal relative number of patients with uterine scars (Table 1).
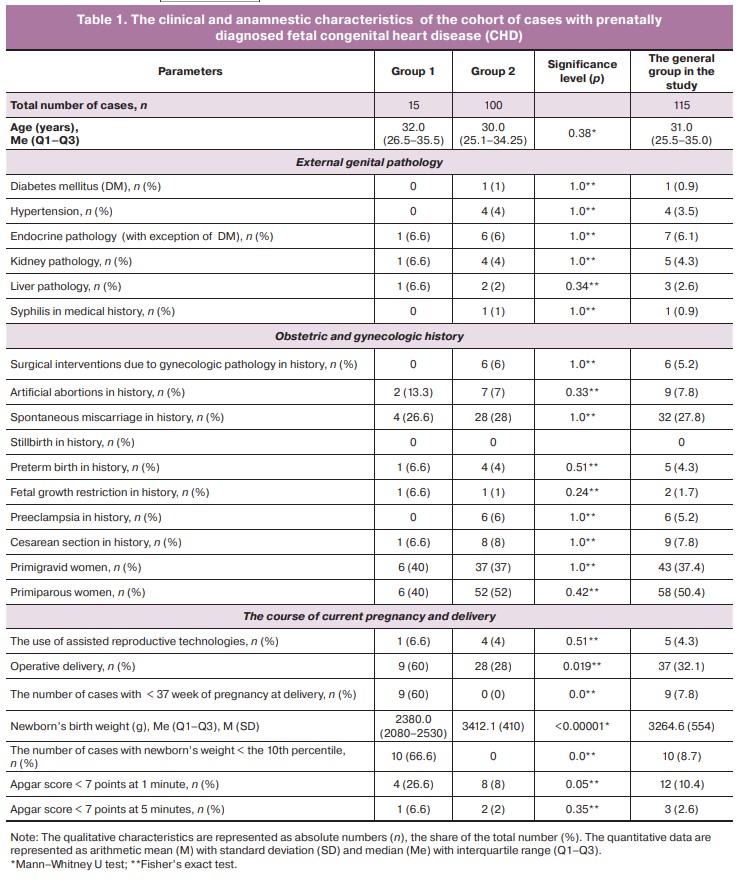
The results of postnatal examination of placentas in the general cohort, in each group, as well as statistical significance of differences are represented in Table 2.
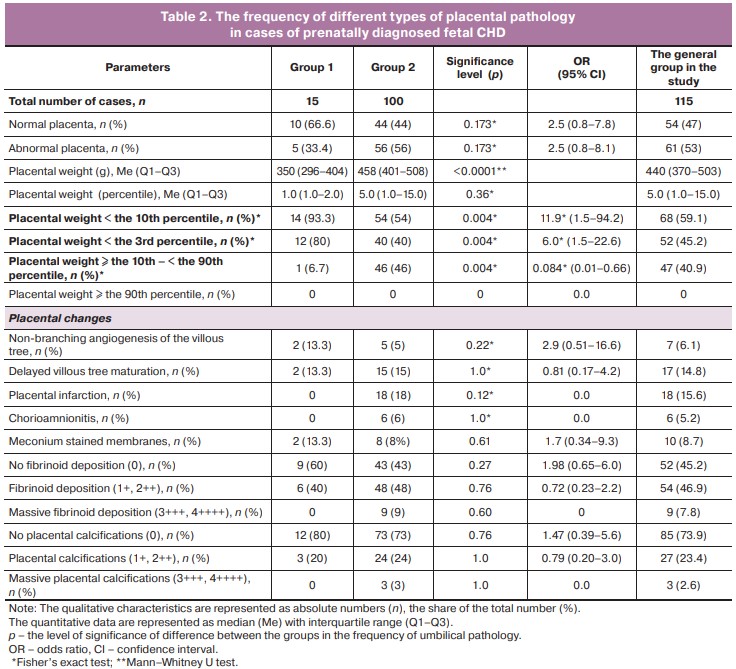
The analysis found that small for gestational age is the most common placental pathologic feature, which was identified in 68/115 (59.1%) of cases of fetal CHD of the total number in the general cohort in the study (Table 2). Abnormal placenta was in 61/115 (53%), placental infarctions were in 18/115 (15.6%), delayed villous tree maturation was in 17/115 (14.8%), non-branching angiogenesis of the villous tree was in 7/115 (6.1%) cases. Meconium stained membranes was found in 10/115 (8.7%) cases, massive fibrinoid deposition in в 9/115 (7.8%), massive calcifications in 3/115 (2.6%) cases (Table 2).
The comparative analysis of the studied characteristics between the groups of patients with CHD showed pronounced differences exclusively in placental weight. Both the absolute values and the number with cases with placental mass < the 3rd and < the 10th percentile were significantly higher in cases of small for gestational age and/or premature infants: 12/15 (80%) versus 40/100 (40%), 14/15 (93.3%) versus 54/100 (54%) in group 1 and in group 2, respectively (р<0.01). It was found that OR for the birth of premature/or small for gestational age infants with CHD was 6.0 and 11.9 with placental weight < the 3rd and < the 10th percentile, respectively (Table 2).
On the contrary, normal placental weight, which was more specific for term and non-small for gestational age fetuses with CHD – 46/100 (46%) versus 1/15 (6.7%) (in group 2 and in group 1, respectively, р<0.01), significantly reduced the chances for birth of premature and/or small for gestational age babies (OR=0.084) (Table 2).
Discussion
There is an urgent necessity to discuss placental problems in fetal heart defects due to the data obtained by foreign researchers on high frequency of anomalies of the macro- and microscopic structures of the placenta, as well as insufficient antenatal growth, which have a negative impact on short-term and long-term prognoses in the patient population under discussion [7, 9, 13]. Another reason is the available data on the role of the placenta in stillbirth [28]. According to world literature [29], in cases of stillbirth the frequency of detection of placental damage varied from 11.2% to 64.8%.
The data obtained by us were the results of the Russian pilot multidisciplinary project on the study of placentation features in case of fetal CHD. In the cohort of 115 cases, which was formed at the prenatal stage, postnatal examination of the placenta found a significant number of cases – 68/115 (59.1%) cases of small for gestational age placentas and association between placental weight and newborns’ weight. Among the group of term, but not-small for gestational age babies, placental weight < the 10th percentile was in 54/100 (54%) cases, that exceeded the data obtained in the study of 96 placentas in the Netherlands, among which small for gestational age was in 28% of cases [18], as well as in the study in the USA, where small for gestational age was found in one third of 306 placentas [30]. Despite the spread of the values of this parameter between the above published studies, the fact of reductions in placental weight remains unchanged. It is one of the most important factors of antenatal impact on weight and brain volume growth in most cases of fetal CHD compared to the general population [31].
Identification rates of infarctions and delayed villous tree maturation conformed to the results of examination of 120 placentas after the birth of term babies with CHD in the study by Rychik J. еt al. (2018) [13], and were 15.6% and 14.8% versus 17% and 15%, in the studies by the Russian and American [13] researches, respectively. In the study by O'Hare C.B. et al. (2023) [14] the rate of villous immaturity was 19% among 194 cases. Nijman M. et al. (2024) [18] reported 30% among 94 cases.
The demonstrated scattering of data obtained from different groups of authors indicates the need to continue the analysis, increase sample size of the cohort studies and conduct multicenter studies.
In our study, a comparative analysis of the detected changes between the groups of small for gestational age and/or premature infants and the cases of birth of term, but non-small for gestational age infants was made. There was statistically significant difference between the groups only in placental weight. The cases of small for gestational age babies predominated in group 1 (14/15 (93.3%) versus 54/100 (54%). The cases of normal placenta for the gestational age predominated in group 2 (1/15 (6.7%) versus 46/100 (46%). The odd ratio was calculated respectively. There was increased risk of including the baby with CHD in group 1 in case of small placenta – OR=11.9 (95% CI 1.5–94.2), and, on the contrary, odds reduction was with normal placental weight – OR=0.084 (95% CI 0.01–0.66) (р=0.004 for both variants).
Additional reasons for classifying the cases with fetal CHD as a group at increased risk of placental dysfunction are both intuitively obvious and research-based fact that pregnant women experience long-term maximum stress intensity after detection of fetal cardiac pathology [32, 33], as wells as association between maternal stress and reduction in placental size, that was reported by Williams A. et al. (2023) [34].
Rajagopalan V. et al. (2023) using functional prenatal magnetic resonance imaging confirmed the existence of the negative impact of maternal pregravid risk factors, especially metabolic syndrome, on placentation and fetal development in congenital heart disease, that is also understandable from a general clinical point of view [35].
Additional interest in antenatal assessment of the placenta is generated by the findings from the study by Rychik J. et al. (2018) [13]. Their study did not find any association between the values of ultrasound Doppler parameters, which in the general population are considered to reflect placental function, and postnatally detected placental abnormalities in fetal CHD [13].
All of the above results of our study and literature data indicate the need to organize a real functioning system for providing comprehensive interdisciplinary medical and psychological care to the families with fetal CHD [33–35], an integral part of which should be detailed prenatal ultrasound examination of the placenta. Given the fact that the protocol for the performance of ultrasound examination of pregnant women includes mandatory requirement for only a visual assessment of placental structure matching for the gestational age [36], it is necessary to develop a protocol for extensive examination of the placenta, based on multiplanar approaches to assessment of the area, thickness, and estimated volume of the placenta, the indicators, which help to predict FGR [37]. Moreover, taking into account the available data on the increased frequency of umbilical cord abnormalities in structural fetal malformations [21], it is necessary to study the structural features of the umbilical cord in cases of fetal congenital heart disease, and that will be the next stage of our research.
Conclusion
The increasing amount of convincing scientific evidence indicates a significant influence of structural and functional changes in the placenta on formation of the cardiovascular system in a fetus, fetal growth and development and condition at birth. Our study was the first Russian study of the cohort of 115 cases with prenatally diagnosed fetal CHD. The study found multiple macro- and microscopic signs of pathologic changes in the placenta and demonstrated a significant association between low placental weight and the birth of small for gestational age or premature infant with cardiac pathology. The obtained results indicate the need to expand the scope of prenatal ultrasound examination for searching the markers of placental dysfunction in diagnosing heart defects in fetuses.
References
- Burton G.J., Fowden A.L., Thornburg K.L. Placental origins of chronic disease. Physiol. Rev. 2016; 96(4): 1509-65. https://dx.doi.org/10.1152/physrev.00029.2015.
- Barker D.J., Thornburg K.L. Placental programming of chronic diseases, cancer and lifespan: a review. Placenta. 2013; 34(10): 841-5. https://dx.doi.org/10.1016/j.placenta.2013.07.063.
- Ярыгина Т.А., Гасанова Р.М., Марзоева О.В., Сыпченко Е.В., Леонова Е.И. Ишемическая болезнь сердца как следствие эпигенетических модификаций и фетального программирования при задержке роста плода. Креативная кардиология. 2023; 17(2): 189-202. [Yarygina T.A., Gasanova R.M., Marzoeva O.V., Sypchenko E.V., Leonova E.I. Ischemic heart disease as a consequence of epigenetic modifications and fetal programming in fetal growth retardation. Creative Cardiology. 2023; 17(2): 189-202. (in Russian)]. https://dx.doi.org/10.24022/1997-3187-2023-17-2-189-202.
- Maslen C.L. Recent advances in placenta-heart interactions. Front. Physiol. 2018; 9: 735. https://dx.doi.org/10.3389/fphys.2018.00735.
- Liu H.J., Liu P.C., Hua J., Zhao Y., Cao J. Placental weight and size in relation to fetal growth restriction: a case-control study. J. Matern. Fetal Neonatal Med. 2021; 34(9): 1356-60. https://dx.doi.org/10.1080/14767058.2019.1636371.
- Salavati N., Smies M., Ganzevoort W., Charles A.K., Erwich J.J., Plösch T. et al. The possible role of placental morphometry in the detection of fetal growth restriction. Front. Physiol. 2019; 9: 1884. https://dx.doi.org/10.3389/fphys.2018.01884.
- Albalawi A., Brancusi F., Askin F., Ehsanipoor R., Wang J., Burd I. et al. Pla-cental characteristics of fetuses with congenital heart disease. J. Ultrasound Med. 2017; 36(5): 965-72. https://dx.doi.org/10.7863/ultra.16.04023.
- Aliasi M., Snoep M.C., van Geloven N., Haak M.C. Birthweight and isolated congenital heart defects - a systematic review and meta-analysis. BJOG. 2022; 129(11): 1805-16. https://dx.doi.org/10.1111/1471-0528.17164.
- Matthiesen N.B., Henriksen T.B., Agergaard P., Gaynor J.W., Bach C.C., Hjortdal V.E. et al. Congenital heart defects and indices of placental and fetal growth in a nationwide study of 924 422 liveborn infants. Circulation. 2016; 134(20): 1546-56. https://dx.doi.org/10.1161/CIRCULATIONAHA.116.021793.
- Best K.E., Tennant P.W.G., Rankin J. Survival, by birth weight and gestational age, in individuals with congenital heart disease: a population-based study. J. Am. Heart Assoc. 2017; 6(7): e005213. https://dx.doi.org/10.1161/JAHA.116.005213.
- Yoshida T., Hiraiwa A., Ibuki K., Makimoto M., Inomata S., Tamura K. et al. Neurodevelopmental outcomes at 3 years for infants with congenital heart disease and very-low birthweight. Pediatr. Int. 2020; 62(7): 797-803. https://dx.doi.org/10.1111/ped.14160.
- Gimeno L., Brown K., Harron K., Peppa M., Gilbert R., Blackburn R. Trends in survival of children with severe congenital heart defects by gestational age at birth: a population-based study using administrative hospital data for England. Paediatr. Perinat. Epidemiol. 2023; 37(5): 390-400. https://dx.doi.org/10.1111/ppe.12959.
- Rychik J., Goff D., McKay E., Mott A., Tian Z., Licht D.J. et al. Characterization of the placenta in the newborn with congenital heart disease: distinctions based on type of cardiac malformation. Pediatr. Cardiol. 2018; 39(6): 1165-71. https://dx.doi.org/10.1007/s00246-018-1876-x.
- O'Hare C.B., Mangin-Heimos K.S., Gu H., Edmunds M., Bebbington M., Lee C.K. et al. Placental delayed villous maturation is associated with fetal congeni-tal heart disease. Am. J. Obstet. Gynecol. 2023; 228(2): 231.e1-231.e11. https://dx.doi.org/10.1016/j.ajog.2022.08.013.
- Щеголев А.И., Туманова У.Н., Шувалова М.П., Фролова О.Г. Сравнительный анализ мертворождаемости в Российской Федерации в 2010 и 2012 гг. Российский вестник перинатологии и педиатрии. 2015; 60(3): 58-62. [Shchegolev A.I., Tumanova U.N., Shuvalova M.P., Frolova O.G. Comparative analysis of stillbirth rates in the Russian Federation in 2010 and 2012. Russian Bulletin of Perinatology and Pediatrics. 2015; (3): 58-62. (in Russian)].
- Щеголев А.И., Туманова У.Н., Чаусов А.А., Шувалова М.П. Мертворожде-ние в Российской Федерации в 2020 году (год пандемии COVID-19). Акушерство и гинекология. 2022; 11: 131-40. [Shchegolev A.I., Tumanova U.N., Chausov A.A., Shuvalova M.P. Stillbirths in the Russian Federation in 2020 (COVID-19 pandemic year). Obstetrics and Gynecology. 2022; (11): 131-40. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.11.131-140.
- Щеголев А.И., Туманова У.Н., Серов В.Н. Роль плаценты в развитии поражений головного мозга новорожденного. Акушерство и гинекология. 2023; 8: 38-47. [Shchegolev A.I., Tumanova U.N., Serov V.N. The role of the placenta in the development of neonatal brain lesions. Obstetrics and Gynecology. 2023; (8): 38-47. (in Russian)]. https://dx.doi.org/10.18565/aig.2023.185.
- Nijman M., van der Meeren L.E., Nikkels P.G.J., Stegeman R., Breur J.M.P.J., Jansen N.J.G. et al.; CHD LifeSpan Study Group. Placental pathology con-tributes to impaired volumetric brain development in neonates with congenital heart disease. J. Am. Heart Assoc. 2024; 13(5): e033189. https://dx.doi.org/10.1161/JAHA.123.033189.
- Цибизова В.И., Первунина Т.М., Артеменко В.А., Бицадзе В.О., Гоциридзе К.Э., Аверкин И.И., Блинов Д.В., Новикова Н.Ю. Ключевая функция плаценты в формировании врожденного порока сердца плода. Акушерство, гинекология и репродукция. 2022; 16(1): 66-72. [Tsibizova V.I., Pervunina T.M., Artemenko V.A., Bitsadze V.O., Gotsiridze K.E., Averkin I.I., Blinov D.V., Novikova N.Yu. Placenta crucially affects formation of fetal congenital heart disease. Obstetrics, Gynecology and Reproduction. 2022; 16(1): 66-72. (in Russian)]. https://dx.doi.org/10.17749/2313-7347/ob.gyn.rep.2022.262.
- Ярыгина Т.А., Гасанова Р.М., Марзоева О.В., Леонова Е.И., Малоземова О.Г., Сыпченко Е.В. Особенности развития и функции плаценты при врожденном пороке сердца у плода. Бюллетень НЦССХ им. А.Н. Бакулева РАМН. Сердечно-сосудистые заболевания. 2023; 24(S6): 192. [Yarygina T.A., Gasanova R.M., Marzoeva O.V., Leonova E.I., Malozemova O.G., Sypchenko E.V. Features of the development and function of the placenta in congenital heart disease in the fetus. Bulletin of Bakoulev Center. Cardiovascular Diseases. 2023; 24(S6): 192. (in Russian)].
- Кравченко Е.Н., Коломбет Е.В., Любавина А.Е. Исследование плода и плаценты при врожденных пороках развития, несовместимых с жизнью. Лечащий врач. 2017; 10: 62. [Kravchenko E.N., Colombet E.V., Lyubavina A.E. Study of the fetus and placenta in congenital malformations incompatible with life. Lechaschi Vrach. 2017; (10): 62. (in Russian)].
- Щеголев А.И., Павлов К.А., Дубова Е.А., Фролова О.Г. Ранняя неонатальная смертность в Российской Федерации в 2010 г. Архив патологии. 2013; 75(4): 15-9. [Shchegolev A.I., Pavlov K.A., Dubova E.A., Frolova O.G. Early neonatal mortality in the Russian Federation in 2010. Russian Journal of Archive of Pathology. 2013; 75(4): 15‑9. (in Russian)].
- Туманова У.Н., Шувалова М.П., Щеголев А.И. Анализ статистических показателей врожденных аномалий как причины ранней неонатальной смерти в Российской Федерации. Российский вестник перинатологии и педиатрии. 2018; 63(6): 60-7. [Tumanova U.N., Shuvalova M.P., Shchegolev A.I. Analysis of statistical indicators of congenital anomalies as causes of early neonatal death in the Russian Federation. Russian Bulletin of Perinatology and Pediatrics. 2018; 63(6): 60-7. (in Russian)]. https://dx.doi.org/10.21508/10274065-2018-63-5-60-67.
- Villar J., Cheikh Ismail L., Victora C.G., Ohuma E.O., Bertino E., Altman D.G. et al.; International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH-21st). International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014; 384(9946): 857-68. https://dx.doi.org/10.1016/S0140-6736(14)60932-6.
- Щеголев А.И. Современная морфологическая классификация повреждений плаценты. Акушерство и гинекология. 2016; 4: 16-23. [Shchegolev A.I. Current morphological classification of damages to the placenta. Obstetrics and Gynecology. 2016; (4): 16-23. (in Russian)]. https://dx.doi.org/10.18565/aig.2016.4.16-23.
- Flatley C., Sole-Navais P., Vaudel M., Helgeland Ø., Modzelewska D., Johansson S. et al. Placental weight centiles adjusted for age, parity and fetal sex. Placenta. 2022; 117: 87-94. https://dx.doi.org/10.1016/j.placenta.2021.10.011.
- Здравоохранение в России. 2023. Статистический сборник. М.: Росстат; 2023. 181с. [Healthcare in Russia. 2023. Statistical collection. Moscow: Rosstat; 2023. 181p. (in Russian)].
- Щеголев А.И., Серов В.Н. Клиническая значимость поражений плаценты. Акушерство и гинекология. 2019; 3: 54-62. [Shchegolev A.I., Serov V.N. Clinical significance of placental lesions. Obstetrics and Gynecology. 2019; (3): 54-62. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.3.54-62.
- Туманова У.Н., Щеголев А.И. Поражения плаценты в генезе мертворождения (обзор литературы). Международный журнал прикладных и фундаментальных исследований. 2017; (3-1): 77-81. [Tumanova U.N., Shchegolev A.I. Placental lesions as the cause of stillbirth (review). International Journal of Applied and Basic Research. 2017; (3-1): 77-81. (in Russian)]. https://dx.doi.org/10.17513/mjpfi.11403.
- Leon R.L., Sharma K., Mir I.N., Herrera C.L., Brown S.L., Spong C.Y. et al. Placental vascular malperfusion lesions in fetal congenital heart disease. Am. J. Obstet. Gynecol. 2022; 227(4): 620.e1-620.e8. https://dx.doi.org/10.1016/j.ajog.2022.05.038.
- Ortinau C.M., Newburger J.W. Placenta-heart-brain connection in congenital heart disease. J. Am. Heart Assoc. 2024; 13(5): e033875. https://dx.doi.org/10.1161/JAHA.124.033875.
- Carvalho J.S., Axt-Fliedner R., Chaoui R., Copel J.A., Cuneo B.F., Goff D. et al. ISUOG Practice Guidelines (updated): fetal cardiac screening. Ultrasound Obstet. Gynecol. 2023; 61(6): 788-803. https://dx.doi.org/10.1002/uog.26224.
- Rychik J., Donaghue D.D., Levy S., Fajardo C., Combs J., Zhang X. et al. Maternal psychological stress after prenatal diagnosis of congenital heart disease. J. Pediatr. 2013; 162(2): 302-7.e1. https://dx.doi.org/10.1016/j.jpeds.2012.07.023.
- Williams A., Saizy S., Mendola P., Grobman W., Subramaniam A., Stevens D.R. et al. Prenatal exposure to perceived stress, maternal asthma, and placental size. Placenta. 2023; 139: 127-33. https://dx.doi.org/10.1016/j.placenta.2023.06.012.
- Rajagopalan V., Schmithorst V., El-Ali A., Reynolds W., Lee V., Wallace J. et al. Associations between maternal risk factors and intrinsic placental and fetal brain functional properties in congenital heart disease. Int. J. Mol. Sci. 2022; 23(23): 15178. https://dx.doi.org/10.3390/ijms232315178.
- Приказ Министерства здравоохранения РФ от 20.10.2020 N1130н "Об утверждении Порядка оказания медицинской помощи по профилю "акушерство и гинекология". 611 с. [Order of the Ministry of Health of Russian Federation dated October 20, 2020 N1130n "On approval of the Procedure for the provision of medical care in the field of obstetrics and gynecology". 611p. (in Russian)].
- Josowitz R., Linn R., Rychik J. The placenta in congenital heart disease: form, function and outcomes. Neoreviews. 2023; 24(9): e569-e582. https://dx.doi.org/10.1542/neo.24-9-e569.
Received 15.05.2024
Accepted 01.06.2024
About the Authors
Tamara A. Yarygina, PhD, Head of the Ultrasound Diagnostics Department, Moscow Regional Research Institute of Obstetrics and Gynecology named after AcademicianV.I. Krasnopolsky, 101000, Russia, Moscow, Pokrovka str., 22a; Researcher at the Perinatal Cardiology Center, A.N. Bakulev National Medical Research Center of Cardiovascular Surgery, Ministry of Health of Russia, 121552, Russia, Moscow, Roublyevskoe Shosse, 135; Associated Professor at the Department of Ultrasound Diagnostics of the Faculty of Continuing Medical Education of the Medical Institute, Peoples’ Friendship University of Russia named after Patrice Lumumba, 127015, Russia, Moscow, Pistsovaya str., 10, +7(495)414-78-75, tamarayarygina@gmail.com, https://orcid.org/0000-0001-6140-1930
Rena M. Gasanova, Dr. Med. Sci., Head of the Perinatal Cardiology Center, A.N. Bakulev National Medical Research Center for Cardiovascular Surgery, Ministry of Health
of Russia, 121552, Russia, Moscow, Roublyevskoe Shosse, 135; Physician of Ultrasound Diagnostics, Department of Ultrasound and Functional Diagnostics, Academician
V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Academician Oparin str., 4, rmgasanova@bakulev.ru, https://orcid.org/0000-0003-3318-1074
Olga V. Marzoeva, PhD, Doctor of Ultrasound Diagnostics, Researcher at the Perinatal Cardiology Center, A.N. Bakulev National Medical Research Center for Cardiovascular Surgery, Ministry of Health of Russia, 121552, Russia, Moscow, Roublyevskoe Shosse, 135, +7(495)414-78-75, ovmarzoeva@bakulev.ru, https://orcid.org/0000-0003-4475-0105
Elena V. Sypchenko, PhD, Doctor of Ultrasound Diagnostics at Perinatal Cardiology Center, A.N. Bakulev National Medical Research Center for Cardiovascular Surgery, Ministry of Health of Russia, 121552, Russia, Moscow, Roublyevskoe Shosse, 135, +7(495)414-78-75, evsypchenko@bakulev.ru, https://orcid.org/0000-0002-8809-7913
Elena I. Leonova, Doctor of Ultrasound Diagnostics at Perinatal Cardiology Center, A.N. Bakulev National Medical Research Center for Cardiovascular Surgery, Ministry of Health of Russia, 121552, Russia, Moscow, Roublyevskoe Shosse, 135, +7(495)414-78-75, eileonova@bakulev.ru https://orcid.org/0000-0002-6140-7950
Vyacheslav M. Lyapin, Pathologist of the Pathology Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Academician Oparin str., 4, +7(495)531-44-44, v_lyapin@oparina4.ru
Alexander I. Shchegolev, Dr. Med. Sci., Professor, Head of the 2nd Pathoanatomical Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Academician Oparin str., 4, +7(495)531-44-44, ashegolev@oparina4.ru,
https://orcid.org/0000-0002-2111-1530
Alexander I. Gus, Dr. Med. Sci., Professor, Chief Researcher at the Department of Ultrasound and Functional Diagnostics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Academician Oparin str., 4; Head of the Department of Ultrasound Diagnostics of the Faculty of Continuing Medical Education of the Medical Institute, Peoples’ Friendship University of Russia named after Patrice Lumumba, 127015, Russia, Moscow, Pistsovaya str., 10, a_gus@oparina4.ru, https://orcid.org//0000-0003-1377-3128
Corresponding author: Tamara A. Yarygina, tamarayarygina@gmail.com



